Abstract
Objective: To provide recommendations regarding the critical elements of the assessment package in treatment-resistant depression (TRD) consultation programs. This is a complementary manuscript to Part I, which discusses practical and logistical considerations for developing and sustaining a subspecialized TRD consultation program.
Participants: A group of 12 clinicians, researchers, administrators, and patient advocates from the National Network of Depression Centers (NNDC) TRD Task Group.
Evidence: The recommendations are based on expert opinion. This consensus statement reflects the effort of the NNDC’s TRD Task Group to reach agreement on a set of principles that those interested in establishing new consultation programs could use to guide their effort and a set of recommendations that could serve as a basis for future empirical work.
Consensus Process: Each member of the NNDC TRD Task Group provided a written description of the procedures used at their home institution, which were used during a day-long forum to achieve consensus on recommendations for each component of a TRD consultation program. Subgroups were formed to draft recommendations, and points of disagreement were resolved at subsequent meetings of the full task group.
Conclusions: We describe consensus recommendations regarding the goals of a TRD consultation, which include establishing the primary diagnosis and comorbidities, clarifying medical and psychiatric symptoms, identifying goals, documenting treatment history, identifying treatment barriers, and developing actionable treatment recommendations. We detail important components of the consultation evaluation process, the assessment tools to consider in establishing a TRD consultation program, and the qualifications of providers.
J Clin Psychiatry 2025;86(2):24cs15336
Author affiliations are listed at the end of this article.
See part I by Voytenko et al
Evidence-based assessment is an important component of high-quality care but must be adequately comprehensive, efficient, and flexible. Treatment-resistant depression (TRD) consultation programs represent a unique clinical service specializing in patients for whom front-line treatments have not generated an adequate response. Careful, evidence-based assessment is critical in these settings to ensure diagnostic accuracy and informed treatment planning to facilitate the best outcomes for the patient.
TRD is a common and debilitating condition associated with profound dysfunction, multimorbidity, and early mortality.1–3 TRD is typically defined as an episode in which 2 evidence-based treatments have failed to improve a patient’s condition.4,5 Lifetime major depressive disorder (MDD) is highly prevalent, and approximately 30% of people with MDD will eventually meet criteria for TRD.4,6 The prevalence of TRD is especially elevated in people with bipolar disorder,7 and is higher still (∼50%) when the definition of TRD considers failure to achieve sustained symptom remission.8,9
Given the high prevalence,10 specialized TRD consultation programs can provide referring clinicians and patients with guidance about enhanced, individualized treatment options.11 The National Network of Depression Centers (NNDC) has detailed important considerations for the establishment of TRD consultation clinics in Part I of the series, Developing a TRD Consultation Program.12 We encourage the reader to refer to the first installment for a description of the consensus process and for key considerations related to embedding a TRD consultation program in healthcare settings at the systems level.
Because the rapid evolution of treatments for depression is leading to a rise in the number of TRD specialty clinics, we believe that close attention to the components of the TRD evaluation process is necessary. In the following sections, we describe a framework integrating consensus on the practical needs of TRD assessment (efficiency, cost-effectiveness, and flexibility) with the need for reliable and valid measurement in standard care for TRD patients. This preliminary “roadmap” for TRD assessment is expected to quickly evolve with advances in TRD intervention science and methods to address health disparities.
PATIENT ASSESSMENT
Assessment Goals
The ultimate purpose of assessment is to guide effective treatment. Accurate assessment is particularly vital in the context of a TRD consultation. Patients presenting to TRD clinics have typically attempted several prior treatments that have not been adequately effective. Given the high morbidity and mortality associated with TRD, it is critical that assessments in these clinics (1) clarify the nature of the patient’s illness, (2) identify the patient’s goals for treatment, and (3) ultimately help to determine which interventions are most likely to be effective for this particular patient at this specific time. To accomplish the latter objective, it is essential to obtain a careful history of the patient’s prior treatment experiences, determine past and current barriers to treatment success, and identify patient characteristics that could guide treatment selection in line with the patient’s goals. Below, we describe each of these considerations in greater detail.
Goal 1: Determine the nature of the patient’s illness.
Clarify the primary diagnosis. A critical objective for an assessment in a TRD consultation clinic is to clarify the primary diagnosis. Several other psychiatric and medical conditions can contribute to presentations involving prolonged dysphoric affect, anhedonia, appetite, sleep disturbances, and other symptoms of depression. If the patient’s symptoms have previously been incompletely characterized, there may be little reason to expect that standard treatments on their own would be effective.
Among psychiatric conditions, key differential (or co-occurring) diagnoses for TRD include alcohol/ substance use, eating, sleep, psychotic, posttraumatic stress, and personality disorders.13,14 When these disorders are significant drivers of the patient’s presentation, different, more specifically targeted treatments may be appropriate. Depending on the nature of the condition, these alternative treatments might be presented prior to or concurrently with the next recommended depression treatment. Other issues, such as gender identity concerns, can likewise present as treatment resistance when they have not been appropriately addressed.9 In addition, several medical conditions can lead to symptoms of depression that are unlikely to resolve until the underlying medical cause is better managed. These include hypothyroidism, Cushing disease, Parkinson disease, metabolic disorders, cardiovascular events, dementia, and certain cancers.9,15 Treatments for medical illnesses, particularly treatments involving corticosteroids, interferons, and calcium channel blockers,14 can themselves cause or exacerbate depressive symptoms. A recent report16 observed a linear relationship between the number of nonpsychiatric medications a patient is taking that have a side effect profile that includes depressive symptoms and the likelihood of inadequate response to standard depression treatments. Whether and how thoroughly to screen for each of these considerations will be determined by a number of factors, including patient characteristics (eg, age), the nature of the presenting complaints, family history, and practical considerations at each clinic.
If the depressive symptoms are determined to result from a primary mood disorder, the nature of that disorder needs to be clarified. Two key determinations are whether there is evidence of an underlying bipolar pathology and whether psychotic symptoms are present. The delay between seeking treatment and receiving a correct diagnosis of bipolar disorder can last more than a decade.17,18 Moreover, nearly 10% of individuals diagnosed with unipolar depression convert to a diagnosis of bipolar disorder over the following decade,19 and nearly 20% of patients hospitalized with a diagnosis of unipolar depression go on to experience at least 1 manic episode within the next 15 years.20 Furthermore, even subthreshold levels of lifetime mania-related psychopathology are associated with poorer prognosis21 and with different underlying neurobiological mechanisms.22 Thus, a notable subset of individuals with TRD may have an undiagnosed or subthreshold bipolar pathology23,24 to be considered in making treatment recommendations.
Psychotic features in the context of a depressive illness can be overlooked, particularly when the abnormalities in thinking are mood-congruent.9 Even when these symptoms are clearly present, it can be difficult to determine whether the presentation represents a depressive disorder with psychotic features, schizoaffective disorder, or the initial emergence of schizophrenia.25 Each of these possibilities would lead to different treatment recommendations, and each could contribute to inadequate response from standard depression therapies.26
Identify relevant comorbidities. The disorders noted above can complicate the treatment of depression, even if they are judged not to be primary. Several additional comorbidities can influence the efficacy of treatments. Most prevalent are the anxiety disorders, which are commonly comorbid with depression.27 The STAR*D study observed that depressed patients with comorbid anxiety were substantially less likely and took longer to remit compared to those without anxiety.28 Determining the extent of the patient’s alcohol and substance use is also key, even if it does not rise to the level of a diagnosable disorder. The effects of alcohol and substances can impact mood and functioning, as well as response to treatments.14 The presence of comorbid personality pathology can likewise affect treatment response, and the direction of these effects appears to depend on the nature of the antidepressant treatment provided.29,30 Comorbid sleep disorders, such as narcolepsy and sleep apnea, as well as disturbances in sleep, including chronic insomnia and hypersomnia, are associated with increased symptoms of depression and can impact response to standard treatments for depression.31–34 Finally, cognitive and meta-cognitive deficits are often present among individuals with TRD13 and represent some of the strongest predictors of poor functioning even when symptoms have improved.35 These deficits may contribute to an individual’s sense that their treatment goals have not been met.
Medical comorbidities can affect treatments for depression in several ways. As noted above, certain medical conditions are themselves associated with the emergence or exacerbation of depressive symptoms, as are certain medical treatments. Other conditions and medications can alter or interfere with the pharmacokinetics and pharmacodynamics of certain medications for depression.9 Furthermore, chronic conditions, like chronic pain, multiple sclerosis, and diabetes, can impact a person’s functioning and complicate the treatment for depression, particularly when poorly controlled. A comprehensive treatment plan should include optimizing treatment of the medical conditions that may be exacerbating depression. If a medication prescribed for comorbidities is suspected of contributing substantially to a patient’s depression, the treatment plan should include a discussion of potential alternatives with the prescriber.
Clarify symptoms and establish a current baseline. In addition to determining primary and secondary diagnoses, another core objective of the TRD assessment is to clarify the nature and extent of the patient’s current symptoms and functioning. The severity of depressive symptoms at the assessment can be used as a baseline against which to evaluate the efficacy of the recommended treatment(s). Also important is determining the nature of the depressive symptoms (eg, suicidal thinking) as this information may be used to inform treatment recommendations and to determine whether the patient needs a higher level of care. This is particularly critical as up to 30% of individuals with TRD attempt suicide at some point in their illness course.36
Goal 2: Establish treatment goals.
Symptom reduction. It is often appropriately assumed that patients have the ultimate goal of complete symptom relief. There is, however, growing recognition that complete and sustained symptom remission may not always be possible. Moreover, some9 have argued that a singular focus on eliminating all symptoms can ultimately lead to an unhelpful pattern of continuous treatment switching with little benefit to a patient’s overall quality of life. Therefore, part of the goal of a TRD consultation is to achieve realistic goal setting with each patient. Dovetailing with the assessment of symptoms and functioning, the consultation team can take the opportunity to understand the patient’s perspective on whether specific symptoms are particularly distressing or contribute to functional impairment. These more focal treatment targets could be incorporated into treatment recommendations and monitored as treatment progresses.
Functional recovery. Symptoms of depression can be debilitating across a wide range of functional domains. In addition to mitigating symptoms, treatment goals can focus on the recovery of functioning in important areas including education, employment, relationships, spiritual well-being when relevant, and valued leisure activities.37,38 As in the case of other chronic illnesses,9 treatments can focus on helping to improve the patient’s quality of life despite the persistence of some level of depressive symptoms. Established psychotherapeutic treatments (eg, cognitive behavioral therapy and interpersonal therapy) can target specific goals in education, employment, and relationships functioning.39,40 Similarly, short-term psychodynamic psychotherapy for depression has been shown to lead to functional improvement in addition to symptom reduction.41 Finally, newer interventions (eg, recovery-focused cognitive behavioral therapy42 and augmented depression therapy43) have been developed, in part, to help patients achieve functional recovery goals.
Goal alignment. Consultation in a TRD assessment clinic provides a valuable opportunity to determine whether the patient and the treatment team agree on the primary goals for treatment.
In some cases, patients and providers are misaligned with respect to prioritizing reduction of particular symptoms over others or prioritizing improvements in functioning over improvements in symptoms.5,44,45 In other cases, the patient and the treatment team may have different expectations for the treatment’s effects or a different understanding of each party’s role in bringing about change. It is also vital to consider that patients who have experienced chronic interpersonal challenges, patients with complex experiences of trauma, and patients with certain personality pathologies may have experienced ruptures in aspects of the alliance with prior providers and may be particularly sensitive to the challenges in managing relationships and recommendations from multiple providers. Consultation at a TRD clinic can help to identify and address these issues when present and explicitly clarify roles, expectations, and goal priorities in the treatment plan.
Goal 3: Identify which interventions are most likely to work for this patient at this time. Assessment in a TRD consultation clinic is concretely directed towards determining which treatment strategy is most likely to be effective at achieving the patient’s goals, given their current diagnostic and symptom presentation, at this particular time in the patient’s illness course. Barring frank mismanagement of the illness, the fact that the patient is presenting to a TRD clinic indicates that their illness is unlikely to resolve in the short term either on its own or as a result of the supportive factors common to all treatment settings. The critical task during TRD consultation is to determine which of the available treatment options are most likely to be effective if provided next, given the patient’s symptoms, characteristics, treatment history, barriers to treatment, and personal strengths. These recommendations will be personalized to each patient. As such, a complete description of all possible recommendations is beyond the scope of the current project but could include combinations of: medication augmentation or switching strategies,46 fast-acting antidepressants like ketamine or esketamine,47 empirically supported psychotherapies,48 interventional psychiatry services like transcranial magnetic stimulation (TMS),49 electroconvulsive therapy (ECT),50 or vagus nerve stimulation.51
Treatment history. Collecting detailed information about the patient’s treatment experiences not only helps to verify that an individual does in fact have a difficult-to-treat form of depression, it directly informs the consultation team about which classes of treatment have already been adequately pursued. A careful assessment of treatment history includes information about the specific treatments, timing, dose, duration, side effects, degree of response, and reasons for and circumstances surrounding treatment discontinuation. This information is often difficult to ascertain. With medications, for example, it is important to determine the type of medication that was prescribed as well as the dose, frequency, and duration of treatment as it was actually taken by the patient. Relying on self-report alone can be challenging as many patients do not recall details of the medications that they have attempted. Medical records may provide more information about medication type, dosing, and duration, but they often do not contain information about medication adherence. We recommend combining information from multiple sources, including the patient’s report, medical record review, pharmacy records when available, and potentially gathering collateral information from important others in the patient’s life who were involved in managing their illness. Collateral information can be particularly helpful for clarifying key symptoms, perception of response to various treatments, and additional delineation of functional impairment and barriers to care.
Assessing the nature and adequacy of other kinds of therapeutics for depression can be even more challenging. These include psychotherapies and interventional approaches like TMS, ECT, and newer agents like ketamine and esketamine. In many cases, patients may not be aware of core elements of the treatment (eg, the specific form of psychotherapy they received or the parameters of the interventional approaches). In the case of psychotherapy, targeted questions such as “What did you learn in your psychotherapy? What happened in therapy sessions? Did your therapist suggest homework or action plans to be done outside treatment sessions?” may assist in determining the method and adequacy of psychotherapy. Across prior treatment modalities, careful record review and collateral information from the prior treatment provider can be particularly helpful in determining the nature and adequacy of these treatment trials.
Barriers to treatment. As important as determining which prior treatments were insufficiently effective for a patient is determining reasons that could explain the lack of adequate treatment response. For example, was a particular side-effect intolerable? Did the patient have difficulty accessing the treatment? Were there challenges for the patient in participating fully in the treatment plan, either due to factors internal to the patient (eg, concerns, attitudes, or beliefs about engaging in a particular treatment) or to external factors related to their life circumstances?
In some cases, prior barriers may become more addressable or may have been resolved, opening an opportunity to recommend a type of treatment that would previously have not been feasible. Regardless, it will be critical to determine what barriers might interfere with new treatment recommendations. Including a description of these in the treatment plan will help the treatment team not only to address them but also to monitor for their influence over the course of treatment.
Patient characteristics. There is a large, active, and growing body of research examining whether certain patient characteristics, other than symptoms, could be used to predict which type of treatment is most likely to be effective for a particular patient (see, eg, Cohen and DeRubeis53). These characteristics include demographic features (such as employment status, marital status, age, and gender), environmental factors such as current stressors, and historical information such as exposure to childhood trauma or maltreatment, among other factors. Although there is not yet a gold-standard prescriptive tool that could be used to guide treatment selection across the range of treatment options, considering these characteristics can help to suggest a potentially effective next treatment course, given the individual’s prior treatment history.53–56
ELEMENTS OF AN ASSESSMENT PACKAGE
Treatment History
As noted above, a core component of the consultation is a thorough assessment of the nature, adequacy, and effects of prior treatments. This assessment can be greatly enhanced by collecting information prior to the consultation both from the patient and from medical and pharmacy records. Patient self-report in a preconsultation questionnaire can provide valuable information about the patient’s understanding of the treatment that they received, whether they adhered to the prescribed treatment regimen, and any barriers to accessing or participating in the treatment. Official medical and, where possible, pharmacy records can provide more detailed information about the specific treatments that were prescribed. Obtaining this information prior to the consultation can help to more efficiently guide the clinical interview below and rule in or out potential recommendations for the next treatment option.
Clinical Interview
Nearly all consultations are likely to include an expert clinical interview with the patient. These interviews, conducted by a clinician with experience in this field, provide a vital opportunity to collect or elaborate on much of the information outlined above and to personalize the assessment to ensure that considerations specific to each individual patient are adequately probed. The clinical interview and mental status examination happen concurrently and can be an extraordinarily rich source of information. Open-ended and follow-up questions that are by nature not part of standardized assessment tools often give insight into an individual’s unique course of illness and its relationship to life events. Even in a one-time consultation encounter, establishment of a therapeutic alliance between patient and clinician facilitates interaction important in creating a comprehensive biopsychosocial formulation of the patient’s illness. Further, patients may have been previously reluctant to disclose or even not consciously aware of relevant factors that may be contributing to their illness (eg, interpersonal relationship patterns, trauma, role of substance use, etc.). Clinicians with specialized expertise in TRD are aware of these possible contributing factors and can be intentional about probing in directions that may have been previously unexplored. In many patients with TRD who have been ill for years, attitudes toward previous treaters and previous medications or therapeutic modalities may provide useful insights about prior barriers to treatment. Many interviewers examine the patient’s previous treatment history through a specific lens that intentionally explores the meaning and beliefs that patients attach to medications, as well as interpersonal factors in pharmacologic treatment (eg, Mintz57). Such an approach may offer a helpful psychological explanation for inadequate previous treatment trials due to previously unexplained adverse reactions to medications or other treatments (eg, particular sensitivities to side effects, nocebo effects, lack of trust, or reluctance to fully disclose with members of a prior treatment team). A case formulation informed by these interviews may prove extremely helpful in designing a personalized treatment plan that addresses the psychological complexity of the patient’s previous treatment experiences, thus maximizing the likelihood of future treatment effectiveness.
Diagnostic and Semistructured Interviews
Consultation clinics can consider incorporating more formal structured or semistructured interviews into the assessment package. The primary rationale for including these measures is that they can increase the chances that all aspects of the patient’s psychiatric presentation are captured. Across psychiatric disorders and clinical settings, semistructured diagnostic tools tend to lead to more accurate and reliable diagnoses compared to clinical interviews alone.58–60 This is perhaps particularly salient for TRD consultation clinics in which it is often the case that critical aspects of the patient’s illness or circumstances may have been overlooked during prior assessments. A key benefit of the clinical interview is that clinicians have complete discretion to ask whichever question they choose and to gather deeper information about any topic they determine to be important. A key challenge with this process is that it can be impacted by known cognitive biases, such as the primacy and recency effects, confirmation bias, and search satisficing (eg, Baron61) that can interfere with accurate diagnoses in medical and psychiatric settings. Examples of semistructured diagnostic instruments include the Structured Diagnostic Interview for DSM-5 Disorders (SCID), the Mini International Psychiatric Interview (MINI), and the Diagnostic Interview for Anxiety, Mood, and OCD and Related Neuropsychiatric Disorders (DIAMOND). Notably, some of these tools have versions (eg, the Quick Structured Clinical Interview for DSM-5 Disorders [QuickSCID-5]) that are designed to be brief (<30 minutes) and to be administered by staff with less clinical expertise.
Self-Report Measures
Brief self-report measures can also be used to help guide the assessment. For facilities that intend to enroll patients into their own clinics, these assessments can serve as baseline symptom assessments to help evaluate treatment efficacy. When considering the particular screening tools to use, we recommend that clinics prioritize the sensitivity of a measure over its specificity to help ensure that important elements of the clinical presentation are not overlooked.62 Elevated scores on these measures can then be used to guide more focused probes of clinical features or comorbidities that may have been overlooked previously.
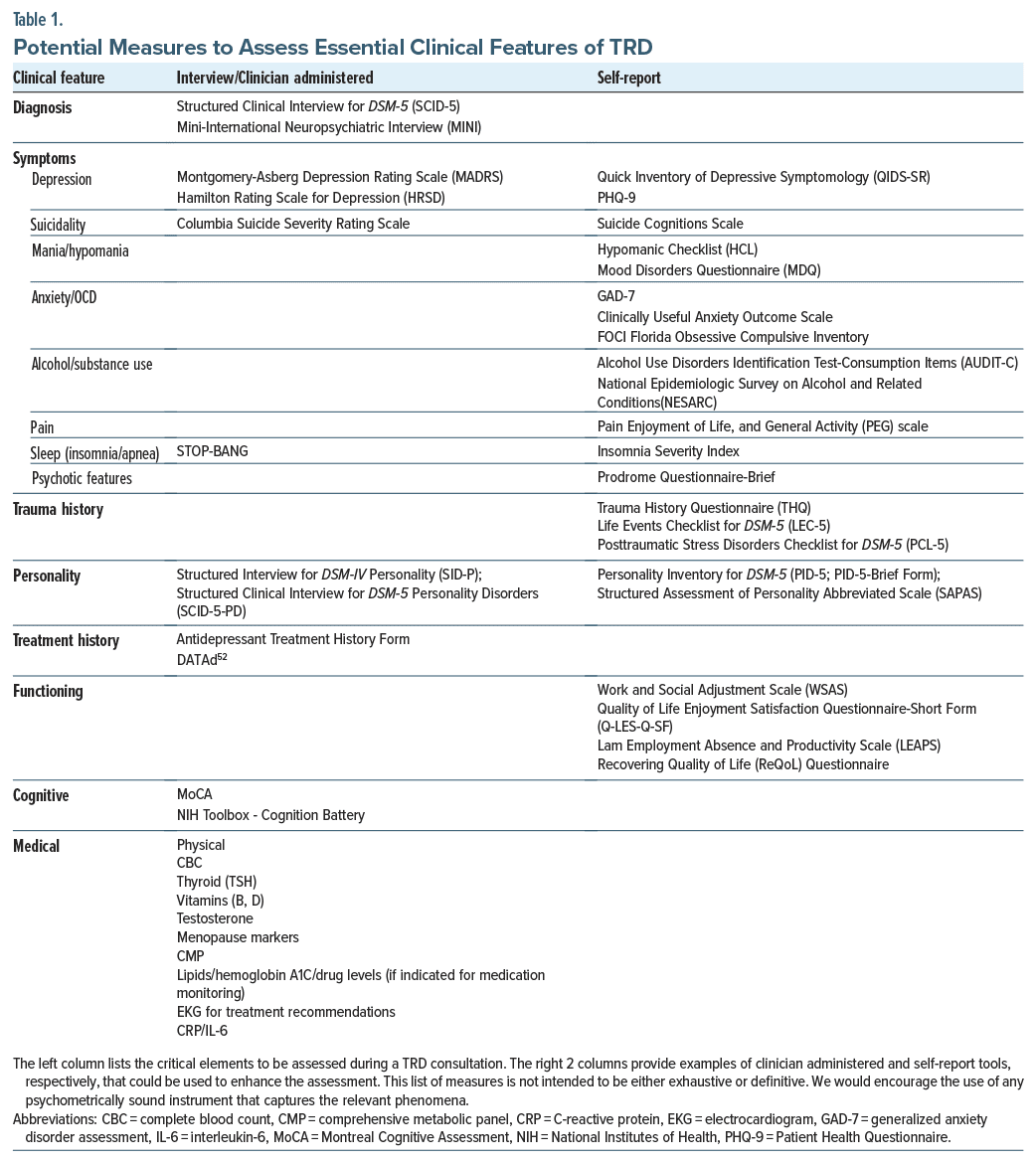
Table 1 highlights the critical elements to be assessed during a TRD consultation and provides examples of self-report or clinical interview tools that could be used to enhance the expert clinical interview. We do not intend for this list of measures to be either exhaustive or definitive. We recognize that some centers and settings may not have the infrastructure to support the use of an exhaustive battery of measures. Rather, we provide this list as a guide. We would encourage the use of any psychometrically sound instrument that captures the relevant phenomena.
ASSESSMENT PROCESS
Each consultation clinic will likely have distinct opportunities and challenges in implementing the elements of TRD consultation, given the nature of the organization in which they may be embedded (see Part I). The assessment process will also depend a great deal on the specific elements that a clinic chooses to implement and the available staffing support. Recognizing this, we recommend the following process elements for consideration, in settings where they are possible to implement.
First, several of the screening tools referenced in Table 1 are self-report instruments that could be completed by the patient prior to their consultation. In some settings, these could be embedded in the electronic medical record; in others, stand-alone secure online assessment portals such as REDCap or Qualtrics could be used; in still others, these could be printed and mailed to participants to be filled out on paper. A primary reason to include these tools is to flag the possibility of a comorbid diagnosis or symptom cluster that may have contributed to the patient’s illness or treatment course. Reviewing responses to these screening tools prior to the consultation can be valuable for helping providers focus assessments to probe those areas that would be particularly important for the specific patient.
Second, medical records can be requested prior to the consultation. Where possible, this task could be completed by a clinic manager or patient care navigator. Again, this information can help focus the conversation between provider and patient regarding both existing diagnoses and prior treatment history, adherence, efficacy, and side effects. This step can be particularly helpful for identifying periods of symptom exacerbation and care escalation, as well as for identifying diagnoses that can be difficult to detect in any single assessment visit, including the potential for a bipolar spectrum illness, personality pathology, or alcohol or substance use disorder.
Third, in multidisciplinary settings, the assessments completed during the consultation can be divided among providers. Clinical psychologists bring expertise in psychodiagnostic, symptom, and neurocognitive testing. Psychiatrists are likewise expert in clinical and diagnostic interviewing and, given their medical and psychopharmacologic expertise, are well-suited to assess medical comorbidities and treatment history. Dividing the assessment across providers creates the opportunity for more accurate diagnoses, as it typically allows for a broader selection of assessment tools and foci than would be possible by 1 provider alone; it allows the providers to communicate between assessments so that any diagnostic questions following the first portion of the assessment can be clarified in the second, and it provides the opportunity for 2 experts to meet with the patient and reach consensus on diagnosis and treatment recommendations.
Qualifications of Providers
Although subspecialty psychiatry fellowship training opportunities in mood disorders exist, as do specialized postdoctoral training opportunities in mood disorders for clinical psychologists, these often focus on research training. Depending on the nature of the program, considerable clinical experience in the evaluation and treatment of TRD can be gained in these programs. Still, to our knowledge, no unifying standard set of competencies for TRD consultants has been delineated. Depending on the clinician’s specific role, we believe based on our collective practice experience that TRD consultants should have knowledge and/or skills in the areas outlined in Box 1. These are intended to be broad in scope and, at the same time, outline a minimum level of expertise needed to define subspecialty expertise in TRD. As such, the elements included in Box 1 may not represent all of the specific requirements for TRD expertise across all practice settings.
Timing and Cost
The timing, duration, and cost of the consultation will likely differ between settings and will depend on the staffing involved and the elements of the consultation that the clinic chooses to implement. At minimum, the patient would meet with a physician for approximately 60–90 minutes for the expert clinical interview. The details of the patient’s medical history and current symptoms would dictate the extent of the follow-up laboratory and medical tests that would be necessary before making treatment recommendations. At the other end of the spectrum, centers in the NNDC that utilize the tools described in Table 1 typically divide the assessment process across patient care navigators, clinical psychologists, psychiatrists, and professional psychometrician technologists. In these settings, the assessment is typically split across 2 visits and can take a combined total of 4 hours to complete. Although a larger number of providers and staff are involved, new codes have been released (eg, 96127, 96136, 96138, 96130) to cover staff and provider time. In our experience, it is possible to structure such a compressive testing clinic to be fiscally sustainable over time.
FUTURE DIRECTIONS
Several additional assessment tools are actively being studied to improve clinical decision-making in the treatment of depression. Each of the following, if validated, may prove useful for TRD consultation clinics:
- Markers of brain structure and function. There are 2 potential advantages of leveraging techniques like functional magnetic resonance imaging and electroencephalography in clinical assessment. First, they can serve as more objective indicators than self-report or clinical interview assessments, and second, they hold the promise of identifying specific neurobiological targets associated with an individual’s illness state that could be used to guide individualized treatment selection. A substantial body of work has emerged over the last 2 decades aiming to identify neurobiologically based biotypes of depression (eg, 67,68) and to identify brain-imaging based predictors of response to different treatments for depression.69,70 Although promising, the use of neuroimaging to guide assessment remains experimental at present. To date, effect sizes have been small, replication remains an aspirational goal,69,71–74 and the reliability of neuroimaging-based metrics at the individual patient level is not yet sufficiently strong to support clinical decision-making.70 With additional development, these approaches could provide a future actionable pathway for personalized interventions that target mechanisms specific to the individual patient.
- Pharmacogenomics. While there is great potential for application of genetic data to guide treatment decisions, large controlled studies do not so far support current routine implementation of pharmacogenomic testing panels.75–78 Of note, a significant proportion of participants enrolled in each of the 3 large pharmacogenomic trials published to date met TRD criteria. In regard to the individual genes commonly included in current pharmacogenomic panels, consensus guidelines for using genetic data to guide antidepressant treatment recommend considering 4 pharmacokinetic genes (CYPs 2D6, 2D19, 2B6, and 2B19). These guidelines do not recommend the use of 2 commonly tested pharmacodynamic serotonin-related genes, SLC6A4 and HTR2A, in treatment decisions.79 Again, more work is needed to replicate findings and validate the use of genetic testing for clinical decision-making.
- Passive sensing/ecological momentary assessment. In light of the limitations of traditional assessments, including reliance on remote recall and the assessment of overlapping symptoms that are common to multiple diagnoses, there has been a concerted effort in the field to harness the capabilities of passive sensing or Ecological Momentary Assessment (EMA) to accurately monitor symptoms of depression in real time.80–84 Smartphone-based EMAs, which involve repeated sampling of the patient’s experiences, symptoms, and behaviors in real time within their natural environment (eg, at home, work), offer the advantage of capturing data during the patient’s lived experience. Passive data collection holds the promise of capturing data without active self-report, providing continuous information about the patient’s experiences. Paired with machine learning, these data have been used to successfully predict depressive episodes.85,86 While clinical application of these emerging technologies is currently experimental, it is an exciting emerging technology.
SUMMARY
Quality evidence-based assessment of TRD is a critical part of a TRD Consultation Program. Goals of a TRD assessment include clarifying the nature of a patient’s illness, including diagnostic and relevant comorbid disorder clarification, establishing personalized treatment goals, establishing and aligning goals accounting for symptom reduction and functional improvement, treatment team-patient goal alignment, and identifying the next steps for a personalized intervention strategy (Box 2). Treatment recommendations should individualize next intervention steps, considering treatment history, potential barriers to treatment, and patient characteristics. Elements of an assessment package, including the clinical interview, self-report, and clinician-rated measures where possible, as well as laboratory/diagnostic testing, are discussed. Together with Part I of this series that discusses practical and logistical considerations, we hope that these recommendations are helpful to those seeking to develop or enhance a TRD consultation program.
Article Information
Published Online: May 7, 2025. https://doi.org/10.4088/JCP.24cs15336
© 2025 Physicians Postgraduate Press, Inc.
Submitted: March 8, 2024; accepted January 28, 2025.
To Cite: Fournier JC, Voytenko VL, Docherty AR, et al. Developing a treatment resistant depression consultation program, part II: assessment. J Clin Psychiatry 2025;86(2):24cs15336.
Author Affiliations: Depression Recovery Center, Department of Psychiatry and Behavioral Health, The Ohio State University Wexner Medical Center and College of Medicine, Columbus, Ohio (Fournier, Virk); Department of Psychiatry and Department of Medical Ethics, Humanities, and Law, Western Michigan University Homer Stryker M.D. School of Medicine, Kalamazoo, Michigan (Voytenko); Division of Psychiatry and Behavioral Medicine, Michigan State University College of Human Medicine, East Lansing, Michigan (Voytenko); Department of Psychiatry, University of Utah School of Medicine & the Huntsman Mental Health Institute, Salt Lake City, Utah (Docherty); Department of Psychiatry and Behavioral Sciences, University of Louisville School of Medicine, Louisville, Kentucky (Wright); Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia (Riva Posse); National Network of Depression Centers, Ann Arbor, Michigan (Flood, Burnett); Center for Interventional Psychiatry, Faillace Department of Psychiatry and Behavioral Sciences, McGovern Medical School, The University of Texas Health Science Center at Houston (UTHealth Houston), Houston, Texas (Quevedo); Department of Behavioral Sciences and Social Medicine, Florida State University College of Medicine, Tallahassee, Florida (Bobo); Department of Psychiatry, Indiana University School of Medicine, Indianapolis, Indiana (Conroy); Department of Psychiatry and Depression Center, University of Michigan, Ann Arbor, Michigan (Parikh).
Corresponding Author: Jay C. Fournier, PhD, Department of Psychiatry and Behavioral Health, The Ohio State University College of Medicine, 1670 Upham Dr, Columbus, OH 43210 ([email protected]).
Fournier and Voytenko contributed equally as first authors. Conroy and Parikh contributed equally as senior authors.
Relevant Financial Relationships: Dr Fournier receives royalties from Guilford Press. Dr Wright has equity interest and serves as a consultant to Mindstreet, Inc., serves as consultant to Otsuka Pharmaceutical, and receives royalties from American Psychiatric Publishing, Inc, and Guilford Press. Dr Virk has received research funding from Johnson & Johnson (Janssen), Otsuka, Boeringer, Compass, Neumora, Allergan, DeNovo, Neurocrine, Novartis, Relmada. Dr Riva Posse has served as consultant to LivaNova, Abbott, Janssen, and MotifNeuro and receives editorial fees from Elsevier. Dr Quevedo received clinical research support from LivaNova; has speaker bureau membership with Myriad Neuroscience, and AbbVie; is a consultant for Eurofarma; is a stockholder at Instituto de Neurociencias, and receives copyrights from Artmed Editora, Artmed Panamericana, and Elsevier/Academic Press. Dr Bobo’s research has been supported by the National Institutes of Health, Agency for Healthcare Research and Quality, National Science Foundation, Watzinger Foundation, Myocarditis Foundation, Blue Gator Foundation, and Mayo Foundation for Medical Education & Research, and he has contributed chapters to UpToDate regarding the pharmacological management of bipolar major depression. Dr Conroy has received research funding from Johnson and Johnson. Dr Parikh reports research funding in the past 2 years from Aifred, Janssen, Compass, Sage, and Merck; consulting income from Mensante, Sage, Aifred, Boehringer-Ingelheim, Otsuka, and Janssen; and shares in Mensante. The other authors report no financial conflicts of interest.
Funding/Support: This work was supported by a Momentum Grant from the National Network of Depression Centers (NNDC), Ann Arbor, Michigan.
Role of the Sponsor: NNDC has had no influence on the conduct and publication of the project.
Clinical Points
- Although many patients with treatment resistant depression (TRD) seek a diagnostic consultation to receive a comprehensive evaluation of their condition and to determine the next course of treatment, there is currently little agreement in the field as to what such a consultation evaluation should include.
- The goals of an assessment in a specialty TRD consultation service are to establish the primary diagnosis and relevant comorbidities, clarify medical and psychiatric symptoms, identify treatment and functioning goals, document treatment history, identify treatment barriers, and develop actionable treatment recommendations.
References (86)

- Mrazek DA, Hornberger JC, Altar CA, et al. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65(8):977–987. PubMed CrossRef
- Johnston KM, Powell LC, Anderson IM, et al. The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord. 2019;242:195–210. PubMed CrossRef
- Lundberg J, Cars T, Lööv SÅ, et al. Association of treatment-resistant depression with patient outcomes and health care resource utilization in a population-wide study. JAMA Psychiatry. 2023;80(2):167–175. PubMed CrossRef
- Trevino K, McClintock SM, McDonald Fischer N, et al. Defining treatment-resistant depression: a comprehensive review of the literature. Ann Clin Psychiatry. 2014;26(3):222–232. PubMed
- Zimmerman M, McGlinchey JB, Posternak MA, et al. How should remission from depression be defined? The depressed patient’s perspective. Am J Psychiatry. 2006;163(1):148–150. PubMed CrossRef
- Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68(suppl 8):17–25. PubMed
- Ghaemi SN, Rosenquist KJ, Ko JY, et al. Antidepressant treatment in bipolar versus unipolar depression. Am J Psychiatry. 2004;161(1):163–165. PubMed CrossRef
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Rush AJ, Aaronson ST, Demyttenaere K.. Difficult-to-treat depression: a clinical and research roadmap for when remission is elusive. Aust N Z J Psychiatry. 2019;53(2):109–118. PubMed CrossRef
- Rizvi SJ, Grima E, Tan M, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349–357. PubMed CrossRef
- Vieta E. Bipolar units and programmes: are they really needed? World Psychiatry. 2011;10(2):152. PubMed CrossRef
- Voytenko VL, Conroy S, Docherty A, et al. Developing a treatment-resistant depression consultation program, part 1: practical and logistical considerations. J Clin Psychiatry. 2025;86(2):24cs15335. PubMed CrossRef
- McAllister-Williams RH, Arango C, Blier P, et al. The identification, assessment and management of difficult-to-treat depression: an international consensus statement. J Affect Disord. 2020;267:264–282. PubMed CrossRef
- Gaynes BN. Identifying difficult-to-treat depression: differential diagnosis, subtypes, and comorbidities. J Clin Psychiatry. 2009;70(suppl 6):10–15. PubMed CrossRef
- Rush AJ, Sackeim HA, Conway CR, et al. Clinical research challenges posed by difficult-to-treat depression. Psychol Med. 2022;52(3):419–432. PubMed CrossRef
- Mojtabai R, Amin-Esmaeili M, Spivak S, et al. Use of non-psychiatric medications with potential depressive symptom side effects and level of depressive symptoms in major depressive disorder. J Clin Psychiatry. 2023;84(4):22m14705. PubMed CrossRef
- Lish JD, Dime-Meenan S, Whybrow PC, et al. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31(4):281–294. PubMed CrossRef
- Hirschfeld RMA, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder. J Clin Psychiatry. 2003;64(2):161–174. PubMed CrossRef
- Akiskal HS, Maser JD, Zeller PJ, et al. Switching from’unipolar'to bipolar II: an 11-year prospective study of clinical and temperamental predictors in 559 patients. Arch Gen Psychiatry. 1995;52(2):114–123. PubMed CrossRef
- Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265–1270. PubMed CrossRef
- Cassano GB, Rucci P, Frank E, et al. The mood spectrum in unipolar and bipolar disorder: arguments for a unitary approach. Am J Psychiatry. 2004;161(7):1264–1269. PubMed CrossRef
- Fournier JC, Keener MT, Mullin BC, et al. Heterogeneity of amygdala response in major depressive disorder: the impact of lifetime subthreshold mania. Psychol Med. 2013;43(2):293–302. PubMed CrossRef
- Sharma V, Khan M, Smith A. A closer look at treatment resistant depression: is it due to a bipolar diathesis? J Affect Disord. 2005;84(2–3):251–257. PubMed CrossRef
- Correa R, Akiskal H, Gilmer W, et al. Is unrecognized bipolar disorder a frequent contributor to apparent treatment resistant depression? J Affect Disord. 2010;127(1-3):10–18. PubMed CrossRef
- DeBattista C, Rothschild AJ, Schatzberg AF. A dynamic algorithm for the treatment of psychotic major depression. Psychiatr Ann. 2002;32(11):681–691. CrossRef
- Wijkstra J, Lijmer J, Balk FJ, et al. Pharmacological treatment for unipolar psychotic depression: systematic review and meta-analysis. Br J Psychiatry. 2006;188:410–415. PubMed CrossRef
- Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM-IV anxiety and mood disorders: implications for assessment and treatment. Psychol Assess. 2009;21(3):256–271. PubMed CrossRef
- Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Aust J Pharm. 2008;165(3):342–351. PubMed CrossRef
- Fournier JC, DeRubeis RJ, Shelton RC, et al. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry. 2008;192(2):124–129. PubMed CrossRef
- Bagby RM, Quilty LC, Segal ZV, et al. Personality and differential treatment response in major depression: a randomized controlled trial comparing cognitive-behavioural therapy and pharmacotherapy. Can J Psychiatry. 2008;53(6):361–370. PubMed CrossRef
- Barateau L, Lopez R, Chenini S, et al. Depression and suicidal thoughts in untreated and treated narcolepsy: systematic analysis. Neurology. 2020;95(20):e2755–e2768. PubMed CrossRef
- Millman RP, Fogel BS, McNamara ME, et al. Depression as a manifestation of obstructive sleep apnea: reversal with nasal continuous positive airway pressure. J Clin Psychiatry. 1989;50(9):348–351. PubMed
- Li X, Sanford LD, Zong Q, et al. Prevalence of depression or depressive symptoms in patients with narcolepsy: a systematic review and meta-analysis. Neuropsychol Rev. 2021;31(1):89–102. PubMed CrossRef
- Mak MSB, Gebara MA, Lenze EJ, et al. Poor sleep is common in treatment-resistant late-life depression and associated with poorer antidepressant response: findings from the OPTIMUM clinical trial. Am J Geriatr Psychiatry. 2025;33(1):63–72. PubMed CrossRef
- Hammar Å, Årdal G. Cognitive functioning in major depression – a summary. Front Hum Neurosci. 2009;3:26. PubMed CrossRef
- Bergfeld IO, Mantione M, Figee M, et al. Treatment-resistant depression and suicidality. J Affect Disord. 2018;235:362–367. PubMed CrossRef
- Kennedy N, Foy K, Sherazi R, et al. Long-term social functioning after depression treated by psychiatrists: a review. Bipolar Disord. 2007;9(1-2):25–37. PubMed CrossRef
- McIntyre RS, Lee Y, Mansur RB. Treating to target in major depressive disorder: response to remission to functional recovery. CNS Spectr. 2015;20(Suppl 1):20–31. PubMed CrossRef
- Hundt NE, Mignogna J, Underhill C, et al. The relationship between use of CBT skills and depression treatment outcome: a theoretical and methodological review of the literature. Behav Ther. 2013;44(1):12–26. PubMed CrossRef
- Renner F, Cuijpers P, Huibers MJH. The effect of psychotherapy for depression on improvements in social functioning: a meta-analysis. Psychol Med. 2014;44(14):2913–2926. PubMed CrossRef
- Driessen E, Hegelmaier LM, Abbass AA, et al. The efficacy of short-term psychodynamic psychotherapy for depression: a meta-analysis update. Clin Psychol Rev. 2015;42:1–15. PubMed CrossRef
- Beck AT, Grant P, Inverso E, et al. Recovery-Oriented Cognitive Therapy for Serious Mental Health Conditions. Guilford Publications; 2020.
- Dunn BD, Widnall E, Reed N, et al. Evaluating Augmented Depression Therapy (ADepT): study protocol for a pilot randomised controlled trial. Pilot Feasibility Stud. 2019;5:63. PubMed CrossRef
- Demyttenaere K, Donneau AF, Albert A, et al. What is important in being cured from depression? Discordance between physicians and patients (1). J Affect Disord. 2015;174:390–396. PubMed CrossRef
- Kan K, Jörg F, Buskens E, et al. Patients’ and clinicians’ perspectives on relevant treatment outcomes in depression: qualitative study. BJPsych Open. 2020;6(3):e44. PubMed CrossRef
- Lam RW, Kennedy SH, Adams C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults: Réseau canadien pour les traitements de l’humeur et de l’anxiété (CANMAT) 2023: Mise à jour des lignes directrices cliniques pour la prise en charge du trouble dépressif majeur chez les adultes. Can J Psychiatry. 2024;69(9):641–687. PubMed CrossRef
- Zaki N, Chen LN, Lane R, et al. Long-term safety and maintenance of response with esketamine nasal spray in participants with treatment-resistant depression: interim results of the SUSTAIN-3 study. Neuropsychopharmacology. 2023;48(8):1225–1233. PubMed CrossRef
- Nakagawa A, Mitsuda D, Sado M, et al. Effectiveness of supplementary cognitive-behavioral therapy for pharmacotherapy-resistant depression: a randomized controlled trial. J Clin Psychiatry. 2017;78(8):1126–1135. PubMed CrossRef
- Chu HT, Cheng CM, Liang CS, et al. Efficacy and tolerability of theta-burst stimulation for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110168. PubMed CrossRef
- UK ECT Review Group. Lancet. 2003;361(9360):799–808.
- Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83–93. PubMed CrossRef
- Riva-Posse P, Bobo WV, Quevedo J, et al. DATAd: A Consensus Proposal for a New Tracking System in Treatment-Resistant Depression. Unpublished Tool. NNDC TRD Task Group.
- Cohen ZD, DeRubeis RJ. Treatment selection in depression. Annu Rev Clin Psychol. 2018;14:209–236. PubMed CrossRef
- Hack LM, Tozzi L, Zenteno S, et al. A cognitive biotype of depression and symptoms, behavior measures, neural circuits, and differential treatment outcomes: a prespecified secondary analysis of a randomized clinical trial. JAMA Netw Open. 2023;6(6):e2318411. PubMed CrossRef
- Rajpurkar P, Yang J, Dass N, et al. Evaluation of a machine learning model based on pretreatment symptoms and electroencephalographic features to predict outcomes of antidepressant treatment in adults with depression: a prespecified secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3(6):e206653. PubMed CrossRef
- Kessler RC, van Loo HM, Wardenaar KJ, et al. Using patient self-reports to study heterogeneity of treatment effects in major depressive disorder. Epidemiol Psychiatr Sci. 2017;26(1):22–36. PubMed CrossRef
- Mintz D. Psychodynamic Psychopharmacology: Caring for the Treatment-Resistant Patient. American Psychiatric Association Publishing; 2022.
- Miller PR, Dasher R, Collins R, et al. Inpatient diagnostic assessments: 1. Accuracy of structured vs. unstructured interviews. Psychiatry Res. 2001;105(3):255–264. PubMed CrossRef
- Steiner JL, Tebes JK, Sledge WH, et al. A comparison of the structured clinical interview for DSM-III-R and clinical diagnoses. J Nerv Ment Dis. 1995;183(6):365–369. PubMed CrossRef
- Zimmerman M. Diagnosing personality disorders. A review of issues and research methods. Arch Gen Psychiatry. 1994;51(3):225–245. PubMed CrossRef
- Baron J. Thinking and Deciding. Cambridge University Press; 2006. CrossRef
- Zimmerman M. Screening for bipolar disorder: confusion between case-finding and screening. Psychother Psychosom. 2014;83(5):259–262. PubMed CrossRef
- Silverman JJ, Galanter M, Jackson-Triche M, et al. The American psychiatric association practice guidelines for the psychiatric evaluation of adults. Am J Psychiatry. 2015;172(8):798–802. PubMed CrossRef
- American Psychological Association. APA clinical practice guideline for the treatment of depression across three age cohorts. American Psychological Association; 2019. https://www.apa.org/depression-guideline
- King RA. Practice parameters for the psychiatric assessment of children and adolescents. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry. 1997;36(10 suppl):4S–20S. PubMed CrossRef
- Conroy SK, Holtzheimer PE. Neuromodulation strategies for the treatment of depression. Am J Psychiatry. 2021;178(12):1082–1088. PubMed CrossRef
- Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38. PubMed CrossRef
- Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3(5):472–480. PubMed CrossRef
- Fonseka TM, MacQueen GM, Kennedy SH. Neuroimaging biomarkers as predictors of treatment outcome in Major Depressive Disorder. J Affect Disord. 2018;233:21–35. PubMed CrossRef
- Taylor JJ, Kurt HG, Anand A. Resting state functional connectivity biomarkers of treatment response in mood disorders: a review. Front Psychiatry. 2021;12:565136. PubMed CrossRef
- Fournier JC, Price RB. Focus. Psychotherapy and neuroimaging. 2014;12(3):290–298. PubMed CrossRef
- Beijers L, Wardenaar KJ, van Loo HM, et al. Data-driven biological subtypes of depression: systematic review of biological approaches to depression subtyping. Mol Psychiatry. 2019;24(6):888–900. PubMed CrossRef
- First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(9):915–916. PubMed CrossRef
- Abi-Dargham A, Moeller SJ, Ali F, et al. Candidate biomarkers in psychiatric disorders: state of the field. World Psychiatry. 2023;22(2):236–262. PubMed CrossRef
- Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111:59–67. PubMed CrossRef
- Oslin DW, Lynch KG, Shih MC, et al. Effect of pharmacogenomic testing for drug-gene interactions on medication selection and remission of symptoms in major depressive disorder: the PRIME care randomized clinical trial. JAMA. 2022;328(2):151–161. PubMed CrossRef
- Perlis RH, Dowd D, Fava M, et al. Randomized, controlled, participant- and rater-blind trial of pharmacogenomic test-guided treatment versus treatment as usual for major depressive disorder. Depress Anxiety. Depress Anxiety. 2020;37(9):834–841. PubMed CrossRef
- Frye MA, Nemeroff CB. Pharmacogenomic testing for antidepressant treatment selection: lessons learned and roadmap forward. Neuropsychopharmacology. 2024;49(1):282–284. PubMed CrossRef
- Bousman CA, Stevenson JM, Ramsey LB, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A genotypes and serotonin reuptake inhibitor antidepressants. Clin Pharmacol Ther. 2023;114(1):51–68. PubMed CrossRef
- De Angel V, Lewis S, White K, et al. Digital health tools for the passive monitoring of depression: a systematic review of methods. NPJ Digit Med. 2022;5(1):3. PubMed CrossRef
- Narziev N, Goh H, Toshnazarov K, et al. STDD: short-term depression detection with passive sensing. Sensors. 2020;20(5):1396. PubMed CrossRef
- Ebner-Priemer UW, Trull TJ. Ecological momentary assessment of mood disorders and mood dysregulation. Psychol Assess. 2009;21(4):463–475. PubMed CrossRef
- aan het Rot M, Hogenelst K, Schoevers RA. Mood disorders in everyday life: a systematic review of experience sampling and ecological momentary assessment studies. Clin Psychol Rev. 2012;32(6):510–523. PubMed CrossRef
- Colombo D, Fernández-Álvarez J, Patané A, et al. Current state and future directions of technology-based ecological momentary assessment and intervention for major depressive disorder: a systematic review. J Clin Med. 2019;8(4):465. PubMed CrossRef
- Chikersal P, Doryab A, Tumminia M, et al. Detecting depression and predicting its onset using longitudinal symptoms captured by passive sensing: a machine learning approach with robust feature selection. ACM Trans Comput-Hum Interact. 2021;28(1):1–41. CrossRef
- Jacobson NC, Chung YJ. Passive sensing of prediction of moment-to-moment depressed mood among undergraduates with clinical levels of depression sample using smartphones. Sensors. 2020;20(12):3572. PubMed CrossRef
This PDF is free for all visitors!