ABSTRACT
Objective: To determine the proportion of adults treated for depression in the US who achieve remission and, among those not achieving remission, the proportion receiving augmentation treatment.
Methods: Using data from the US National Health and Nutrition Examination Survey (NHANES) for years 2013–2014, 2015–2016, and 2017–2018, we identified 869 adults who reported using antidepressant medications for depression for at least 3 months. This sample was partitioned into remitted (score < 5) and non-remitted (score ≥ 5) respondents based on 9-item Patient Health Questionnaire (PHQ-9) score—a questionnaire based on the DSM-IV criteria for major depressive disorder. Among the non-remitted group, the proportion receiving antidepressant augmentation with another antidepressant medication of a different class or other medications was also assessed.
Results: An estimated 43.5% of adults receiving antidepressant medications for depression were in remission when assessed. Among those not in remission, 28.1% were using augmentation treatment, which in most cases was another antidepressant medication from a different class. As compared to depressed adults without any mental health contact in the past year, those with such contact had significantly higher odds of using augmentation treatment (adjusted odds ratio = 2.72; 95% CI, 1.56–4.76; P = .001)
Conclusions: The low percentage of US adults treated with antidepressants for depression that achieves remission represents a missed clinical and public health opportunity to optimize depression treatment. Closer monitoring of symptoms through measurement-based care and setting symptom remission as a goal can help improve outcomes for adults with depression.
J Clin Psychiatry 2021;82(6):21m13988
To cite: Mojtabai R, Amin-Esmaeili M, Spivak S, et al. Remission and treatment augmentation of depression in the United States. J Clin Psychiatry. 2021;82(6):21m13988.
To share: https://doi.org/10.4088/JCP.21m13988
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland
bDepartment of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland
cIranian National Center for Addiction Studies (INCAS), Tehran University of Medical Sciences, Tehran, Iran
dDepartment of Psychiatry, Vagelos College of Physicians and Surgeons, Columbia University, and New York State Psychiatric Institute, New York, New York
*Corresponding author: Ramin Mojtabai, MD, PhD, MPH, 624 N. Broadway, Room 797, Baltimore, MD 21205 ([email protected]).
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. This activity is free.
CME Objective
After studying this article, you should be able to:
- Try evidence-based strategies such as augmentation when measurement-based care for depression indicates that patients have not achieved symptom remission
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by the ACCME.
Release, Expiration, and Review Dates
This educational activity was published in November 2021 and is eligible for AMA PRA Category 1 Credit™ through December 31, 2023. The latest review of this material was October 2021.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past 3 years, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the Independent Data Safety and Monitoring Committee for Janssen (Johnson & Johnson) and Novartis; and has served on advisory boards for Eliem and Sage. As an employee of Massachusetts General Hospital (MGH), Dr Freeman works with the MGH National Pregnancy Registry, which receives funding from Alkermes, Aurobindo, AuroMedics, Johnson & Johnson/Janssen, Otsuka, Sage, Sunovion, Supernus, and Teva, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
See commentary by Rush
Many patients treated for major depressive disorder (MDD) continue to experience depressive symptoms after weeks of antidepressant treatment.1–5 In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial,4 for example, only 28% of patients achieved remission after up to 14 weeks of citalopram monotherapy. Because not achieving remission is associated with increased risk of recurrence and worse outcomes,6–8 full remission of depressive symptoms—usually, within 6–12 weeks of treatment initiation for acute MDD9—is a widely accepted treatment goal.1,9,10
Remission can be achieved in specialty mental health or primary care treatment settings.11 Several strategies to enhance antidepressant treatment response have evidence-based support, including dose optimization,12 switching antidepressants within or across classes, augmentation with an antidepressant from a different class,13 or augmentation with other medications.14,15 While most guidelines support reviewing diagnosis, assessing treatment adherence, and optimizing antidepressant dose as first steps in managing partial response or nonresponse to antidepressant treatment,9,16,17 there is little agreement regarding the superiority of medication switching compared to medication augmentation strategies.18–21 Reviewing the available evidence, the 2016 Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines16 recommended that the choice between switching and augmentation strategies should be individualized based on clinical factors, including level of initial treatment response. There appears to be a consensus that augmentation is the preferred strategy when there is an initial partial response to treatment, whereas switching within or across antidepressant classes is preferred for nonresponse to initial treatment.17,22
The 2010 American Psychiatric Association’s Practice Guideline for the Treatment of Patients With Major Depressive Disorder9 recommended 3 augmentation strategies “with moderate clinical confidence”: atypical antipsychotic medications, lithium, and thyroid hormone. In the intervening years, evidence has grown supporting use of these medications23 and other medications for augmentation treatment, including the anticonvulsant lamotrigine,24 stimulant medications, and the dopamine agonist pramipexole.25,26 However, the extent to which these augmentation strategies are used in the US is not known. One European study27 suggested that few of these strategies are used in day-to-day practice and that patients with residual depression symptoms often continue taking the same medications for months.
In the present study, we use data from a nationally representative general population survey to examine the prevalence of non-remission and use of medication augmentation in adults currently receiving antidepressant treatment for depression. While past general population surveys in the US have provided estimates of prevalence and treatment of depression,28–30 and depression remission,31,32 they have not examined the prevalence of augmentation treatment. These new findings can potentially inform efforts to improve the care of adult depression.
METHODS
Sample
Data were drawn from 3 successive cycles of the National Health and Nutrition Examination Survey (NHANES): 2013–2014, 2015–2016, and 2017–2018. NHANES is a nationally representative survey of the general population conducted by the National Center for Health Statistics.33 Starting in 1999, the NHANES has been conducted biennially. Completed interviews were obtained from 17,961 adults aged 18 years and over. Response rates for the NHANES, as defined by the proportion with completed interviews among screened potential respondents, ranged from 71.0% in 2013–2014 to 57.1% in 2017–2018. Computerized interviews were conducted in the respondents’ homes. The NHANES data are deidentified, publicly available, and exempted from institutional ethical review. Further details on study design and sample description are provided elsewhere.34 The study sample was limited to 869 adult respondents of the 3 NHANES cycles who reported using antidepressant medications for at least 3 months for depression and whose remission status could be ascertained (see the next section), representing 6.9% (weighted) of the US adult population.
Assessments
Antidepressant treatment for depression was defined by self-reported past-month use of these medications for depression. Antidepressants comprised serotonin reuptake inhibitors (SSRIs), including citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, vilazodone, and vortioxetine; serotonin-norepinephrine reuptake inhibitors (SNRIs), including venlafaxine, desvenlafaxine, duloxetine, milnacipran, and levomilnacipran; tricyclic antidepressants (TCAs), including amitriptyline, clomipramine, desipramine, imipramine, nortriptyline, protriptyline, trimipramine, and amoxapine; monoamine oxidase inhibitors (MAOIs), including isocarboxazid, phenelzine, tranylcypromine, and selegiline transdermal; and other antidepressants, including bupropion, agomelatine, maprotiline, mirtazapine, and nefazodone.
The interviewers inspected and confirmed medication packages for 87.7%–89.4% of medications across the survey cycles. The length of time the respondent had been taking each medication was also recorded. For respondents taking more than one antidepressant, the length of medication use was based on the longest used medication. To help ensure that respondents were in the maintenance phase of treatment, the analyses were limited to respondents using at least one antidepressant for at least 3 months.
Respondents were also asked about the “main reason” for taking each medication. These reasons were translated into the International Classification of Diseases, 10th Edition, Clinical Modification (ICD-10-CM) categories (https://www.icd10data.com/ICD10CM/Codes) by the National Center for Health Statistics using explicit guidelines. Analyses were restricted to respondents who reported taking antidepressants for MDD coded as “major depressive disorder, single episode, unspecified” (F32.9) and “major depressive disorder, recurrent, unspecified” (F33.9). The details of the coding strategy are available elsewhere (https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/RXQ_RX_J.htm).
Remission was ascertained by the 9-item Patient Health Questionnaire (PHQ-9),35 a commonly used measure that assesses the frequency of 9 Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) symptom criteria for major depressive episodes in the past 2 weeks. Frequency of each symptom ranges from “not at all” (0) to “nearly every day” (3). Total scores, computed by summing the symptom scores, can range from 0 to 27. PHQ-9 scores of < 5 indicate remission of depression.35,36 In sensitivity analyses, an alternative cutoff PHQ-9 score of < 10 was also assessed. The validity of PHQ-9 ratings has been established in past research.35,37
Following questions about specific depressive symptoms, respondents who reported any symptoms were asked, “How difficult have these problems made it for you to do your work, take care of things at home, or get along with people?” Responses included “not at all difficult” (0), “somewhat difficult” (1), “very difficult” (2), and “extremely difficult” (3).
Antidepressant augmentation strategies were limited to use of another antidepressant from a different class, an atypical antipsychotic medication, thyroid hormone, lithium, lamotrigine, modafinil, lisdexamfetamine, and pramipexole.9,24–26
MAOIs (with the exception of transdermal selegiline) and trazodone were not considered as augmentation treatments because of limited evidence supporting their efficacy for this purpose and because trazodone is commonly prescribed as a sleep aid medication.38
Atypical antipsychotic medications included aripiprazole, asenapine, brexpiprazole, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone.
No specific ICD-10-CM categories exist for augmentation treatment. Therefore, we developed an algorithm to identify uses of these medications that are more likely to be for antidepressant augmentation than other clinical indications (Supplementary Appendix 1).
Other variables in the analyses included sex, age, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other), income compared to Federal Poverty Level (FPL), education, common chronic medical conditions, use of health services, any mental health visits in the past year, and health insurance (private, Medicaid, Medicare, other types of health insurance). Chronic health conditions included arthritis, lung disease (asthma, chronic obstructive pulmonary disease, and bronchitis), diabetes mellitus, hypertension, and heart disease (coronary heart disease or history of heart attacks). Health services were assessed as the number of times respondents reported seeing a doctor or other health care professional during the past 12 months.
Statistical Analyses
Analyses were conducted in 3 stages. First, the prevalence of remission among respondents using antidepressants for depression was assessed. The prevalence of different PHQ-9 symptoms and the level of difficulty caused by symptoms were also compared among respondents whose depression had and had not remitted.
Second, factors associated with non-remission were assessed using multivariable logistic regression models. Models were adjusted for sociodemographic characteristics, chronic medical conditions, use of health services, health insurance, length of antidepressant treatment, and type of antidepressants (SSRI vs other types of antidepressants).
Third, the prevalence of antidepressant augmentation and factors associated with augmentation among respondents whose depression had not remitted were examined using multivariable logistic regression models adjusting for the same variables noted above.
In sensitivity analyses, correlates were assessed for remission defined as PHQ-9 score < 10 and for augmentation treatment among those not remitted based on this definition.
Svy routines of STATA 16 software (StataCorp, 2020; College Station, Texas) were used for analyses to account for the complex survey design of NHANES. All percentages reported are weighted. The weighted percentages may not match the actual numbers of respondents. A P < .05 (2-tailed) significance level was used.
RESULTS
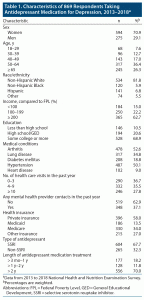
Table 1 presents characteristics of respondents using antidepressants for depression. A majority were female, aged 50 years or older, non-Hispanic White, with family income ≥ 200% FPL, college education, private insurance, and at least one medical condition. SSRIs were the most common class of antidepressants used for depression. More than two-thirds had been using the same medication for more than 2 years (Table 1).
Remission
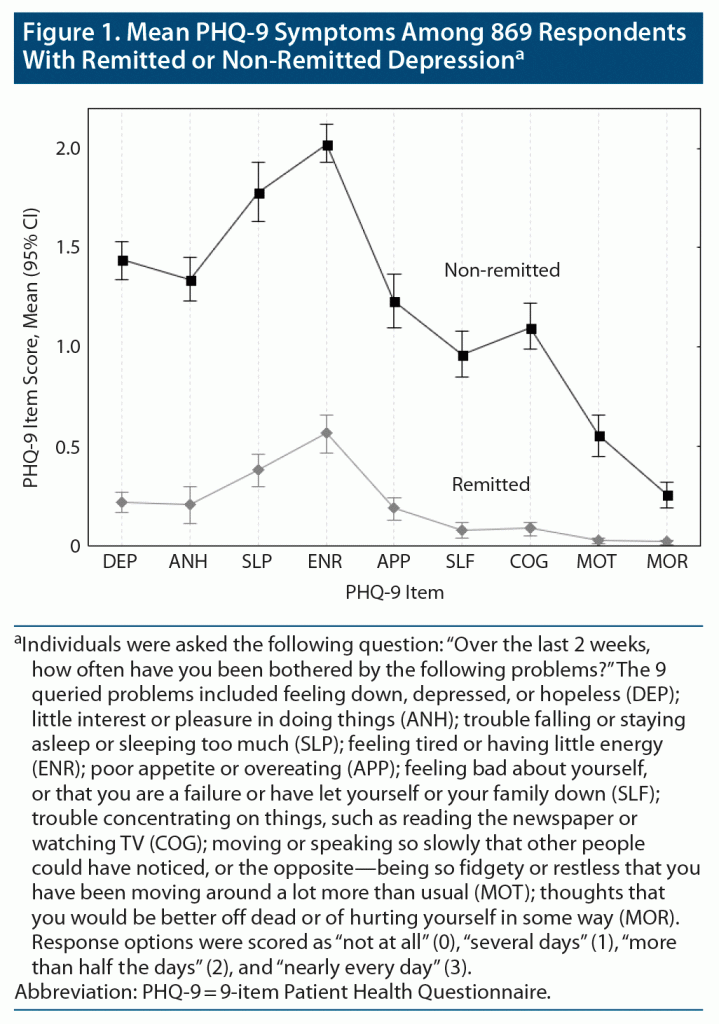
Among the 869 respondents using antidepressants for depression for at least 3 months, 43.5% (n = 329) were in remission; 56.5% (n = 540) experienced residual symptoms (PHQ-9 score ≥ 5). The most frequent residual symptoms were “feeling tired or having little energy” and “trouble falling or staying asleep or sleeping too much” (Figure 1). A total of 41.5% (n = 227) of non-remitted respondents reported feeling tired or having little energy and 36.7% (n = 197) reported sleep problems nearly every day in the past 2 weeks (data not shown).
Compared to respondents not in remission, those in remission reported much less difficulty in work, taking care of things at home, and getting along with people (mean difficulty score = 1.01 vs 0.18, regression coefficient = −0.84, SE = 0.05, P < .001). While 22.7% of respondents not in remission reported that the depression had made it very or extremely difficult to complete these tasks, less than 0.01% of those whose depression had remitted reported this level of difficulty.
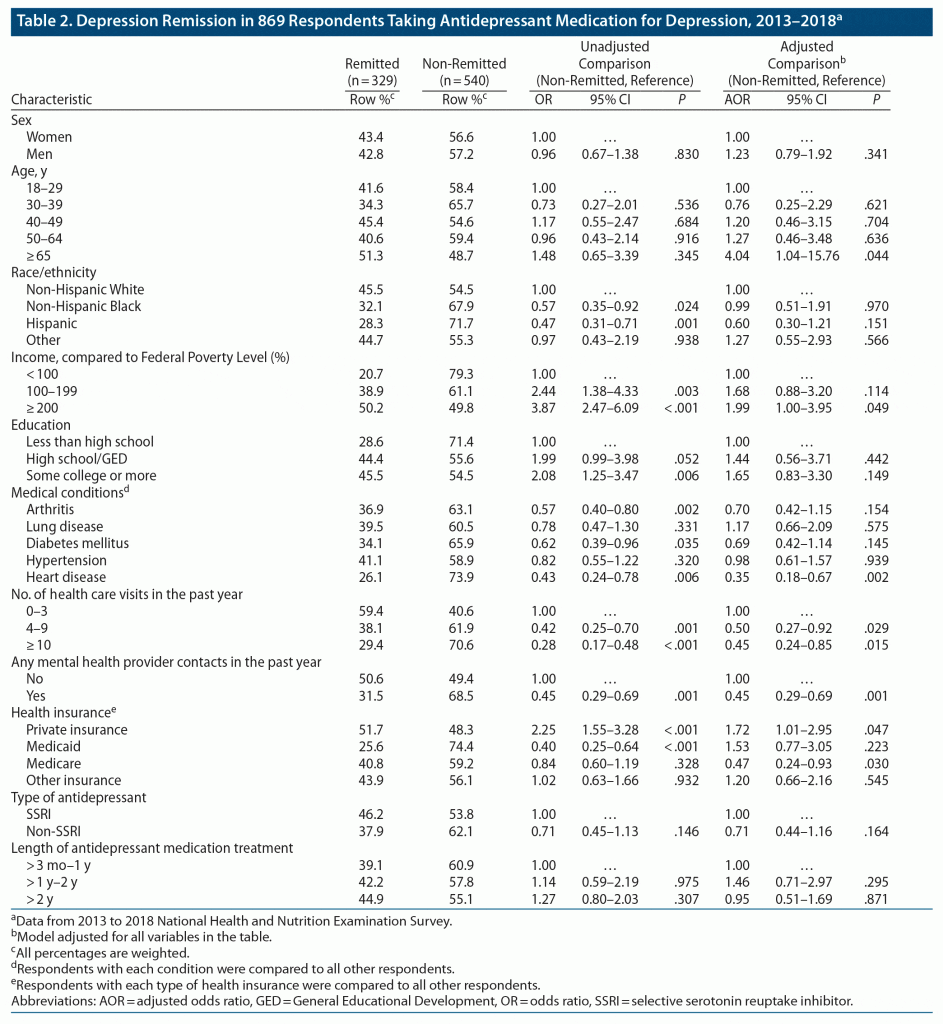
In adjusted analyses, adults in the ≥ 65 years age group had higher odds of remitting than respondents aged 18–29 years (51.3% vs 41.6%; adjusted odds ratio [AOR] = 4.04; 95% CI, = 1.04–15.76, P = .044), as did respondents with family incomes in the ≥ 200% FPL compared to those with family income < 100% FPL (50.2% vs 20.7%; AOR = 1.99; 95% CI, 1.00–3.95; P = .049) (Table 2).
Among physical health comorbidities, only heart disease was significantly associated with lower adjusted odds of remission compared to no heart disease (26.1% vs 45.1%; AOR = 0.35; 95% CI; 0.18%–0.67%; P = .002). A larger number of health care visits in the past year was also associated with lower odds of remission compared to 0–3 visits (38.1% vs 59.4%; AOR = 0.50; 95% CI, 0.27–0.92; P = .029 for 4–9 visits; 29.4% vs 59.4%; AOR = 0.45; 95% CI, 0.24–0.85; P = .015 for ≥ 10 visits), as was any mental health contact in the past year compared to no contact (31.5% vs 50.6%; AOR = 0.45; 95% CI, 0.29–0.69; P = .001) (Table 2).
Of different types of insurance, private insurance was significantly associated with higher odds of remission compared to not having this coverage (51.7% vs 32.2%; AOR = 1.72; 95% CI, 1.01–2.95; P = .047) and Medicare with lower odds of remission (40.8% vs 44.9%; AOR = 0.47; 95% CI, 0.24–0.93; P = .030). The length of antidepressant treatment was not significantly associated with remission (Table 2).
In sensitivity analyses, 28.3% (n = 296) of the 869 respondents scored ≥ 10 on PHQ-9 and 71.8% (n = 573) scored < 10. The results of multivariable analyses for correlates of a PHQ-9 score < 10 were similar to those of the main analyses (Supplementary Appendix 2). Adults in the ≥ 65 years age range had higher odds compared to the 18–29 years age group, as did those with college education compared to less than high school education and those with private insurance compared to those without such insurance. In contrast, respondents with heart disease had lower odds of scoring in this range compared to those without heart disease, as did respondents with more health care visits, and those with mental health care provider contacts compared to those without such contacts.
Antidepressant Augmentation
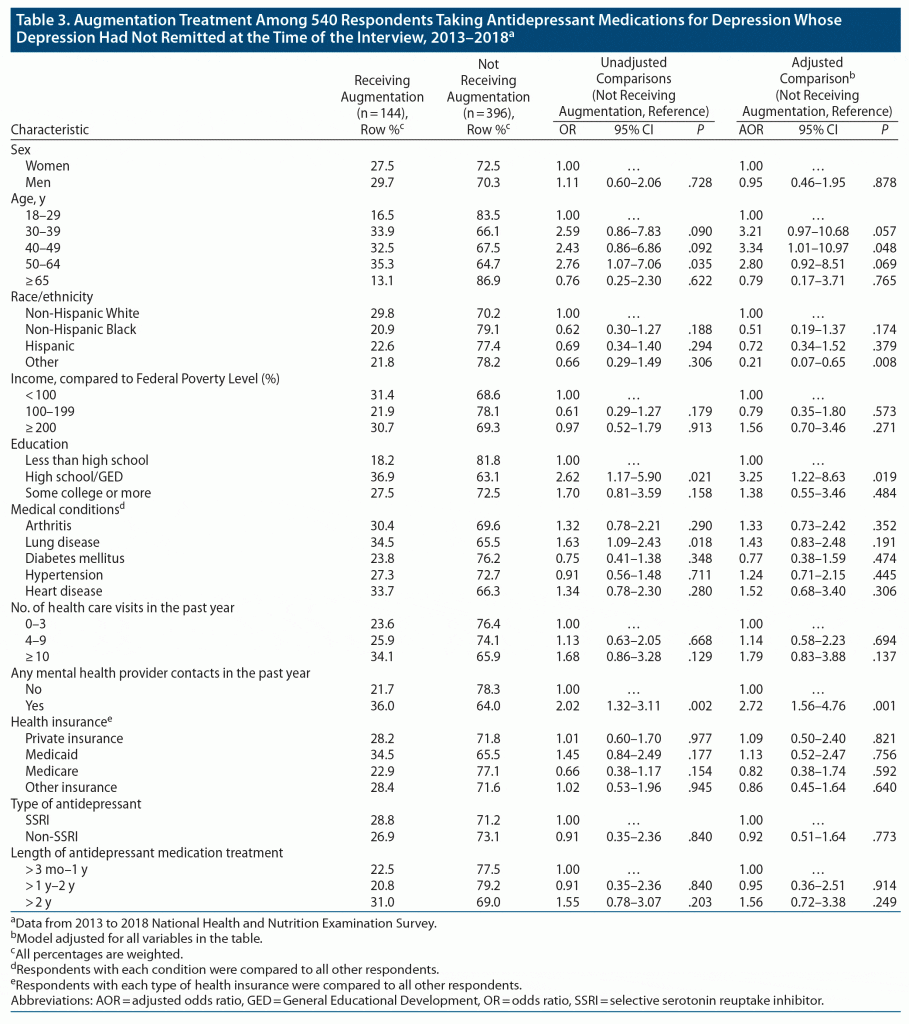
A total of 28.1% of respondents whose depression had not remitted were using augmentation medication. A list of various augmentation regimens is provided in Supplementary Appendix 3. The most common augmentation treatments were antidepressants from a different class (n = 104, 71.7% of respondents using augmentation), followed by atypical antipsychotics (n = 38, 25.7%).
In adjusted analyses, adults aged 40–49 years had significantly higher odds of using augmentation than those aged 18–29 years (32.5% vs 16.5%; AOR = 3.34; 95% CI, 1.01–10.97; P = .048), as did respondents with high school or GED education compared to those with less education (36.9% vs 18.2%; AOR = 3.25; 95% CI, 1.22–8.63; P = .019) and respondents who had mental health provider contact compared to those who did not (36.0% vs 21.7%; AOR = 2.72; 95% CI, 1.56–4.76; P = .001) (Table 3). In contrast, adults from the Other racial/ethnic group had lower odds of using augmentation treatment compared to non-Hispanic White respondents (21.8% vs 29.8%; AOR = 0.21; 95% CI, 0.07–0.65; P = .008).
In sensitivity analyses, 32.6% of respondents who scored ≥ 10 on PHQ-9 reported using augmentation treatment. The results of multivariate analyses of augmentation treatment among these respondents were similar to results of the main analyses (Supplementary Appendix 4). Adults aged 30–39 years had higher odds of using augmentation treatment compared to those aged 18–29 years, as did those with income ≥ 200 FPL compared to those with FPL < 100, respondents with high school/GED compared to respondents with less education, respondents with heart disease compared to those without, and those who had mental health provider contacts compared to those without such contacts. In contrast, respondents from the Other racial/ethnic groups had lower odds of using augmentation treatment compared to non-Hispanic White respondents.
DISCUSSION
This study presents a broad overview of the prevalence and correlates of depression non-remission and antidepressant augmentation treatment use among US adults. There were two main findings. First, most respondents currently on antidepressant treatment for depression for 3 months or longer experienced residual depressive symptoms and were not in remission based on a PHQ-9 cutoff score ≥ 5. They also experienced difficulties in daily living associated with these symptoms. These findings in a nationally representative sample are consistent with results from past clinical studies.1–5,39 In the STAR*D study,4 for example, most patients did not achieve remission by the end of a 14-week citalopram trial.
Persisting depressive symptoms put patients at increased risk of relapse and are associated with other adverse outcomes.6–8 A lower prevalence of remission among adults with lower income and heart disease highlights the importance of socioeconomic and health factors in depression remission. By contrast, lower remission rates among respondents with more health care contacts and with any mental health contact likely represent greater service use and greater likelihood of receiving care from mental health providers in those with more severe and persistent depression.40
In previous clinical and general population studies,2,5,41,42 poor physical health and lower socioeconomic status have been consistent predictors of poor response to treatment and course of depression, highlighting the role of social and health adversities in the course of depression. The association between heart disease and non-remission underscores the need for coordinated mental and physical health care. Collaborative care interventions have been shown to improve care and outcomes in patients with depression43 and provide opportunities to address both physical and mental health needs. The greater likelihood of augmentation treatment in respondents who had contact with mental health providers also points to potential benefits of collaborative care.43
A second finding was that augmentation was received by only a small fraction of antidepressant-treated adults with non-remitted depression. Low use of proven augmentation strategies, such as lithium, is especially noteworthy and consistent with past research.44,45 Augmentation strategies, along with optimizing the antidepressant dose or medication switching, can help increase the likelihood of remission.46 In the STAR*D program,2,47 most patients who completed the different stages of treatment finally achieved remission, sometimes after a series of medication switches and augmentation attempts. In contrast, most respondents taking antidepressant medications in the present study had not achieved remission, even though a majority stayed on the same medication for more than 2 years. This finding suggests missed opportunities to optimize medication regimens to improve chances of full remission.
Few past studies have examined the prevalence of depression augmentation treatment in the general population.27,40 A European study of patients hospitalized for depression27 reported that over 84% of these patients had continued to receive the same medication for many weeks. Only about 14% received augmentation treatment. Another study based on the US National Ambulatory Medical Care Survey40 reported that prescription of atypical antipsychotic medications among patients with non-psychotic depression increased from 4.6% in 1999–2000 to 12.5% in 2009–2010. Other forms of augmentation treatment were not assessed. Furthermore, the remission status of patients could not be assessed in either study.
Non-recognition of residual symptoms of depression may contribute to low uptake of antidepressant augmentation. Monitoring treatment response with validated measures and adjusting treatment accordingly—generally known as “measurement-based care”—can potentially improve recognition of residual symptoms and their management.36,48
In interpreting these results, several limitations should be considered. First, the NHANES did not capture symptom ratings at the start of antidepressant treatment. Second, other strategies such as antidepressant dose change or medication switching, as well as possible previous augmentation attempts,. Also, information on psychotherapy was not collected. However, a sizeable proportion of the sample, especially those with non-remitted depression, had contact with mental health providers. Third, the most common residual depressive symptoms were fatigue and sleep problems, which are difficult to distinguish from similar complaints in physical conditions. Fourth, many patients who start antidepressants remit and stop the medication shortly thereafter.49 These patients would be undercounted in a cross-sectional sample of patients currently on antidepressant treatment, whereas long-term users of medications would be overrepresented.50 Fifth, a causal relationship between physical health conditions and non-remission of depression cannot be established in this cross-sectional study. The relationship between physical health conditions and depression is thought to be bidirectional.51–55 Finally, depression diagnoses were based on self-reported symptoms for which respondents were prescribed antidepressants rather than research diagnoses.
In the context of these limitations, this study offers an overview of the prevalence of non-remission and medication augmentation treatment in individuals receiving antidepressant treatment for depression in the US. The high prevalence of residual symptoms in individuals who had stayed on the same antidepressant medications for extended periods is concerning and calls for greater attention to evidence-based strategies to improve the pharmacologic management of adult depression.
Submitted: March 4, 2021; accepted July 29, 2021.
Published online: November 2, 2021.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, fluvoxamine, milnacipran, levomilnacipran, clomipramine, agomelatine, asenapine, lurasidone, olanzapine, paliperidone, risperidone, ziprasidone, thyroid hormone, lithium, lamotrigine, modafinil, lisdexamfetamine, and pramipexole are not approved by the US Food and Drug Administration for the treatment of major depressive disorder.
Financial disclosure: None.
Funding/support: None.
Additional information: The original data set for the National Health and Nutrition Examination Survey (NHANES) is available from the website of the survey sponsored by the National Center for Health Statistics, Centers for Disease Control and Prevention (https://wwwn.cdc.gov/nchs/nhanes/).
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Only 43.5% of US adults receiving antidepressant medications for depression are in remission at any time.
- Only 28.1% of US adults with non-remitted depression receiving antidepressant medications are using augmentation treatment, which in most cases is another antidepressant medication from a different class.
References (55)

- Thase ME. The clinical, psychosocial, and pharmacoeconomic ramifications of remission. Am J Manag Care. 2001;7(11 suppl):S377–S385. PubMed
- Mojtabai R. Nonremission and time to remission among remitters in major depressive disorder: revisiting STAR*D. Depress Anxiety. 2017;34(12):1123–1133. PubMed CrossRef
- Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41–50. PubMed CrossRef
- Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. PubMed CrossRef
- Vuorilehto MS, Melartin TK, Isometsä ET. Course and outcome of depressive disorders in primary care: a prospective 18-month study. Psychol Med. 2009;39(10):1697–1707. PubMed CrossRef
- Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2–3):97–108. PubMed CrossRef
- Judd LL, Paulus MJ, Schettler PJ, et al. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am J Psychiatry. 2000;157(9):1501–1504. PubMed CrossRef
- Judd LL, Akiskal HS, Maser JD, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55(8):694–700. PubMed CrossRef
- Gelenberg AJ, Freeman MP, Markowitz JC, et al; Work Group on Major Depressive Disorder. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
- Trivedi MH. Treating depression to full remission. J Clin Psychiatry. 2009;70(1):e01. PubMed CrossRef
- Dawson MY, Michalak EE, Waraich P, et al. Is remission of depressive symptoms in primary care a realistic goal? a meta-analysis. BMC Fam Pract. 2004;5(1):19. PubMed CrossRef
- Waldschmitt C, Vogel F, Pfuhlmann B, et al. Duloxetine serum concentrations and clinical effects: data from a therapeutic drug monitoring (TDM) survey. Pharmacopsychiatry. 2009;42(5):189–193. PubMed CrossRef
- Onder E, Tural U. Faster response in depressive patients treated with fluoxetine alone than in combination with buspirone. J Affect Disord. 2003;76(1-3):223–227. PubMed CrossRef
- Mohamed S, Johnson GR, Chen P, et al; and the VAST-D Investigators. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132–145. PubMed CrossRef
- Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243–1252. PubMed CrossRef
- Kennedy SH, Lam RW, McIntyre RS, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3, pharmacological treatments. Can J Psychiatry. 2016;61(9):540–560. PubMed CrossRef
- Malhi GS, Bell E, Singh AB, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: major depression summary. Bipolar Disord. 2020;22(8):788–804. PubMed CrossRef
- Tundo A, de Filippis R, Proietti L. Pharmacologic approaches to treatment resistant depression: evidences and personal experience. World J Psychiatry. 2015;5(3):330–341. PubMed CrossRef
- Zisook S, Johnson GR, Hicks P, et al. Continuation phase treatment outcomes for switching, combining, or augmenting strategies for treatment-resistant major depressive disorder: a VAST-D report. Depress Anxiety. 2021;38(2):185–195. PubMed CrossRef
- Köhler S, Unger T, Hoffmann S, et al. Comparing augmentation with non-antidepressants over sticking to antidepressants after treatment failure in depression: a naturalistic study. Pharmacopsychiatry. 2013;46(2):69–76. PubMed
- Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114–119. PubMed CrossRef
- Goldberg JF, Freeman MP, Balon R, et al. The American Society of Clinical Psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders. Depress Anxiety. 2015;32(8):605–613. PubMed CrossRef
- Papadimitropoulou K, Vossen C, Karabis A, et al. Comparative efficacy and tolerability of pharmacological and somatic interventions in adult patients with treatment-resistant depression: a systematic review and network meta-analysis. Curr Med Res Opin. 2017;33(4):701–711. PubMed CrossRef
- Barbee JG, Thompson TR, Jamhour NJ, et al. A double-blind placebo-controlled trial of lamotrigine as an antidepressant augmentation agent in treatment-refractory unipolar depression. J Clin Psychiatry. 2011;72(10):1405–1412. PubMed CrossRef
- Pae CU. Pramipexole augmentation in treatment-resistant major depressive disorder. Expert Rev Neurother. 2014;14(1):5–8. PubMed CrossRef
- Cusin C, Iovieno N, Iosifescu DV, et al. A randomized, double-blind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J Clin Psychiatry. 2013;74(7):e636–e641. PubMed CrossRef
- Herzog DP, Wagner S, Ruckes C, et al. Guideline adherence of antidepressant treatment in outpatients with major depressive disorder: a naturalistic study. Eur Arch Psychiatry Clin Neurosci. 2017;267(8):711–721. PubMed CrossRef
- Blazer DG, Kessler RC, McGonagle KA, et al. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151(7):979–986. PubMed CrossRef
- Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. PubMed CrossRef
- Kessler RC, Berglund P, Demler O, et al; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105. PubMed CrossRef
- Sargeant JK, Bruce ML, Florio LP, et al. Factors associated with 1-year outcome of major depression in the community. Arch Gen Psychiatry. 1990;47(6):519–526. PubMed CrossRef
- Agosti V. Predictors of remission from chronic depression: a prospective study in a nationally representative sample. Compr Psychiatry. 2014;55(3):463–467. PubMed CrossRef
- National Health and Nutrition Examination Survey. National Center for Health Statistics, Centers for Disease Control and Prevention. 2017. Cited June 20, 2017. https://www.cdc.gov/nchs/nhanes/
- Chen TC, Clark J, Riddles MK, et al. National Health and Nutrition Examination Survey, 2015–2018: sample design and estimation procedures. Vital Health Stat 2. 2020;(184):1–35. PubMed
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. PubMed CrossRef
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry. 2009;70(suppl 6):26–31. PubMed CrossRef
- Huang FY, Chung H, Kroenke K, et al. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21(6):547–552. PubMed CrossRef
- Yi XY, Ni SF, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25–32. PubMed CrossRef
- Hierholzer R. Remission rates for depression in STAR*D study. Am J Psychiatry. 2006;163(7):1293, author reply 1293–1294. PubMed CrossRef
- Gerhard T, Akincigil A, Correll CU, et al. National trends in second-generation antipsychotic augmentation for nonpsychotic depression. J Clin Psychiatry. 2014;75(5):490–497. PubMed CrossRef
- Perlman K, Benrimoh D, Israel S, et al. A systematic meta-review of predictors of antidepressant treatment outcome in major depressive disorder. J Affect Disord. 2019;243:503–515. PubMed CrossRef
- Mojtabai R, Olfson M. Major depression in community-dwelling middle-aged and older adults: prevalence and 2- and 4-year follow-up symptoms. Psychol Med. 2004;34(4):623–634. PubMed CrossRef
- Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56(12):1109–1115. PubMed CrossRef
- Nelson JC, Baumann P, Delucchi K, et al. A systematic review and meta-analysis of lithium augmentation of tricyclic and second generation antidepressants in major depression. J Affect Disord. 2014;168:269–275. PubMed CrossRef
- Bauer M, Döpfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 2000;20(2):287. PubMed CrossRef
- Strawbridge R, Carter B, Marwood L, et al. Augmentation therapies for treatment-resistant depression: systematic review and meta-analysis. Br J Psychiatry. 2019;214(1):42–51. PubMed CrossRef
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Guo T, Xiang YT, Xiao L, et al. Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004–1013. PubMed CrossRef
- Samples H, Mojtabai R. Antidepressant self-discontinuation: results from the collaborative psychiatric epidemiology surveys. Psychiatr Serv. 2015;66(5):455–462. PubMed CrossRef
- Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the US National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75(2):169–177. PubMed CrossRef
- Jeon SW, Chang Y, Lim SW, et al. Bidirectional association between blood pressure and depressive symptoms in young and middle-age adults: A cohort study. Epidemiol Psychiatr Sci. 2020;29:e142. PubMed CrossRef
- Tang B, Yuan S, Xiong Y, et al. Major depressive disorder and cardiometabolic diseases: a bidirectional Mendelian randomisation study. Diabetologia. 2020;63(7):1305–1311. PubMed CrossRef
- Wium-Andersen MK, Wium-Andersen IK, Prescott EIB, et al. An attempt to explain the bidirectional association between ischaemic heart disease, stroke and depression: a cohort and meta-analytic approach. Br J Psychiatry. 2020;217(2):434–441. PubMed CrossRef
- Pan A, Keum N, Okereke OI, et al. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35(5):1171–1180. PubMed CrossRef
- Demakakos P, Zaninotto P, Nouwen A. Is the association between depressive symptoms and glucose metabolism bidirectional? evidence from the English Longitudinal Study of Ageing. Psychosom Med. 2014;76(7):555–561. PubMed CrossRef
This PDF is free for all visitors!