In recent years, esketamine and dextromethorphan combined with bupropion have gained US Food and Drug Administration approval for treatment-sresistant depression (TRD) and major depressive disorder (MDD), respectively.1–6 Unlike most other monoaminergic oral antidepressants, dextromethorphan/bupropion and esketamine both antagonize the N-methyl-D-aspartate (NMDA) receptor, with dextromethorphan having stronger affinity for the NMDA receptor.7 Thus, there is an urgent need to identify the efficacy and tolerability of coadministration of these two rapidly acting antidepressants.
The following is a case series of 3 patients with TRD who had no or partial response to 4 weeks of biweekly esketamine 84 mg monotherapy as part of their participation in an open-label (OL) clinical trial (NCT045998558) who then received augmentation with dextromethorphan/bupropion 45 mg/105 mg daily for 3 days, which was then increased to twice a day. All participants provided informed consent as part of their participation in this clinical trial, and their information has been deidentified for this case series.
Case 1
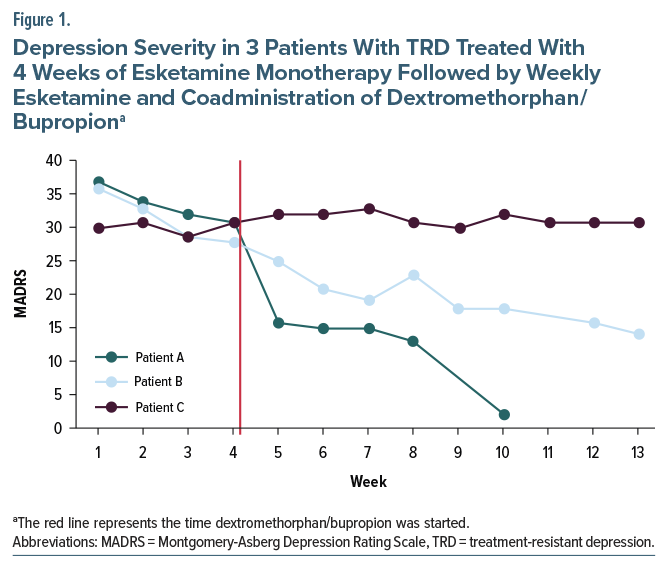
Patient A is 30-year-old male with MDD, single episode; social anxiety disorder; and generalized anxiety disorder whose current depressive episode had lasted 21 years. During this episode, he had failed to respond to adequate trials of escitalopram, venlafaxine, and bupropion. His Montgomery-Asberg Depression Rating Scale (MADRS) score prior to OL esketamine treatment was 37, which decreased to 31 after 4 weeks and then decreased to 2 after 6 weeks of augmentation with dextromethorphan/bupropion (Figure 1). He reported no adverse events during the duration of treatment.
Case 2
Patient B is a 35-year-old female with MDD, recurrent; generalized anxiety disorder; and attention-deficit/hyperactivity disorder whose depressive episode had lasted 22 months. During this episode, she had failed to respond to adequate trials of paroxetine, bupropion, and duloxetine. Her MADRS score prior to OL esketamine treatment was 36, which decreased to 28 after 4 weeks and then decreased to 14 after 9 weeks of augmentation with dextromethorphan/bupropion (Figure 1). She reported no adverse events during the duration of treatment.
Case 3
Patient C is a 27-year-old male with MDD, recurrent; social anxiety disorder; and generalized anxiety disorder whose current depressive episode had lasted 4 years. During this episode, he had failed to respond to adequate trials of venlafaxine, bupropion, imipramine augmented with aripiprazole, tranylcypromine, sertraline, and vortioxetine. His MADRS score prior to OL esketamine treatment was 30, which increased to 31 after 4 weeks and remained the same after 9 weeks of augmentation with dextromethorphan/bupropion. He reported no adverse events during the duration of treatment.
Discussion
To our knowledge, this is the first report of efficacy and tolerability of esketamine and dextromethorphan/ bupropion coadministration. Caution is warranted regarding any conclusions, as this case series included only 3 patients; however, 2 of the 3 patients had a clinical improvement with coadministration. In addition, among these 3 patients, no adverse events were reported, including no significant changes in blood pressure or somnolence.
The decrease in MADRS scores for Patient A is consistent with the potential of rapid-acting antidepressants, as MADRS scores dropped rapidly after augmentation with dextromethorphan/bupropion. Patient B’s response to augmentation was less dramatic, and, considering the slope of down-trending MADRS scores prior to augmentation that appears consistent after augmentation, it is unclear to what degree dextromethorphan/bupropion contributed to clinical improvement. Patient C had no response to esketamine monotherapy or augmentation with dextromethorphan/ bupropion, which may be due to his level of treatment resistance, as he failed more antidepressants during the current depressive episode, including a monoamine oxidase inhibitor.
This case series provides the first step in understanding the safety and potential efficacy of antidepressant medications that interact with the NMDA receptor. Further studies are warranted to examine the tolerability and efficacy of esketamine and dextromethorphan/bupropion coadministration.
Article Information
Published Online: November 3, 2025. https://doi.org/10.4088/JCP.25cr16082
© 2025 Physicians Postgraduate Press, Inc.
Submitted: August 12, 2025; accepted September 15, 2025.
To Cite: Eloge JC, Joneydian T, McCall K, et al. Efficacy and tolerability of esketamine augmented with dextromethorphan/bupropion for treatment-resistant depression: a case series. J Clin Psychiatry 2026;87(1):25cr16082.
Author Affiliations: Rush University Medical Center, Chicago, Illinois (Eloge and Zajecka); Integrated Healthcare Association Medical Group (Joneydian); Intempo Psychiatry, Skokie, Illinois (McCall); Psychiatric Medicine Associates, LLC, Skokie, Illinois (Mackey and Zajecka).
Corresponding Author: Joshua C. Eloge, MD, Rush University Medical Center, Department of Psychiatry and Behavioral Sciences, 1645 W Jackson Blvd, Suite 600, Chicago, IL 60612 ([email protected]).
Relevant Financial Relationships: Dr Eloge has received research support from Compass Pathways. Mr Mackey is a paid consultant for Johnson & Johnson and Axsome Therapeutics. Dr Zajecka has received research support from Abbott, Axsome, Boehringer-Ingelheim, Compass, Cybin, Hoffman-LaRoche, Johnson & Johnson/Janssen, LivaNova, Otsuka, Neurocrine Bioscience, Reunion Neuroscience, and Sage Therapeutics and has been paid as a consultant or advisory board for Alpha Sigma, Johnson & Johnson/Janssen, LivaNova, and Paragon. Drs Joneydian and McCall report no disclosures.
Funding/Support: This case series was part of an open-label clinical trial of esketamine in treatment-resistant depression (NCT04599855) sponsored by Janssen Pharmaceuticals. While Janssen sponsored this clinical trial, the decision to use dextromethorphan/bupropion for augmentation of esketamine during the reported open-label phase was the investigators’. The sponsor was not involved in the conception, writing, or submission of this case series. The sponsor did review the manuscript prior to submission.
Patient Consent: Informed consent to participate in this clinical trial was obtained from each patient. Their information has been de-identified to protect anonymity.
References (8)

- Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620–630. PubMed CrossRef
- Daly E, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139–148. PubMed CrossRef
- FDA Approves New Nasal Spray Medication For Treatment-Resistant Depression; Available Only At A Certified Doctor’s Office Or Clinic [News Release]. US Food and Drug Administration. 2019. Accessed April 16, 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm632761.htm
- Spravato (Esketamine). Prescribing information. Janssen Pharmaceuticals, Inc; 2019.
- Tabuteau H, Jones A, Anderson A, et al. Effect of AXS-05 (Dextromethorphan-bupropion) in major depressive disorder: a randomized double-blind controlled trial. Am J Psychiatry. 2022;179(7):490–499. PubMed CrossRef
- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4):21m14345. PubMed
- Stahl S. Dextromethorphan/bupropion: a novel oral NMDA (N-methyl-d-aspartate) receptor antagonist with multimodal activity. CNS Spectr. 2019;24(5):461–466. PubMed CrossRef
- Janik A, Qiu X, Lane R, et al. Esketamine monotherapy in adults with treatment-resistant depression: a randomized clinical trial. JAMA Psych. 2025;82(9):877–887. PubMed CrossRef
This PDF is free for all visitors!