Abstract
Objective: To evaluate the long-term effects of once-daily valbenazine (40 or 80 mg) on tardive dyskinesia (TD) in elderly adults (age ≥65 years).
Methods: Data were pooled from two 48-week studies, KINECT 3 extension (NCT02274558) and KINECT 4 (NCT02405091), with analyses performed over time in elderly participants (≥65 years) and at Week 48 by age group (<65 and ≥65 years). Outcomes included mean change in Abnormal Involuntary Movement Scale (AIMS) total score, AIMS response thresholds (≥30% and ≥50% improvement from baseline), and response threshold for Clinical Global Impression of Change–TD (CGI-TD) and Patient Global Impression of Change (PGIC), defined as a score ≤2 (“much improved” or “very much improved”). Safety assessments included treatment-emergent adverse events (TEAEs) and psychiatric symptom scales (eg, Positive and Negative Syndrome Scale).
Results: Of the pooled analysis population (N = 304), 55 (18.1%) were ≥65 years of age. The mean change from baseline in AIMS total score (±standard error) in the ≥65-year age group increased from Week 8 (−4.5 ± 0.7; first visit after dose escalation in KINECT 4) through Week 48 (−8.8 ± 0.9). These robust and sustained improvements were consistent with the percentage who met response thresholds at Weeks 8, 24, and 48: AIMS ≥30% improvement (58.0%, 88.5%, and 89.3%); AIMS ≥50% improvement (40.0%, 65.4%, and 82.1%); CGI-TD score ≤2 (33.3%, 88.5%, and 92.9%); and PGIC score ≤2 (43.1%, 84.6%, and 85.7%). The most common TEAEs among elderly participants were urinary tract infections (10.9%) and somnolence (10.9%). Psychiatric status remained stable during the studies.
Conclusion: Valbenazine was safe and effective in elderly adults who received up to 48 weeks of treatment. Long-term treatment led to substantial and sustained TD improvements per clinician assessment (AIMS, CGI-TD) and patient report (PGIC), with no impact on psychiatric stability.
J Clin Psychiatry 2025;86(2):24m15550
Author affiliations are listed at the end of this article.
Older age is associated with an increased risk for tardive dyskinesia (TD), a persistent and often disabling hyperkinetic movement disorder associated with exposure to antipsychotics and other dopamine receptor blocking agents (DRBAs).1 Per the American Psychiatric Association, TD symptoms generally emerge after 3 or more months of DRBA exposure and can appear long after the offending agent is stopped. However, individuals aged 60 years and older may develop TD after as little as 1 month of exposure.2
The association between older age and increased TD risk was demonstrated in 2 prospective studies of patients ≥55 years old and initiating antipsychotic treatment. One study found the cumulative incidence of TD to be 25% after 1 year of exposure to first-generation antipsychotics (FGAs) and 53% after 3 years, which was 3–5 times higher than had been reported in younger adults.3 The other study found the cumulative risk for TD with second-generation antipsychotics (SGAs) to be 5%–7% after 1 year of exposure and 7%–11% after 2 years, with even higher risks among women, African Americans, and patients with no prior antidepressant treatment.4 Consistent with these results, a meta analysis of data from 41 studies showed a significant association between higher TD prevalence and increased mean age.5
As a movement disorder that can affect all body regions, TD can have substantial impacts in elderly adults.6–9 Abnormal involuntary movements in the lower limbs can lead to impaired gait or balance.10,11 Orofacial movements can cause or exacerbate eating/swallowing difficulties and dental problems, while truncal dyskinesia can impede breathing and complicate respiratory conditions.10,12–14 Moreover, elderly adults may be particularly prone to the social isolation often associated with TD and have a greater need for caregiver assistance.8,15,16 In a real-world study of patients with clinician-confirmed suspected TD, older patients (≥55 years) reported that their uncontrolled movements had “some” or “a lot” of impact on their ability to continue daily activities (41.4%), socialize (40.5%), or take care of themself (29.7%).8
Valbenazine is a once-daily, single-capsule, vesicular monoamine transporter 2 (VMAT2) inhibitor that is approved for the treatment of TD in the US, Japan, Singapore, Thailand, Indonesia, Malaysia, and South Korea and is under regulatory review in Taiwan. In the US, it is also approved for chorea associated with Huntington disease (HD).17,18 Valbenazine is the only US Food and Drug Administration–approved medication for TD with enough data in the elderly to specifically have a statement in the label that no dose adjustment is required for patients aged 65 years and older.17
The characteristics of valbenazine (ie, no titration required to reach an effective and tolerable dose, once daily dosing, no extended-release coating) and its formulations (ie, 3 effective dosage strengths sprinkle formulation that can be mixed with soft foods) make it potentially uniquely appropriate for elderly patients with TD. Data from in vitro studies indicate that acceptable levels of valbenazine are recovered when contents of the capsules are removed, manually crushed, and added to a wide range of soft foods and liquids or dispersed in water and passed through a gastrostomy tube.19 These alternative modes of delivery may be particularly valuable for elderly patients who have difficulty swallowing whole pills due to TD or other impairments. The prescribing information for deutetrabenazine (also approved for TD and HD chorea) specifically prohibits chewing, crushing, or breaking the tablets, including the extended-release form.20
A previous post hoc analysis of valbenazine data in adults aged ≥55 years demonstrated robust TD improvements in this age group.21 However, aging populations are important considerations—both in the US, with the number of people aged ≥65 years projected to increase from 57.8 million in 2022 to 82.1 million by 2050,22 and globally, with the proportion of persons aged ≥65 years projected to increase from 10% in 2022 to 16% in 2050.23 In spite of these demographic trends, there is still a relative paucity of information on valbenazine efficacy and safety in relation to increasing age. To address this gap, data from 2 long-term studies, KINECT 3 extension (NCT02274558) and KINECT 4 (NCT02405091),24,25 were analyzed post hoc in participants aged 65 years and older.
METHODS
Study Design and Participants
Detailed methods and results of the long-term valbenazine clinical trials have been previously published.24,25 Study protocols were approved by the institutional review board at each site, and all participants provided written informed consent.
KINECT 3 included a 6-week double-blind placebo controlled (DBPC) period followed by a 42-week extension period with double-blinded valbenazine dosing (40 or 80 mg, once-daily). In KINECT 4, participants received 48 weeks of open-label valbenazine, starting at 40 mg for 4 weeks followed by an escalation to 80 mg based on efficacy and tolerability. In both studies, participants unable to tolerate 80 mg were allowed a dose reduction to 40 mg; those unable to tolerate 40 mg were discontinued.
Participants in both studies met the following criteria: Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnosis of schizophrenia, schizoaffective disorder, or mood disorder; DSM diagnosis of neuroleptic-induced TD for ≥3 months prior to screening; and psychiatric stability prior to study entry (eg, Brief Psychiatric Rating Scale score <50 at screening). Key exclusion criteria included the following: active, clinically significant, and unstable medical condition within 1 month prior to screening; comorbid movement disorder more prominent than TD; and significant risk for active suicidal ideation, suicidal behavior, or violent behavior. Both studies allowed concomitant use of all medications required to treat participants’ medical and psychiatric conditions, including FGAs and SGAs, antidepressants, and anticholinergics (eg, benztropine, trihexyphenidyl), provided that no changes in dosing, dosing frequency, or medication (starting or discontinuing) were made within 30 days prior to screening.
Post Hoc Analyses
Data were pooled at baseline and postbaseline visits as follows: Week 8 (both studies); Week 12/16 (KINECT 4/KINECT 3); Week 24 (KINECT 4); Week 32/36 (KINECT 3/KINECT 4); Week 48 (both studies) (Supplementary Figure 1). The 40-mg dose group included participants who were randomized to valbenazine 40 mg at baseline in the KINECT 3 DBPC period or did not have a dose escalation in KINECT 4. The 80-mg dose group included participants who were randomized at baseline to valbenazine 80 mg in KINECT 3 and those who were escalated to 80 mg at Week 4 in KINECT 4. Participants who had a dose reduction from 80 mg to 40 mg in either study were included in the 80- mg dose group. Participants who initially received placebo in the KINECT 3 DBPC period were excluded from analyses, along with those from KINECT 4 who had no efficacy assessments at Week 8 (first study visit after dose escalation) or later.
TD Assessments
Changes in TD were assessed using the Abnormal Involuntary Movement Scale (AIMS), as evaluated by blinded central video raters in KINECT 3 and on-site raters in KINECT 4, the Clinical Global Impression of Change-Tardive Dyskinesia (CGI-TD), and the Patient Clinical Global Impression of Change (PGIC).
The following outcomes were analyzed descriptively in elderly participants (≥65 years), with no imputation for missing values: mean change from baseline in AIMS total score (sum of items 1–7); clinically meaningful threshold for AIMS response, defined as ≥30% total score improvement from baseline26; protocol-defined threshold for AIMS response, defined as ≥50% improvement; CGI-TD response, defined as a score ≤2 (1 = “very much improved” or 2 = “much improved”); and PGIC response, also defined as a score ≤2.
At Week 48, these outcomes were analyzed by dose (40 mg, 80 mg) and age (<65 years, ≥65 years). Differences between the age subgroups were analyzed using a 2-sample t test for the mean change in AIMS total score and a chi-square test for AIMS, CGI-TD, and PGIC response.
Safety Assessments
Treatment-emergent adverse events (TEAEs) were monitored in all participants throughout both studies. The Positive and Negative Syndrome Scale (PANSS) and Calgary Depression Scale for Schizophrenia (CDSS) scores were administered to participants with schizophrenia or schizoaffective disorder. The Young Mania Rating Scale (YMRS) and Montgomery-Asberg Depression Rating Scale (MADRS) were administered to those with a mood disorder. The Barnes Akathisia Rating Scale (BARS) and the Simpson-Angus Scale (SAS) scores were administered to all participants.
The incidence of TEAEs, including serious TEAEs and TEAEs leading to discontinuation, were analyzed by age (<65 years, ≥65 years) using a χ2 test. Mean changes from baseline in psychiatric symptom scales (PANSS, CDSS, YMRS, MADRS) and abnormal movement scales (BARS, SAS) were analyzed descriptively by study visit in the elderly subgroup, with no imputation for missing values. At Week 48, these outcomes were analyzed by dose (40 mg, 80 mg) and age (<65 years, ≥65 years). Differences between the age subgroups were analyzed using a 2-sample t test.
RESULTS
Participants
The pooled analysis population included 304 participants, 55 (18.1%) of whom were 65 years and older (“elderly”). Overall, 181 (59.5%) reached the Week 48 visit with no statistically significant difference between elderly participants (50.9% [28/55]) and younger participants (61.4% [153/249]).
Ages in the elderly subgroup ranged from 65 to 83 years, and the mean age was 69.8 years (Table 1). Compared to the younger subgroup, a higher percentage of elderly participants were female (52.7% vs 45.4%) and white (76.4% vs 59.0%). AIMS total score was approximately 12 points at baseline in both age subgroups.
Valbenazine Effects on TD
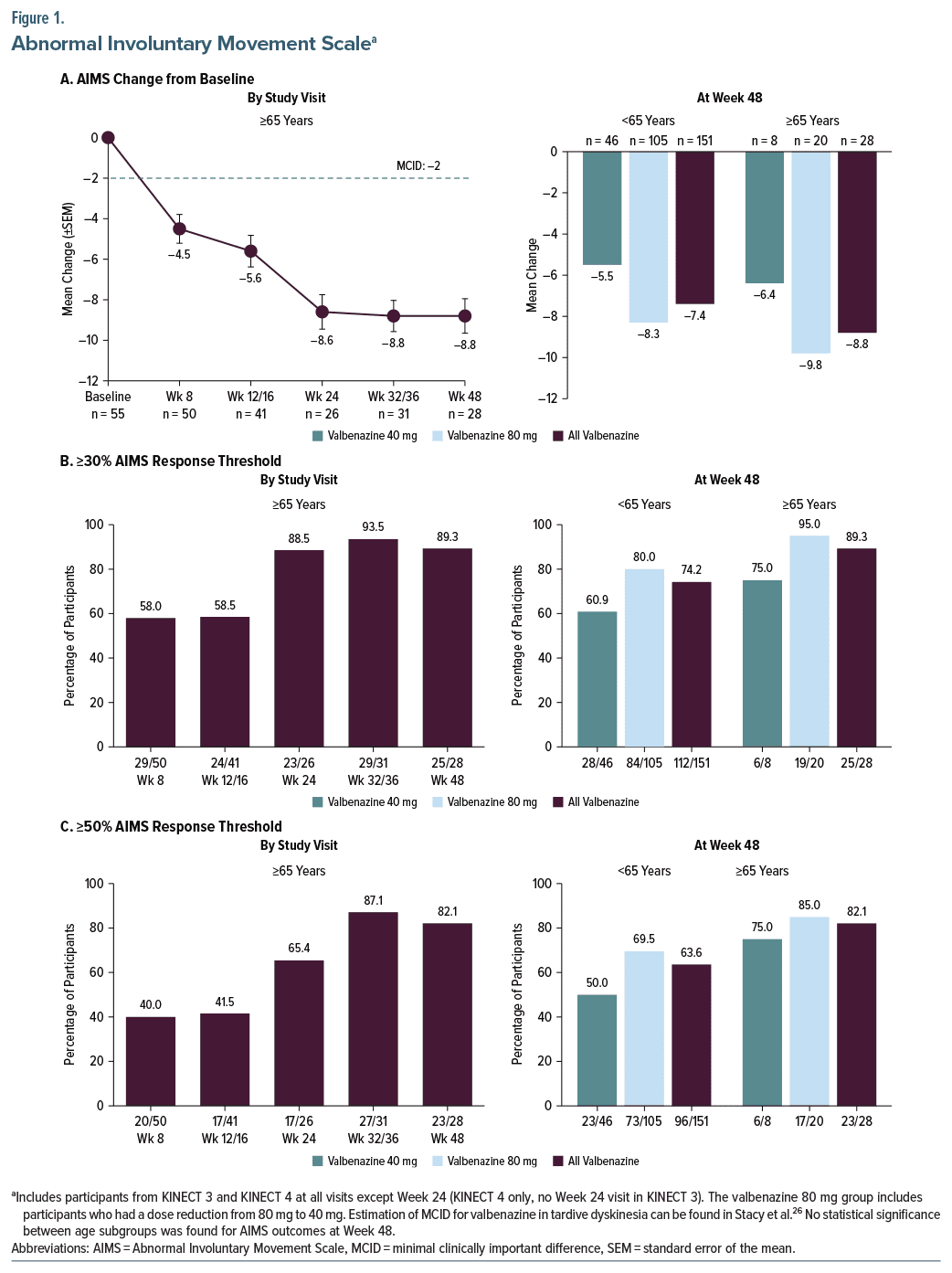
In participants ≥65 years of age who received either dose of valbenazine (40 mg or 80 mg), AIMS total score improvement exceeded the estimated minimal clinically important difference (MCID26) of −2 points for valbenazine in TD at all analysis timepoints (Figure 1A, left panel). Mean changes at Weeks 8, 24, and 48 in the ≥65-year group were −4.5 ± 0.7 (n = 50), −8.6 ± 0.9 (n = 26), and −8.8 ± 0.9 (n = 28), respectively. By end of treatment at Week 48, substantial improvements were observed with both valbenazine doses in both age groups: <65 years (40 mg: −5.5 [n = 46]; 80 mg: −8.3 [n = 105]); ≥65 years (40 mg: −6.4 [n = 8]; 80 mg: −9.8 [n = 20]) (Figure 1A, right panel).
The proportion of elderly participants who met the clinically meaningful ≥30% threshold for AIMS response increased from Week 8 (58.0%) to Week 24 (88.5%) and was sustained through Week 48 (89.3%) (Figure 1B, left panel). Similar results were found using the more stringent ≥50% threshold for response baseline, with percentages increasing from Week 8 (40.0%) to Week 24 (65.4%) and through Week 48 (82.1%) (Figure 1C, left panel). In both age subgroups, a greater proportion of participants met the AIMS response thresholds with valbenazine 80 mg than with 40 mg (Figures 1B and 1C, right panels).
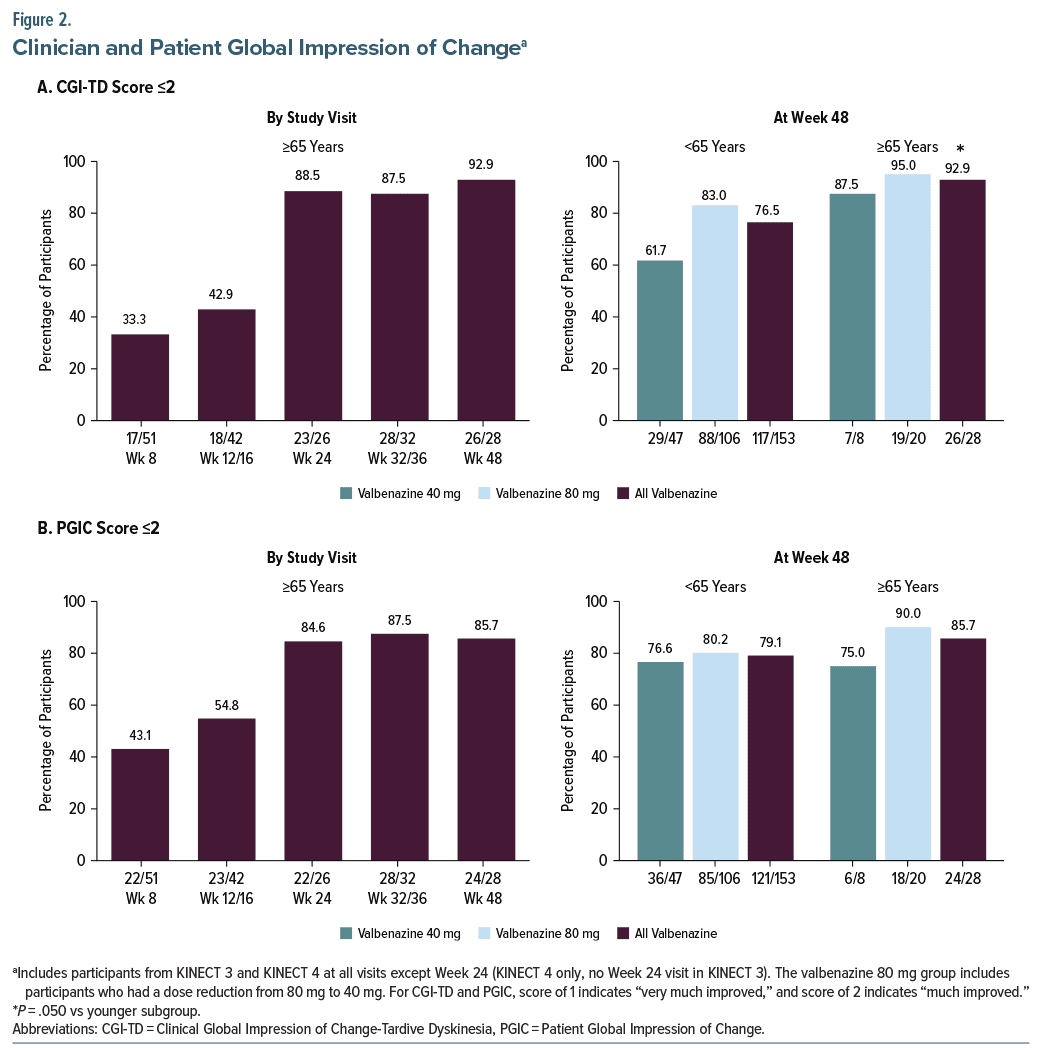
AIMS outcomes in the elderly subgroup were supported by results from the CGI-TD and PGIC analyses. With both scales, the percentage of elderly participants who had a score ≤2 (“much improved” or “very much improved”) increased from Week 8 to Week 24 and then was sustained through Week 48 (Figures 2A and 2B, left panels). At Week 48, 92.9% of elderly participants had a CGI-TD score ≤2, which was significantly higher than the 76.5% of younger participants who met this threshold of improvement (Figure 2A, right panel). For PGIC, 85.7% of elderly participants had a score ≤2 at Week 48, which was not statistically significant relative to the younger subgroup (Figure 2B, right panel).
Adverse Events
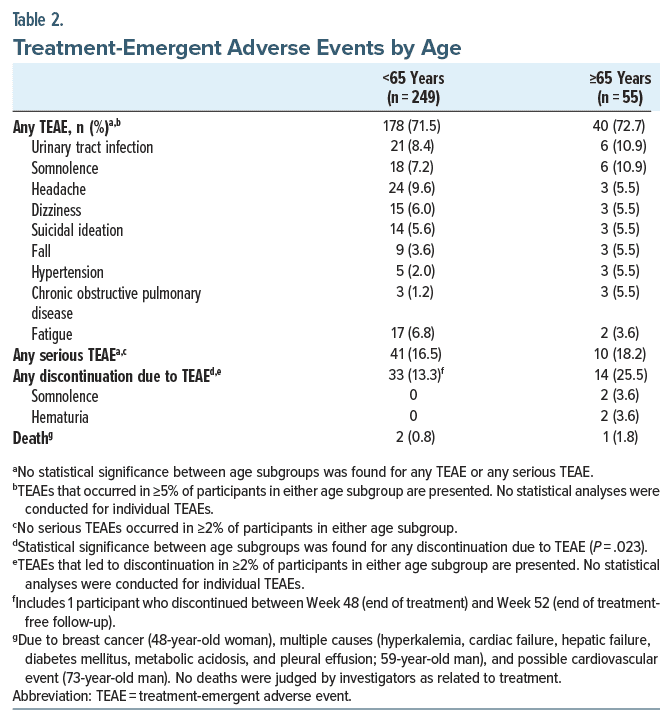
There were no statistically significant differences between age subgroups for the incidence of TEAEs or serious TEAEs (Table 2). Urinary tract infection was common in both elderly and younger participants (10.9% and 8.4%, respectively), as was somnolence (10.9% and 7.2%). A significantly higher percentage of elderly participants discontinued due to a TEAE compared to the younger subgroup (25.5% vs 13.3%, P =.023). Somnolence and hematuria led to discontinuation in 2 elderly participants; all other TEAEs leading to discontinuation occurred in 1 elderly participant.
Two deaths occurred in the younger subgroup during the studies: 1 due to breast cancer and 1 due to multiple causes (hyperkalemia, cardiac failure, hepatic failure, diabetes mellitus, metabolic acidosis, and pleural effusion). One death occurred in the elderly subgroup due to a possible cardiovascular event. Study investigators did not judge any death as related to treatment.
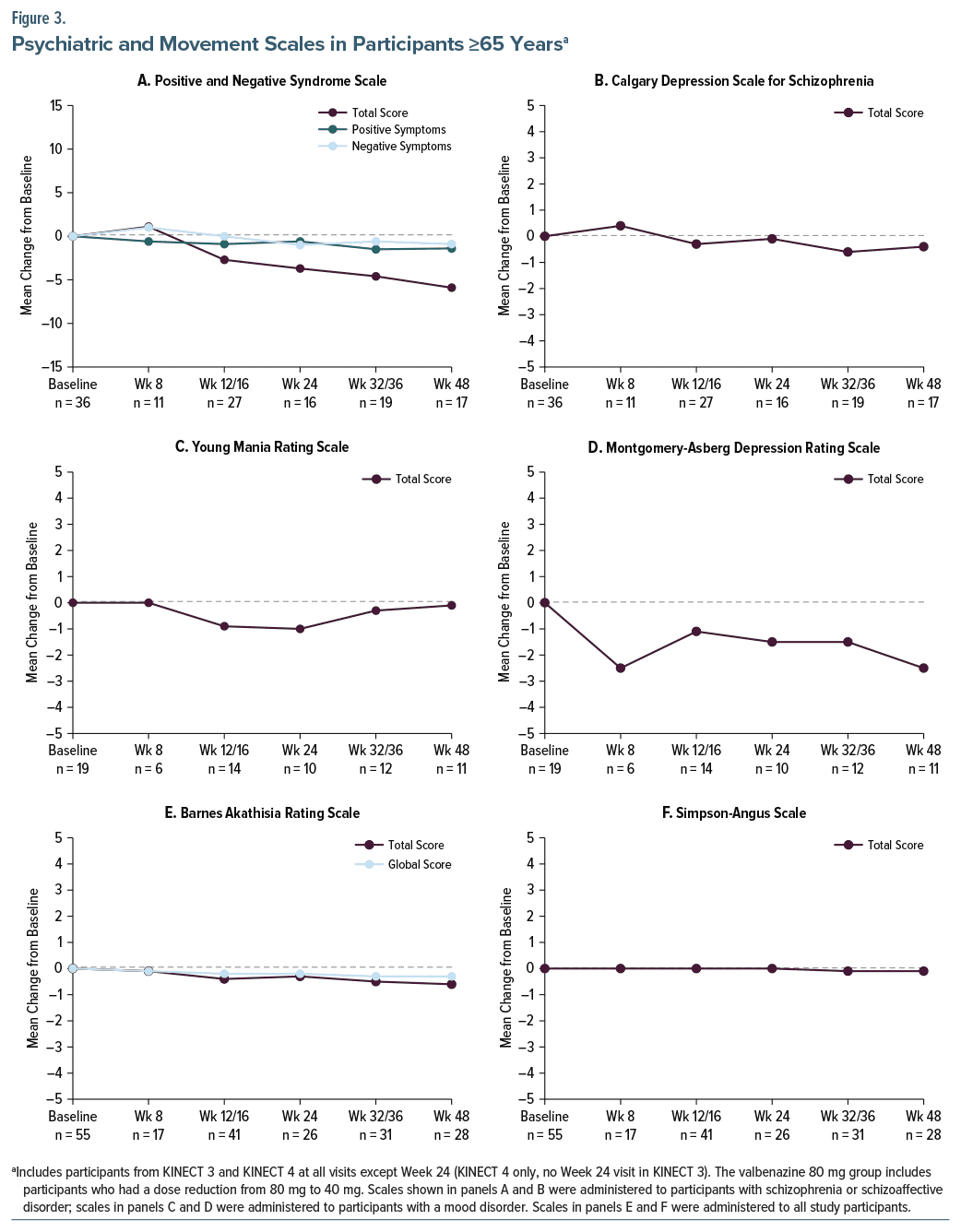
Psychiatric and Movement Scales
Psychiatric status remained stable throughout valbenazine treatment in elderly participants with schizophrenia or schizoaffective disorder (PANSS, CDSS) or a mood disorder (YMRS, MADRS) (Figures 3A–3D). Mean changes from baseline over time in movement scale scores indicated no emergence or worsening of akathisia (BARS) or drug-induced parkinsonism (SAS) (Figures 3E and 3F). Mean changes from baseline to Week 48 in these psychiatric symptom scales and movement scales were minimal, with no significant differences between elderly and younger participants and indicating maintenance of psychiatric stability throughout the studies (Supplementary Table 1).
DISCUSSION
To date, the findings in this report are the first and only available analyses of clinical trial data for a VMAT2 inhibitor in adults with TD who were aged 65 years and older. Given the projected rates of population aging in the US and globally,22,23 along with the increased risk of TD with older age and historic under representation of adults aged ≥65 years in clinical trials,27 these results address an important gap in TD research.
The data in this report are from post hoc analyses of 2 phase 3 trials, KINECT 3 extension and KINECT 4, in which participants received once-daily valbenazine (40 or 80 mg) for up to 48 weeks.24,25 Post hoc analyses of these data suggest that elderly participants achieved clinically meaningful improvements in TD within 8 weeks of valbenazine treatment, with substantial improvement for those who received 24 weeks of treatment, followed by sustained improvement up to 48 weeks.
Two clinician-rated assessments (AIMS, CGI-TD) and 1 patient-reported assessment (PGIC) were used to evaluate the long-term effects of valbenazine in elderly participants. In KINECT 3, the AIMS was scored by central video raters who were blinded to treatment assignment (during the DBPC period), valbenazine dose (during the extension period), and study visit (during both periods). In KINECT 4, blinded central video raters were used for selected visits; however, AIMS scoring by nonblinded study-site investigators was conducted at every visit. For this pooled analysis, all AIMS scores for KINECT 4 were based on the investigators’ ratings. The primary reports of these studies indicated that AIMS scores at baseline were higher (more severe) when abnormal movements were assessed by site investigators than by central video raters.
At baseline, both age subgroups (<65 years, ≥65 years) had an AIMS total score of approximately 12, consistent with moderate-to-severe severity in 1 or more body regions. Based on observed cases, the mean change from baseline in elderly participants at Week 8 (−4.5 [n=50]) exceeded the AIMS MCID for valbenazine (−2 points).26 The mean decrease in AIMS total score was almost doubled at Week 24 (−8.6 [n=26]), Week 32/36 (−8.8 [n=31]), and Week 48 (−8.8 [n=28]).
To evaluate clinical meaningfulness, responder analyses based on individual score changes were conducted. One analysis identified participants who had at least a 30% decrease from baseline in AIMS total score, which was determined to be a clinically meaningful threshold for response.26 The other used a ≥50% threshold, which was based on the definition of AIMS response used in both study protocols. In addition to the AIMS analyses, responder analyses for CGI-TD and PGIC were conducted using a score ≤2 (“much improved” or better) as the response threshold. With all 3 scales, the percentage of elderly participants who met thresholds for response increased over time. Comparisons with the younger subgroup indicated no statistical differences for any of these outcomes at Week 48, except for CGI-TD response (92.9% vs 76.5% [<65 years]; P = .050).
When considered in conjunction with the prior post hoc analyses of AIMS and CGI-TD data in older adults (≥55 years, mean age of 62.4 years),21 the current post hoc analyses in elderly adults (≥65 years, mean age of 69.8 years) provides a glimpse of what might be expected with valbenazine treatment as TD patients advance through later life. In terms of efficacy, the post hoc analyses indicate no loss of valbenazine efficacy as age increases. At Week 48, a similar percentage of older and elderly participants met the response thresholds for AIMS (≥50% improvement: 70.7% and 82.1%, respectively) and CGI-TD (score ≤2: 82.8% and 92.9%).
As in the ≥55-year age group,21 long-term valbenazine treatment was generally well tolerated in the ≥65 years age group. The prescribing information for valbenazine indicates that somnolence/sedation, which includes “somnolence” and “fatigue” (MedDRA terms), was the most common adverse reaction with valbenazine in placebo-controlled trials.17 In this post hoc analysis, 10.9% of elderly participants had somnolence (with 2 discontinuations) and 3.6% had fatigue (compared to 7.2% and 6.8%, respectively, in the younger subgroup). The incidence of dizziness and falls (5.5% for both) was similar to the younger subgroup (dizziness 6.0%, fall 3.6%), but these TEAEs may be of greater concern in the elderly subgroup due to their association with mortality and chronic disability.28 Mean changes from baseline in psychiatric symptom scales were minimal at all pooled study visits, indicating maintenance of psychiatric stability during long-term valbenazine treatment. Mean changes in movement scales were also minimal; however, treatment emergent parkinsonism has been observed with VMAT2 inhibitors, and valbenazine dosing should be reduced if these symptoms appear.17,20
Several important limitations should be considered when interpreting the results of this post hoc analysis. The pooled study population, which included fewer elderly adults (n = 55) than younger adults (n = 249), might not be representative of the broader patient population with TD due to inclusion criteria (eg, requirement for stable psychiatric status prior to study entry); therefore, generalizability of the results is limited. Moreover, the statistical analyses were not corrected for multiple comparisons, and no conclusions can be drawn regarding dose effects because valbenazine dosing in KINECT 4 was determined by site investigators based on each participant’s tolerability and treatment response. Finally, potential selection bias due to study dropouts must be considered, particularly with regard to the higher incidence of TEAEs leading to discontinuation in the elderly subgroup. Nonetheless, findings from this post hoc analysis provide information about valbenazine treatment in adults with TD who are 65 years and older, a group in which clinical trial data are not publicly available for other TD medications.
CONCLUSIONS
This post hoc analysis of data from 2 clinical trials of valbenazine indicates that elderly participants who received up to 48 weeks of treatment experienced substantial and sustained improvements in TD. Moreover, no new TEAEs of clinical concern were found in the elderly participants, and psychiatric stability was maintained through 48 weeks of treatment. These analyses, which are the first to evaluate a VMAT2 inhibitor in patients with TD aged 65 years and older, add to the evidence that suggests valbenazine may be a uniquely appropriate option for managing TD in the elderly age group.
Article Information
Published Online: April 23, 2025. https://doi.org/10.4088/JCP.24m15550
© 2025 Physicians Postgraduate Press, Inc.
Submitted: August 7, 2024; accepted February 3, 2025
To Cite: Sajatovic M, Alexopoulos GS, Jen E, et al. Improvements over time with valbenazine in elderly adults (≥65 years) with tardive dyskinesia: post hoc analyses of 2 long-term studies. J Clin Psychiatry 2025;86(2):24m15550.
Author Affiliations: Case Western Reserve University School of Medicine, Cleveland, Ohio (Sajatovic); University Hospitals of Cleveland Medical Center, Cleveland, Ohio (Sajatovic); Department of Psychiatry, Weill Cornell Medical College, New York, New York (Alexopoulos); Neurocrine Biosciences, Inc, San Diego, California (Jen, Farahmand, Zinger).
Corresponding Author: Martha Sajatovic, MD, Case Western Reserve University School of Medicine; University Hospitals of Cleveland Medical Center, 10524 Euclid Ave, Cleveland, OH 44106 ([email protected]).
Relevant Financial Relationships: Dr Sajatovic has received research grants (past 3 years) from Neurelis, Intra-Cellular, Merck, Otsuka, Alkermes, International Society for Bipolar Disorders (ISBD), National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), and Patient-Centered Outcomes Research Institute (PCORI); has been a consultant (past 1 year) for Alkermes, Otsuka, Lundbeck, Janssen, and Teva; has received royalties (past 1 year) from Springer Press, Johns Hopkins University Press, Oxford Press, and UpToDate; and has received compensation for preparation of/participation in CME activities (past 1 year) from American Physician’s Institute (CMEtoGo), Psychopharmacology Institute, American Epilepsy Society, and Clinical Care Options. Drs Jen and Farahmand are employees of Neurocrine Biosciences, Inc. Dr Zinger is a former employee of Neurocrine Biosciences, Inc. Dr Alexopoulos has participated in the Otsuka Speakers Bureau and in an Otsuka advisory board.
Funding/Support: The studies and analyses in this report were funded by Neurocrine Biosciences, Inc.
Role of the Sponsor: The sponsor was involved in study design, study conduct, data collection, and data analysis.
Previous Presentation: This work was presented at the American Association for Geriatric Psychiatry Annual Meeting; March 3–6, 2023; New Orleans, Louisiana; the 2nd Annual Psychiatry Summit; May 11–13, 2023 (virtual meeting); the American Association of Nurse Practitioners National Conference; June 20–25, 2023; New Orleans, Louisiana; the 37th Annual American Psychiatric Nurses Association Conference; October 4–7, 2023; Lake Buena Vista, Florida; the American Society for Consultant Pharmacists Annual Meeting; October 26–29, 2023; Kissimmee, Florida.
Acknowledgment: Writing and editorial assistance were provided by Prescott Medical Communications Group, a Citrus Health Group, Inc, company (Chicago, Illinois), with support from Neurocrine Biosciences.
ORCID: Martha Sajatovic: https://orcid.org/0000-0002-3073-668X; Eric Jen: https://orcid.org/0000-0001-7322-2403
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- Older patients who take antipsychotics or other dopamine receptor blocking agents have a higher risk of developing tardive dyskinesia (TD), but treatment-related data in more elderly patients (≥65 years) had been lacking. This article reports on data indicating that once-daily valbenazine is effective and well-tolerated in the ≥65-year age group.
- Valbenazine is the only approved TD medication with sufficient clinical trial data indicating that no dose reduction is needed in patients who are ≥65 years of age. Valbenazine has a sprinkle formulation that can be added to soft foods, and there is no prohibition on crushing the compacted powder formulation.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at Psychiatrist.com/contact/karp, or Gary W. Small, MD, at Psychiatrist.com/contact/small.
References (28)

- Citrome L, Isaacson SH, Larson D, et al. Tardive dyskinesia in older persons taking antipsychotics. Neuropsychiatr Dis Treat. 2021;17:3127–3134.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision. American Psychiatric Association;2022.
- Woerner MG, Alvir JM, Saltz BL, et al. Prospective study of tardive dyskinesia in the elderly: rates and risk factors. Am J Psychiatry. 1998;155(11):1521–1528. PubMed CrossRef
- Woerner MG, Correll CU, Alvir JM, et al. Incidence of tardive dyskinesia with risperidone or olanzapine in the elderly: results from a 2-year, prospective study in antipsychotic-naïve patients. Neuropsychopharmacology. 2011;36(8):1738–1746. PubMed CrossRef
- Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry 2017;78(3):e264–e278.
- Caroff SN, Yeomans K, Lenderking WR, et al. RE-KINECT: a prospective study of the presence and healthcare burden of tardive dyskinesia in clinical practice settings. J Clin Psychopharmacol. 2020;40(3):259–268.
- Tanner CM, Caroff SN, Cutler AJ, et al. Impact of possible tardive dyskinesia on physical wellness and social functioning: results from the real-world RE-KINECT study. J Patient Rep Outcomes. 2023;7(1):21.
- Caroff S, Cutler A, Tanner C, et al. RE-KINECT, a real-world, prospective tardive dyskinesia screening study: an evaluation of baseline characteristics in older patients. Am J Geriatr Psychiatry. 2019;27(3 suppl):S217–S219.
- Strassnig M, Rosenfeld A, Harvey PD. Tardive dyskinesia: motor system impairments, cognition and everyday functioning. CNS Spectr. 2018;23(6):370–377. PubMed CrossRef
- Yassa R, Jones BD. Complications of tardive dyskinesia: a review. Psychosomatics. 1985;26(4):305–307, 310, 312–313.
- Jeste DV. Tardive dyskinesia rates with atypical antipsychotics in older adults. J Clin Psychiatry. 2004;65(suppl 9):21–24.
- Yassa R, Lal S. Respiratory irregularity and tardive dyskinesia. A prevalence study. Acta Psychiatr Scand. 1986;73(5):506–510. PubMed CrossRef
- Jeste DV. Tardive dyskinesia in older patients. J Clin Psychiatry. 2000;61(suppl 4):27–32.
- Pandey S. Orofacial dyskinesia in elderly. Mov Disord Clin Pract. 2015;2(4):442.
- McEvoy J, Gandhi SK, Rizio AA, et al. Effect of tardive dyskinesia on quality of life in patients with bipolar disorder, major depressive disorder, and schizophrenia. Qual Life Res. 2019;28(12):3303–3312. PubMed CrossRef
- Cutler AJ, Caroff SN, Tanner CM, et al. Caregiver-reported burden in RE-KINECT: data from a prospective real-world tardive dyskinesia screening study. J Am Psychiatr Nurses Assoc. 2023;29(5):389–399.
- INGREZZA (valbenazine) capsules and INGREZZA SPRINKLE (valbenazine capsules). Prescribing Information. Neurocrine Biosciences, Inc.;2024.
- Regulatory approval of DYSVAL® capsules 40 mg for treatment of tardive dyskinesia in Japan. News release. Mitsubishi Tanabe Pharma; 2022. Accessed July 19, 2024. https://www.mt-pharma.co.jp/e/news/assets/pdf/e_ MTPC220328.pdf
- Sajatovic M, Patel A, Hebert M, et al. Crushing the contents of valbenazine capsules for potential addition to soft foods or administration via gastrostomy tube. Clin Ther. 2023;45(12):1222–1227.
- Austedo XR (deutetrabenazine extended-release) and Austedo (deutetrabenazine) tablets. Prescribing information. Teva pharmaceuticals USA. Inc.;2024.
- Sajatovic M, Alexopoulos GS, Burke J, et al. The effects of valbenazine on tardive dyskinesia in older and younger patients. Int J Geriatr Psychiatry. 2020;35(1):69–79. PubMed CrossRef
- United States Census Bureau. National population projections tables: Main series. 2023. Accessed July 19, 2024. https://www.census.gov/data/tables/2023/ demo/popproj/2023-summary-tables.html
- United Nations Department of Economic and Social Affairs. World Population Prospects 2022: Summary of Results. 2022. Accessed July 19, 2024. https://www. un.org/development/desa/pd/content/World-Population-Prospects-2022
- Factor SA, Remington G, Comella CL, et al. The effects of valbenazine in participants with tardive dyskinesia: results of the 1-year KINECT 3 extension study. J Clin Psychiatry. 2017;78(9):1344–1350.
- Marder SR, Singer C, Lindenmayer JP, et al. A phase 3, 1-year, open-label trial of valbenazine in adults with tardive dyskinesia. J Clin Psychopharmacol. 2019;39(6):620–627.
- Stacy M, Sajatovic M, Kane JM, et al. Abnormal Involuntary Movement Scale in tardive dyskinesia: minimal clinically important difference. Mov Disord. 2019;34(8):1203–1209. PubMed CrossRef
- Schwartz JB. Representative enrolment of older adults in clinical trials: the time is now. Lancet Healthy Longev. 2023;4(7):e301–e303.
- Kapur S, Sakyi KS, Lohia P, et al. Potential factors associated with healthcare utilization for balance problems in community-dwelling adults within the United States: a narrative review. Healthcare (Basel). 2023;11(17):2398.
This PDF is free for all visitors!