Objective: To determine the likelihood of antidepressant response in older adults with major depression as a function of their prior antidepressant trials.
Methods: 500 older adults with major depression as diagnosed by DSM-IV criteria for major depressive episode were treated with venlafaxine extended release for 12 weeks. Participants were recruited from July 2009 to January 2014. For each participant, we collected detailed data on prior antidepressant trials for the current episode of depression. We examined the prospective remission rates as a function of number and class of prior antidepressant trials in a post hoc analysis of pooled data from 2 prior trials.
Results: Remission rates with venlafaxine were inversely correlated with the number of prior adequate medication trials (66% for no prior adequate trials, 45% for 1 prior adequate trial, 23% for 2 or more prior adequate trials; P < .0001). Additionally, if prior treatment trials included a serotonin-norepinephrine reuptake inhibitor, participants were even less likely to achieve remission with venlafaxine (32% for 1 prior adequate trial, 18% for 2 or more prior adequate trials; P < .0001). Those with prior adequate trials were also more likely to require a higher dosage of venlafaxine to achieve remission.
Conclusions: Information on an individual patient’s number and class of prior adequate antidepressant trials can be used to predict the likelihood of a successful treatment outcome with a given antidepressant in older adults with major depression. Further work is needed to refine this approach to provide personalized antidepressant treatment.
Trial Registration: ClinicalTrials.gov identifiers: NCT00892047 and NCT02263248‘ ‹
ABSTRACT
Objective: To determine the likelihood of antidepressant response in older adults with major depression as a function of their prior antidepressant trials.
Methods: 500 older adults with major depression as diagnosed by DSM-IV criteria for major depressive episode were treated with venlafaxine extended release for 12 weeks. Participants were recruited from July 2009 to January 2014. For each participant, we collected detailed data on prior antidepressant trials for the current episode of depression. We examined the prospective remission rates as a function of number and class of prior antidepressant trials in a post hoc analysis of pooled data from 2 prior trials.
Results: Remission rates with venlafaxine were inversely correlated with the number of prior adequate medication trials (66% for no prior adequate trials, 45% for 1 prior adequate trial, 23% for 2 or more prior adequate trials; P < .0001). Additionally, if prior treatment trials included a serotonin-norepinephrine reuptake inhibitor, participants were even less likely to achieve remission with venlafaxine (32% for 1 prior adequate trial, 18% for 2 or more prior adequate trials; P < .0001). Those with prior adequate trials were also more likely to require a higher dosage of venlafaxine to achieve remission.
Conclusions: Information on an individual patient’s number and class of prior adequate antidepressant trials can be used to predict the likelihood of a successful treatment outcome with a given antidepressant in older adults with major depression. Further work is needed to refine this approach to provide personalized antidepressant treatment.
Trial Registration: ClinicalTrials.gov identifiers: NCT00892047 and NCT02263248
J Clin Psychiatry 2019;80(6):18m12483
To cite: Buchalter ELF, Oughli HA, Lenze EJ, et al. Predicting remission in late-life major depression: a clinical algorithm based upon past treatment history. J Clin Psychiatry. 2019;80(6):18m12483.
To share: https://doi.org/10.4088/JCP.18m12483
© Copyright 2019 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Washington University School of Medicine, Saint Louis, Missouri
bCentre for Addiction and Mental Health and Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada
cDepartment of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania
‡Both first authors contributed equally to the work.
*Corresponding author: Benoit H. Mulsant, MD, MS, Department of Psychiatry, CAMH, 250 College St, Toronto, ON, M5T 1R8 Canada ([email protected]).
Late-life depression (LLD), defined as major depressive disorder in those aged > 65 years, is quite common, with prevalence ranging from 1%–5% in community dwelling older adults.1 Its outcomes are deleterious, as LLD often leads to an increased risk of cognitive impairment and cardiovascular, cerebrovascular, and metabolic disease.1–3 Furthermore, LLD is associated with lower quality of life, functional decline, and all-cause mortality.1
In terms of treatment response, LLD responds well to pharmacotherapy, with response being comparable to depression treatment in younger adults.4 Yet, practitioners have little basis for choosing a specific medication or class of medications for a given patient with LLD; putative treatment resistance factors such as comorbid anxiety or executive dysfunction remain controversial as to whether they even predict antidepressant nonresponse, let alone determine a specific treatment course.5 When treating major depressive disorder, most physicians use a selective serotonin reuptake inhibitor (SSRI) as first line treatment because of their ease of use, good tolerability, and safety profile.6 However, approximately two-thirds of patients do not respond to this medication choice even when given a trial of adequate dosage and duration.7 Rates of treatment resistance in randomized controlled trials in LLD are as high as 77% using SSRIs.8,9 Meta-analyses have shown that increasing SSRI dose10 or switching to another antidepressant11 does not improve rates of remission. While a popular treatment option is switching to a serotonin-norepinephrine reuptake inhibitor (SNRI) such as venlafaxine, providers need additional information to predict clinical outcomes and guide treatment planning.
Multiple approaches have been proposed to better define treatment-resistant depression (TRD) and guide treatment planning. First, it is recommended to characterize any cognitive impairment or neurodegenerative disease such as early Alzheimer’s disease, microvascular ischemic disease, or medical or metabolic disease, based on prior studies demonstrating that these factors contribute to treatment resistance in LLD.12–14 Second, it is recommended to ascertain an individual’s prior medication trials that were of adequate dosage and duration.15 For example, Thase and Rush16 determine the level of treatment resistance based on number and type of prior adequate medication trials. Conway et al17 used data from the Sequenced Treatment Alternatives to Relieve Depression study to argue for an “inflection point” at which after having failed 2 adequate trials, patients were unlikely to benefit from a third trial, experiencing only 14% acute remission rate; therefore, they propose that after this inflection point a treatment using novel mechanisms—such as ketamine, nitrous oxide, or repetitive transcranial magnetic stimulation—is warranted.
Although these attempts to better define TRD add to descriptive precision, they do not give practitioners what is most needed: empirical information on the likelihood of achieving remission with a switch to a new antidepressant, augmentation strategy, or neurostimulation approach. To provide a more personalized antidepressant treatment approach, we assessed the likelihood of achieving remission of depressive symptoms with an adequate prospective trial of venlafaxine based on the number and class of prior adequate medication trials using data from the Incomplete Response in Late-Life Depression: Getting to Remission (IRL-GREY) studies.18 We hypothesized that participants’ likelihood of successful remission with venlafaxine would be determined by the number of prior medication trials, with successful remission being inversely associated with number of prior adequate trials. Given prior recommendations that subsequent medication trials should have different mechanisms of action,6,17,19 we also hypothesized that participants who had failed a trial with an SNRI would be less likely to remit than those who had failed to respond to antidepressants from other classes. We also examined the dosage of venlafaxine at which participants achieved remission, as a function of prior antidepressant trials.
METHODS
The participants for this analysis are from 2 National Institute of Mental Health–funded studies, IRL-GREY18 and IRL-GREY-B (Incomplete Response in Late- Life Depression: Getting to Remission with Buprenorphine; unpublished). The studies were approved and overseen by an independent data and safety monitoring board. Both studies were designed to test antidepressant augmentation strategies for TRD in older adults. All participants provided written informed consent. The studies used a common open-label 12-week lead-in phase using venlafaxine extended release (XR) at a dosage up to 300 mg/d, defining treatment resistance as lack of remission from an adequate prospective trial with venlafaxine. The studies also assessed prior medication trials during the current episode. This open-label trial of venlafaxine is the focus of this article (ClinicalTrials.gov identifiers NCT0089204718 and NCT02263248).
Participants and Medication Dosing Strategy
Participants were recruited between July 2009 and January 2014. They were adults 50 years and older who had a major depressive disorder as defined by DSM-IV with at least moderate symptoms as defined by a Montgomery-Asberg Depression Rating Scale (MADRS) score ≥ 15 (ranges 0–60; higher scores indicate greater depression severity). Exclusion criteria included dementia, bipolar disorder, schizophrenia, current psychotic symptoms, and alcohol or substance abuse or dependence within the past 6 months.
Participants were treated with venlafaxine XR for approximately 12 weeks to determine remission status defined as a MADRS20,21 score ≤ 10 at the 2 consecutive final visits. Given that this was an open-label trial, the rater was not blind to treatment assignment. Venlafaxine XR was started at 37.5 mg/d and was titrated to 150 mg/d over 2 weeks (as tolerated). If remission was not achieved by week 6, venlafaxine XR was further titrated up to 300 mg/d (as tolerated). Some patients did not tolerate dosages of 150 or 300 mg/d and remained on lower dosages. In a small number of participants, treatment with venlafaxine XR was extended for up to an additional 4 weeks to clarify remission status.
Assessments
The baseline evaluation included an assessment of the burden of physical illness with the Cumulative Illness Rating Scale-Geriatric22 and an assessment of global cognition with the Mini-Mental State Examination.23 Study clinicians completed the Antidepressant Treatment History Form (ATHF)24 to determine the adequacy in both duration and dosage of prior medication trials for the current depressive episode (ie, the number of failed trials). Information was derived from interviews with participants and review of available medical and pharmacy records. An ATHF score of 0 indicates no prior pharmacotherapy; for each antidepressant trial, a score of 1 indicates a definitely inadequate trial; 2, a probably inadequate trial; 3, a probably adequate trial; 4, a definitely adequate trial; and 5, a definitely adequate trial that included augmentation pharmacotherapy. Augmentation trials with antidepressants were counted as separate trials for our analyses; for example, an adequate trial of citalopram with an augmentation of bupropion was counted as 2 trials. Therefore, participants with ATHF scores of 0 were treatment naïve prior to receiving venlafaxine XR, those with scores of 1–2 had received inadequate treatment, and those with scores of 3–5 had received prior adequate treatment.
Participants who had previous trials of venlafaxine were not excluded, with the rationale that a prior adequate trial of venlafaxine retrospectively was not felt to invalidate the possibility of remission with a full trial of venlafaxine prospectively.
Analytic Strategy
Remission status was examined as a function of number of prior adequate medication trials. When we examined participants with 3 or more prior medication trials, their rate of remission was similar to those of participants with 2 prior trials; therefore, we created a category of “2 or more trials” and analyzed these participants together. Participants were further categorized by whether they had been treated with an SNRI. We also examined the number of prior inadequate medication trials.
We used Pearson χ2 tests to compare remission rates between groups based on prior treatment status, number of prior adequate medication trials, and class of medication trials. To evaluate change in MADRS scores, we fit a mixed effect repeated-measures model with separate average slopes for each group. Complete data were used in this analysis. All statistical analyses were conducted using statistical software (SAS 9.4, SAS Institute; Cary, North Carolina).
RESULTS
Description of Participants
ATHF data were available for 500 participants; 454 were from the first study and 46 were from the second study. Of these participants, 104 (20.8%) withdrew participation from the medication. Reasons for noncompletion included treatment preference (n = 27), side effects or adverse events (n = 32), nonadherence (n = 11), current medical problems (n = 10), and miscellaneous issues such as alcohol or drug use, depression worsening, cognitive impairment/dementia, death, and other administrative reason (n = 24). These participants were removed from further analysis.
As seen in Table 1, the average age of participants was 68.6 years; two-thirds were women, and the majority was Caucasian. On average, participants were high school graduates. The mean baseline MADRS score was 26.7. One third of participants were receiving concomitant benzodiazepine treatment.
Overall Rate of Remission to Venlafaxine XR
Of the initial 500 participants with information on the number of prior antidepressant trials from the ATHF, 396 completed 12 to 16 weeks of treatment with venlafaxine XR. One hundred ninety-two (48%) had remission of symptoms, and 204 (52%) did not fully remit.
Rates of Remission as a Function
of Prior Medication Trials
The breakdown by number of prior adequate medication trials was none: 189 (38.8%); one: 204 (40.8%); two: 78 (15.6%); three: 17 (3.4%); four: 8 (1.6%); five: 2 (0.4%); and seven: 2 (0.4%). In terms of previous history of SNRI use, 57 participants received duloxetine (14 inadequate and 42 adequate trials), 68 participants received venlafaxine (32 inadequate and 36 adequate trials), and 19 participants received desvenlafaxine (7 inadequate and 12 adequate trials). None had a previous history of treatment with levomilnacipran.
With the use of the ATHF, participants were classified as naïve to prior treatment (ie, no prior antidepressant trials, either adequate or inadequate, for this current episode of depression), treatment with only inadequate medication trial(s) due to insufficient medication dosage or duration of treatment, and treatment with at least 1 adequate medication trial.
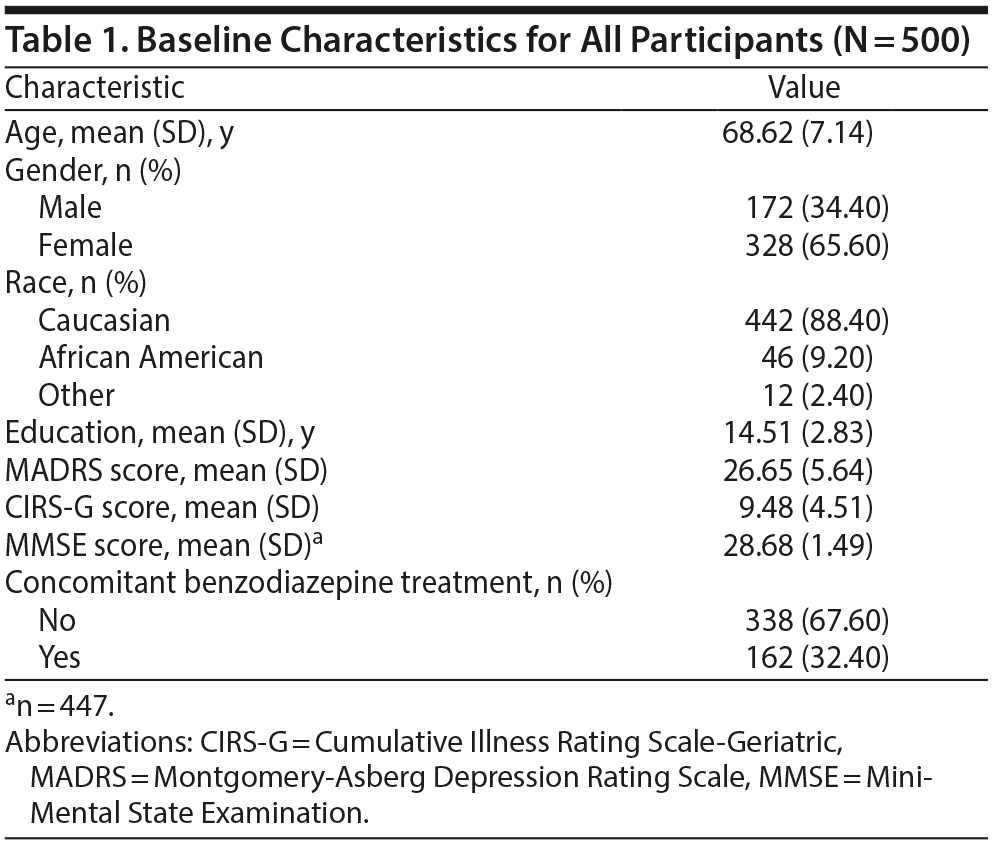
As seen in Figure 1, of the 93 participants who were naïve to prior medication trials, 62 (67%) achieved remission; of the 44 participants with only prior inadequate treatment, 29 (66%) achieved remission; and of the 259 with prior adequate treatment, 101 (39%) achieved remission (χ22 = 27, P < .0001 for the comparison of no prior trials, only inadequate trials, and ≥ 1 adequate trials for likelihood of remission). Given that participants who were naïve or had inadequate treatment had the same response to venlafaxine, they were combined in further analyses as “no prior adequate medication trials.”
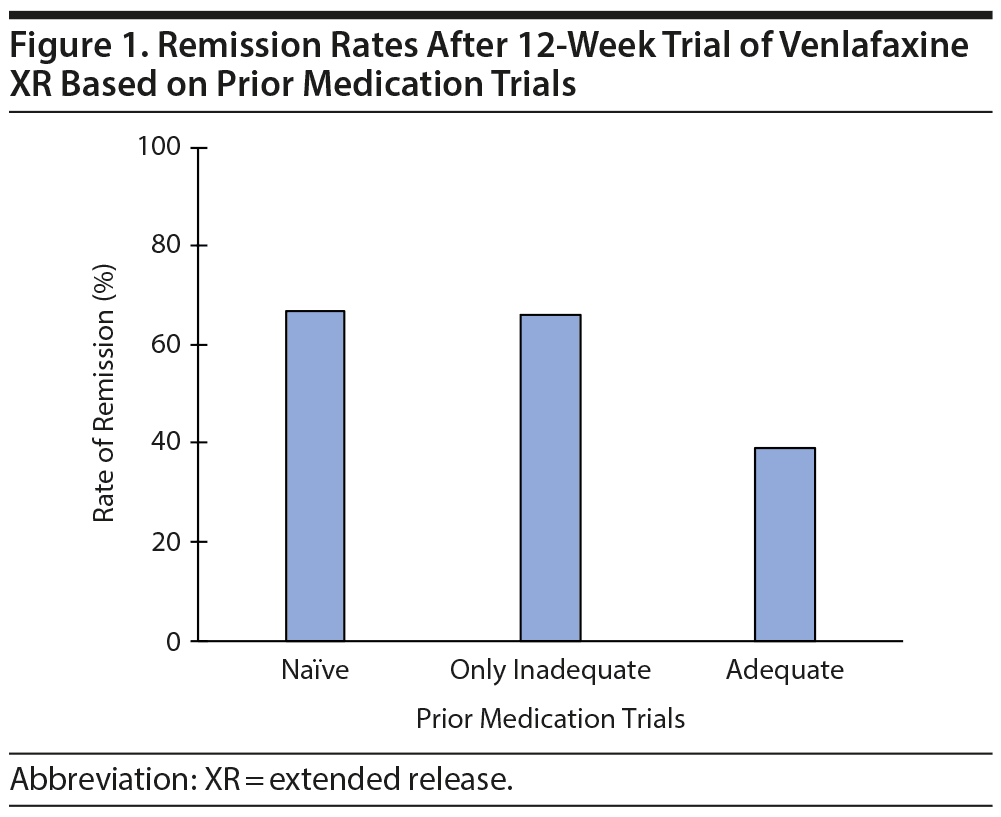
As seen in Figure 2, patients who had more prior adequate medication trials were less likely to achieve remission with venlafaxine: of the 137 participants with no prior adequate medication trials, 91 (66%) achieved remission with venlafaxine; of the 189 participants with 1 prior adequate medication trial, 85 (45%) achieved remission; and of the 70 participants with 2 or more prior adequate medication trials, 16 (23%) achieved remission (χ22 = 37, P < .0001 for the comparison of 0, 1, and ≥ 2 adequate trials for likelihood of remission).
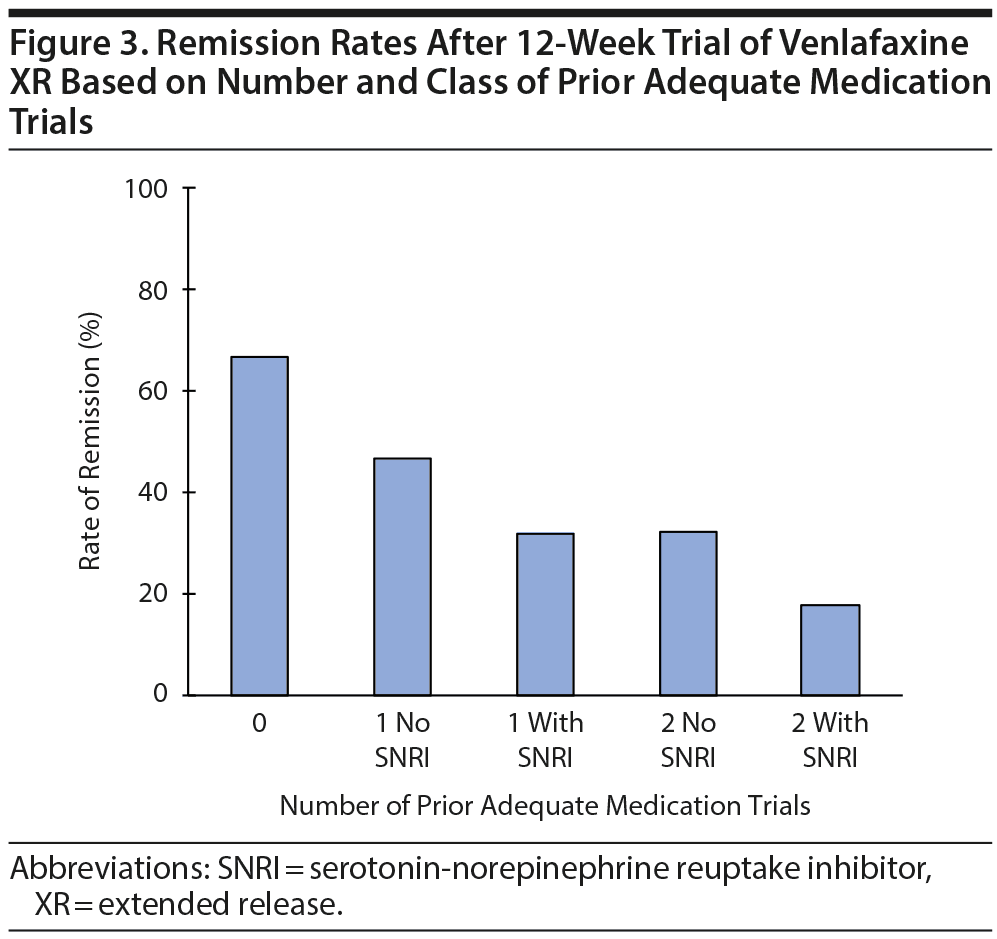
Rate of Remission Based on Prior SNRI Trial
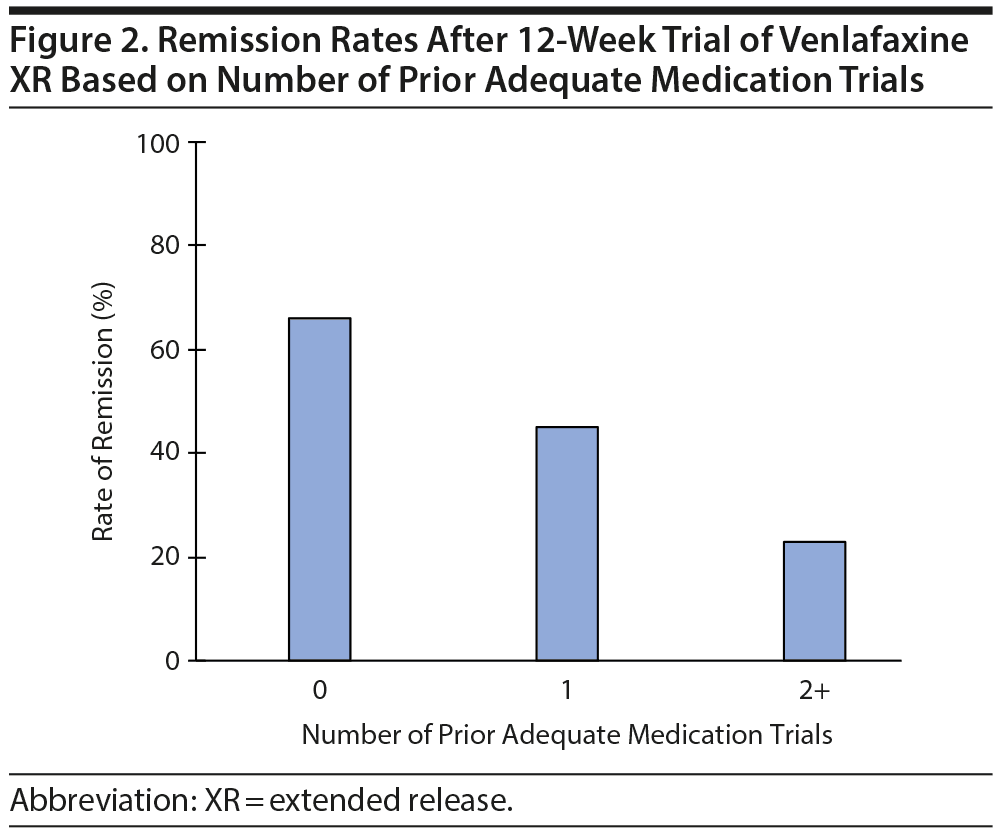
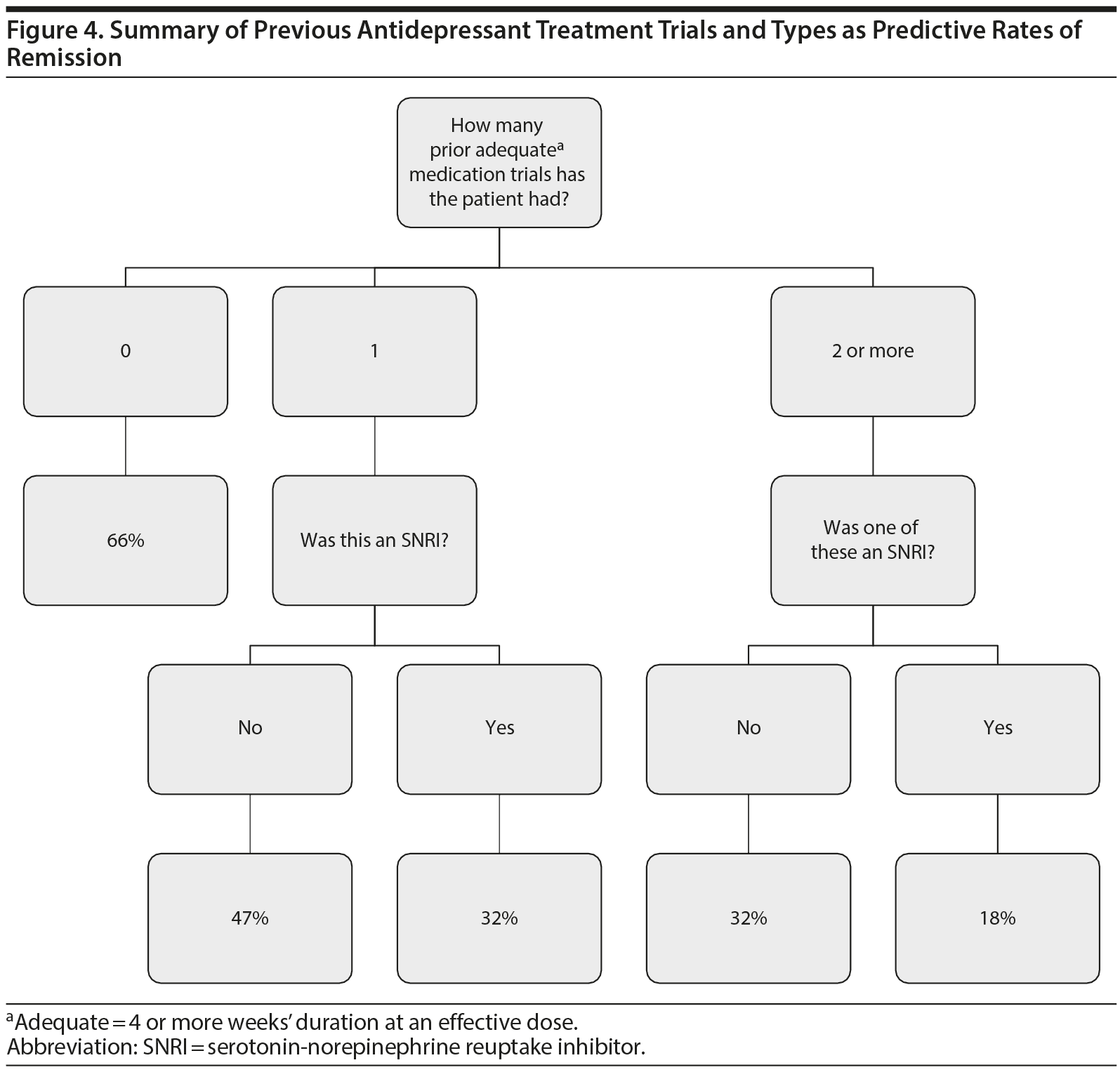
As seen in Figure 3, 22 of the 189 (12%) participants with 1 prior adequate medication trial had been treated with an SNRI previously, and 7 (32%) of them achieved remission with venlafaxine. Of the 70 participants with 2 or more prior adequate medication trials, 45 (64%) had been treated with at least 1 SNRI previously, and 8 (18%) of them achieved remission (χ24 = 40, P < .0001 for the comparison of 0, 1 non-SNRI, 1 SNRI, ≥ 2 non-SNRI, and ≥ 2 including an SNRI adequate trials for likelihood of remission; see Supplementary Table 1). Thus, participants who had been treated previously with an SNRI were less likely to respond to venlafaxine XR than those who had not been treated with an SNRI. This finding did not change when we removed from the analysis participants who had been previously treated with adequate dosage and duration of venlafaxine XR (data not shown). Figure 4 summarizes the likelihood of remission based on both the number of prior antidepressant trials and whether one of these prior trials was of an SNRI.
Venlafaxine XR Dosage Based on
Number and Class of Prior Medication Trials
The final venlafaxine XR dosage was available for 190 of the 192 participants who met criteria for remission. Among the 90 participants with no prior adequate medication trials who remitted with venlafaxine, 58 (64%) remitted on 150 mg/d or less, vs 32 (36%) requiring a dosage higher than 150 mg/d. Among the 77 participants with 1 prior adequate medication trial that was not an SNRI who remitted, 32 (42%) remitted on 150 mg/d or less, while 45 (58%) required a dosage higher than 150 mg/d (χ21 = 8.75, P = .003).
Changes in MADRS Scores
The changes in MADRS scores are presented in Supplementary Figure 1, and the corresponding analyses are presented in Supplementary Appendix 1.
DISCUSSION
We examined prospective remission rates with venlafaxine XR treatment as a function of prior medication trials in a large sample of older adults. Our study had 3 key findings. First, we found that both the number and class of prior adequate medication trials predicted likelihood of remission with an acute trial of venlafaxine. Second, if a participant had previously been treated with 2 or more antidepressants and if one of these prior trials was an SNRI, the participant had a low likelihood to respond to venlafaxine at any dosage, suggesting this threshold of prior antidepressant exposure could predict futility with another SNRI trial. Third, prior trial data also informed the target dosage of venlafaxine needed for remission: participants with no prior medication trials required a lower dosage of venlafaxine to remit, whereas those with at least 1 prior medication trial typically needed a higher dosage of venlafaxine or longer duration of treatment to remit. These findings have practical implications for clinicians as they choose pharmacotherapy options with their older patients: our decision tree (Figure 4) summarizes the likelihood of remission based on both the number of prior antidepressant trials and the class of antidepressant used (ie, SNRI vs no SNRI).
We found that participants resistant to prior adequate medication trials were less likely to respond to venlafaxine, confirming prior findings7 in older depressed adults.7,15 Our analysis went a step further and found that the class of medication trial was also important. Participants with 2 or more medication trials who had never tried an SNRI were more likely to respond than those who had tried an SNRI: those who had failed at least 2 trials, including 1 in the same (SNRI) class, had only 1 chance in 6 of remitting with another SNRI trial. This would seem to demonstrate futility to SNRI use; given this low likelihood of success, we believe that rather than trying another SNRI trial, other treatment strategies, for example, augmenting with atypical antipsychotics,18,25,26 lithium,27 or novel mechanism pharmacotherapies such as vortioxetine28 or vilazodone,29 should be preferred for individuals with this level of prior antidepressant exposure.
This study demonstrates the potential benefits of using idiographic, or patient-specific, data to personalize antidepressant care. To date, most precision medicine research is nomothetic—ie, it uses data gathered from other patient samples and tries to apply observations of that group’s experience to an individual patient. This nomothetic approach has been criticized30 and to date has been unsuccessful in determining consistently reproducible and clinically relevant treatment resistance factors that would inform treatment planning.5 In contrast, we found that using the patient’s own unique experience with medications can robustly inform treatment decisions. This information is limited by recall bias, and other research has shown that patients defined as being treatment resistant only by history, such as the ATHF method, will have a higher subsequent remission rate with an antidepressant compared to those shown to be treatment resistant via a prospective trial.31 In spite of this, our data agree with others that retrospective antidepressant treatment history is a reasonable method for determining the likelihood of treatment resistance.31
Our results could be replicated and extended using a big-data analytic approach examining treatment outcomes as a function of prior trials and the adequacy of those trials, potentially in combination with other putative treatment resistance factors in LLD such as executive dysfunction and comorbid medical burden. It remains to be seen whether the characterization of prior antidepressant treatments in a real-world treatment sample (eg, from an administrative database) would have the predictive value we have shown with clinical trial data.
Our study has limitations that highlight the need for further research with additional samples. First, we only tested 1 antidepressant prospectively (venlafaxine XR) and only in older adults. As well, our data came from open-label clinical trials that provided frequent measurement-guided visits, which may have contributed to a “placebo response” and a relatively high remission rate. Therefore, the high rate of remission with venlafaxine XR we observed in those with no prior trials might not be seen in typical clinical settings, such as primary care, unless they also provide this level of measurement-based care. We also allowed the use of benzodiazepines, which is a reflection of real world clinical care but is not ideal given the risks of these medications (such as falls) in older adults.32 Additionally, tolerability of previous medication trials was not assessed in this study.
In conclusion, this analysis confirms that a careful evaluation of prior adequate medication trials, in terms of both number and class, can help clinicians to optimize the selection of the next step that should be tried when treating depression, including switching antidepressant medications, augmenting the medication, or using a neurostimulation technique. Continued research is needed to further optimize treatment algorithms for depression.
Submitted: July 25, 2018; accepted August 22, 2019.
Published online: December 10, 2019.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, ketamine and nitrous oxide are not approved by the US Food and Drug Administration for the treatment of depression.
Author contributions: Drs Lenze, Reynolds, and Mulsant designed the parent study and wrote the protocol. Drs Buchalter, Oughli, Lenze, Dixon, and Mulsant and Mr Miller developed the hypothesis and secondary data analytic plan, had access to all the data, and analyzed the data. Drs Buchalter, Oughli, Lenze, and Mulsant were responsible for the decision to submit the report and drafted it. All authors read, critically revised, edited, and approved the report.
Financial disclosure: Drs Buchalter, Oughli, Lenze, Dixon, Blumberger, Karp, Reynolds, and Mulsant and Mr Miller have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: This study was supported primarily by the National Institute of Mental Health (R01 MH083660 and P30 MH90333 to University of Pittsburgh, R01 MH083648 to Washington University, and R01 MH083643 and R34 MH101365 to the Centre for Addiction and Mental Health at the University of Toronto). Additional funding was provided by the UPMC Endowment in Geriatric Psychiatry, the Taylor Family Institute for Innovative Psychiatric Research (at Washington University), the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences, and the Campbell Family Mental Health Research Institute at the Centre for Addiction and Mental Health, Toronto. Bristol-Myers Squibb contributed aripiprazole and placebo tablets, and Pfizer contributed venlafaxine extended release capsules for this study.
Role of the sponsor: Funding sources for this study had no role in the design or conduct of the research, including the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclaimer: Dr Karp, JCP Focus on Geriatric Psychiatry Section Editor, was not involved in the editorial review or decision to publish this article.
Acknowledgments: The authors thank the clinical and data management staff and the patient participants of the IRL GREY (Incomplete Response in Late-Life Depression: Getting to Remission) study and the Data and Safety Monitoring Board members: Joel Streim, MD, University of Pennsylvania; Jeff Williamson, MD, Wake Forest University; J. Craig Nelson, MD, University of California San Francisco; Joel Greenhouse, PhD, Carnegie Mellon University; and Steven Roose, MD, Columbia University.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
1. Hall CA, Reynolds III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147–152. PubMed CrossRef
2. Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–335. PubMed CrossRef
3. Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biol Psychiatry. 2013;73(5):406–413. PubMed CrossRef
4. Haigh EAP, Bogucki OE, Sigmon ST, et al. Depression among older adults: a 20-year update on five common myths and misconceptions. Am J Geriatr Psychiatry. 2018;26(1):107–122. PubMed CrossRef
5. Masse-Sibille C, Djamila B, Julie G, et al. Predictors of response and remission to antidepressants in geriatric depression: a systematic review. J Geriatr Psychiatry Neurol. 2018;31(6):283–302. PubMed CrossRef
6. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517–534. PubMed CrossRef
7. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
8. Allard P, Gram L, Timdahl K, et al. Efficacy and tolerability of venlafaxine in geriatric outpatients with major depression: a double-blind, randomised 6-month comparative trial with citalopram. Int J Geriatr Psychiatry. 2004;19(12):1123–1130. PubMed CrossRef
9. Whyte EM, Basinski J, Farhi P, et al. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry. 2004;65(12):1634–1641. PubMed CrossRef
10. Rink L, Braun C, Bschor T, et al. Dose increase versus unchanged continuation of antidepressants after initial antidepressant treatment failure in patients with major depressive disorder: a systematic review and meta-analysis of randomized, double-blind trials. J Clin Psychiatry. 2018;79(3):17r11693. PubMed CrossRef
11. Bschor T, Kern H, Henssler J, et al. Switching the antidepressant after nonresponse in adults with major depression: a systematic literature search and meta-analysis. J Clin Psychiatry. 2018;79(1):16r10749. PubMed CrossRef
12. Geerlings MI, Gerritsen L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: a systematic review and meta-analysis. Biol Psychiatry. 2017;82(5):339–350. PubMed CrossRef
13. Alves GS, Carvalho AF, Sudo FK, et al. Structural neuroimaging findings in major depressive disorder throughout aging: a critical systematic review of prospective studies. CNS Neurol Disord Drug Targets. 2014;13(10):1846–1859. PubMed CrossRef
14. Mahgoub N, Alexopoulos GS. Amyloid hypothesis: is there a role for antiamyloid treatment in late-life depression? Am J Geriatr Psychiatry. 2016;24(3):239–247. PubMed CrossRef
15. Tew JD Jr, Mulsant BH, Houck PR, et al. Impact of prior treatment exposure on response to antidepressant treatment in late life. Am J Geriatr Psychiatry. 2006;14(11):957–965. PubMed CrossRef
16. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23–29. PubMed
17. Conway CR, George MS, Sackeim HA. Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry. 2017;74(1):9–10. PubMed CrossRef
18. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404–2412. PubMed CrossRef
19. Connolly KR, Thase ME. If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71(1):43–64. PubMed CrossRef
20. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
21. Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on the Montgomery-Asberg Depression Rating Scale corresponding to the definition of remission on the Hamilton Rating Scale for Depression. J Psychiatr Res. 2004;38(6):577–582. PubMed CrossRef
22. Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–248. PubMed CrossRef
23. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. PubMed CrossRef
24. Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–17. PubMed
25. Hsu JH, Mulsant BH, Lenze EJ, et al. Impact of prior treatment on remission of late-life depression with venlafaxine and subsequent aripiprazole or placebo augmentation. Am J Geriatr Psychiatry. 2016;24(10):918–922. PubMed CrossRef
26. Hobart M, Skuban A, Zhang P, et al. A randomized, placebo-controlled study of the efficacy and safety of fixed-dose brexpiprazole 2 mg/d as adjunctive treatment of adults with major depressive disorder. J Clin Psychiatry. 2018;79(4):17m12058. PubMed CrossRef
27. Kok RM, Vink D, Heeren TJ, et al. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: an open, randomized, controlled trial. J Clin Psychiatry. 2007;68(8):1177–1185. PubMed CrossRef
28. Schatzberg AF, Blier P, Culpepper L, et al. An overview of vortioxetine. J Clin Psychiatry. 2014;75(12):1411–1418. PubMed CrossRef
29. Pierz KA, Thase ME. A review of vilazodone, serotonin, and major depressive disorder. Prim Care Companion CNS Disord. 2014;16(1):13r01554. PubMed
30. Senn S. Statistical pitfalls of personalized medicine. Nature. 2018;563(7733):619–621. PubMed CrossRef
31. Iovieno N, Papakostas GI. Does the presence of an open-label antidepressant treatment period influence study outcome in clinical trials examining augmentation/combination strategies in treatment partial responders/nonresponders with major depressive disorder? J Clin Psychiatry. 2012;73(5):676–683. PubMed CrossRef
32. Lee D, Martini N, Moyes S, et al. Potentially inappropriate medication use: the Beers’ Criteria used among older adults with depressive symptoms. J Prim Health Care. 2013;5(3):182–190. PubMed CrossRef
This PDF is free for all visitors!