Abstract
Ketamine, introduced as an anesthetic drug, is now used for many indications beyond anesthesia; it is also increasingly a drug of abuse. Long-term recreational use and abuse of ketamine are associated with urological risks. This article discusses ketamine-associated uropathy from the perspective of prevalence, clinical features, mechanisms, and strategies for risk reduction in patients who require long term maintenance therapy with the drug for psychiatric indications. A systematic review and meta-analysis of 37 studies of uropathy in recreational (ab)users obtained prevalences of 44% to 77% for lower urinary tract symptoms and 8% to 30% for upper urinary tract disease; for reasons explained, these findings are potentially misleading and cannot be extrapolated to therapeutic contexts. More recent studies, using different methods of case ascertainment, present lower risks (2% to 27%). A systematic review of 27 studies of ketamine used to treat psychiatric disorders, mainly depression, found urological symptoms in 0% to 24% of patients; however, in 14 randomized controlled trials, urological symptom prevalences differed little between ketamine and comparison arms. The review presented no convincing evidence of ketamine-associated uropathy arising in therapeutic contexts. The literature on ketamine-associated uropathy is critically examined; reasons for false positive uropathy findings are considered. Ketamine pharmacokinetics are described to assist the understanding of how ketamine and its metabolites may predispose to uropathy. Mechanisms of uropathy, arising from exposure to ketamine and its metabolites in urine (rather than in circulation), are summarized. A reasonable conclusion is that higher doses of ketamine, more frequent dosing with ketamine, longer duration of treatment with ketamine, and oral administration of ketamine are all potential risk factors for ketamine-associated uropathy during maintenance therapy. High hydration and frequent voiding of urine on treatment days can reduce exposure of the bladder to ketamine and its metabolites, reducing urological risks. Fortnightly or monthly urine testing is also advisable.
J Clin Psychiatry 2025;86(4):25f16083
Author affiliations are listed at the end of this article.
Ketamine, developed as a phencyclidine congener in 1962, entered the pharmacopoeia as an anesthetic drug in 1965; by 1970, it had already been widely investigated and described in clinical studies.1–4 Ketamine is now used for many indications beyond anesthesia; it is also increasingly a drug of abuse, especially among youth. Long-term recreational use and abuse of ketamine have been associated with urological risks. The urinary bladder is particularly affected, resulting in a range of lower urinary tract symptoms.
This article discusses ketamine-associated uropathy from the perspective of prevalence, clinical features, mechanisms, and strategies for risk reduction in patients who require long-term maintenance therapy with the drug for psychiatric indications.
Use, Misuse, and Abuse
Ketamine is a 1:1 racemic mixture of R-ketamine and S-ketamine (arketamine and esketamine).5 At present, intravenous and transdermal ketamine are approved for anesthesia and postsurgical pain management, respectively, and intranasal esketamine, only for depression. However, ketamine, arketamine, and esketamine have been examined in clinical trials across a wide range of neuropsychiatric and medical indications. Beyond anesthesia, postsurgical pain management, and depression, ketamine and esketamine are being used or have been used, off-label or experimentally, alone or in combination with other treatments (including psychotherapy), for procedural sedation, for emergency sedation, to manage agitation or violence in the community or in the emergency room, to treat acute or chronic pain of different etiologies, and to treat alcohol use disorder, generalized anxiety disorder, obsessive compulsive disorder, posttraumatic stress disorder, migraine, status epilepticus, and bronchial asthma (including status asthmaticus); this list is not exhaustive.
Ketamine is also misused. It has been misused as a date rape drug because it can produce rapid-onset sedation. It is a drug of abuse because it is associated with euphoria, dissociative symptoms, and even psychedelic phenomena, the occurrence and severity of which depend on the dose, the route of administration, and the speed of administration.
Long-Term Use and Risk of Uropathy
Recreational use of ketamine is increasing.6 Long-term recreational use and abuse of ketamine have been associated with uropathy,7 as described in the next section of this article. The risk of uropathy is of concern in clinical contexts because the antidepressant and other benefits of ketamine are temporary; for maintained clinical benefits, either ketamine needs to be continued into the long-term as maintenance therapy or some other suitable maintenance therapy requires to be found. The latter may be difficult when treatment-resistance was the reason why ketamine treatment was initiated.
Maintenance treatment with ketamine or esketamine has been described for up to 6 years and beyond with intravenous, intranasal, and oral treatment.8–11 So, a reasonable question is, might long-term maintenance treatment with ketamine, as with long-term abuse of ketamine, be associated with increased risk of uropathy? Or, for that matter, is short-term use of ketamine-associated with increased risk of uropathy? An attempt is made to answer these and other questions in the sections that follow.
Ketamine-Associated Uropathy: Meta-Analysis
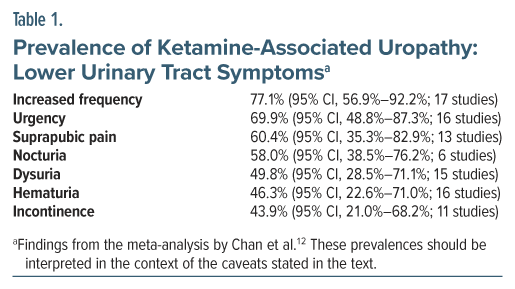
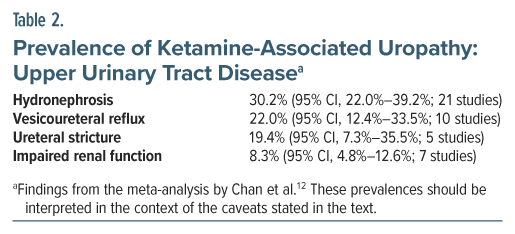
What is the prevalence of lower urinary tract symptoms and other urological disturbances in long-term abusers of ketamine? Chan et al12 attempted to answer this question in a systematic review and meta-analysis of studies on ketamine-associated uropathy. Their search identified 45 studies for qualitative analysis and 37 for quantitative analysis. The pooled sample in the 45 studies comprised 4,890 patients. The mean age of the sample was 25 years. The sample was 72% male. The mean duration of ketamine exposure was 55 months; that is, nearly 5 years.
The study found high rates of both ketamine-associated lower urinary tract symptoms and ketamine-associated upper urinary tract disease (Tables 1 and 2). Although cystitis as a clinical diagnosis was not examined, its presence could be inferred from the symptoms listed.
The findings of this meta-analysis should be interpreted with much caution for several reasons. One, most of the studies that were identified appeared to have originated in a small part of the world, that is, southeast Asia. Two, the authors did not provide the customary table describing the reviewed studies and their samples, but it appeared that most of the studies had been conducted on high dose, high frequency ketamine abusers; so, the findings cannot be generalized to patients who receive ketamine in therapeutic contexts. Three, most of the studies appeared to have been conducted on samples preselected for ketamine-associated uropathy, making high prevalences of individual symptoms and specified urological disorders inevitable; again, the findings cannot be generalized to patients who receive ketamine as a treatment. Four, heterogeneity was very high (I 2 > 90%) in almost all analyses, making it unclear to what population the findings could be generalized. And, five, only crude pooled prevalences were estimated with no comparisons drawn with any control or comparison group.
These concerns are important because the findings of the article almost certainly present an inflated value of risks that, if taken at face value and cited out of context, will provoke apprehension about risks associated with the use of ketamine in therapeutic situations. Regrettably, this meta-analysis was uncritically cited in at least 2 influential reviews.13,14
A reasonable takeaway from this meta-analysis is that ketamine-associated uropathy is a visible problem; there were 37 studies with a pooled sample of 4,314 patients in the quantitative analysis. The meta-analysis, however, did not provide an understanding of the prevalence of the problem in ketamine abusers in the population, or in ketamine-treated patients with depression and other disorders.
Ketamine-Associated Uropathy: Other Studies
It could be challenging to determine the true prevalence of and risk factors associated with ketamine-associated uropathy because of the difficulties involved in identifying and surveying a representative sample of ketamine users and abusers in the population, and because of the difficulties in determining their patterns of use, operationalized as dose per dosing occasion, frequency of dosing, route of administration, duration of use, and total exposure. In this section, 3 examples are provided of studies that used different methods of case ascertainment to examine the prevalence of uropathy in ketamine abusers. Two of the studies were published after the meta-analysis described in the previous section,12 and the third was not included in the meta-analysis.
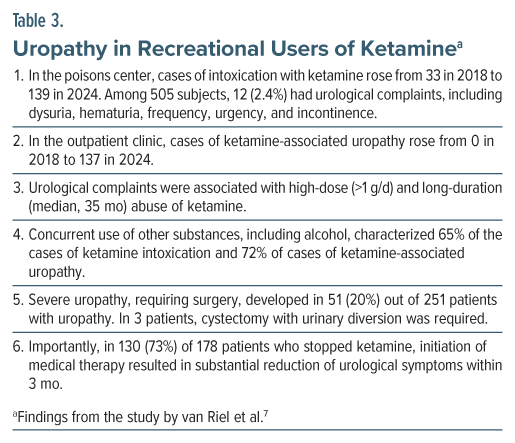
Using 2 different methods of case ascertainment, van Riel et al7 studied the risk of uropathy in recreational users of ketamine. They also examined what dose and what duration of use of ketamine might be associated with uropathy, and what the uropathy outcomes might be.
These authors obtained data on recreational users of ketamine for the years 2018 to 2024, extracted from 2 sources: the Dutch Poisons Information Centre, and the first outpatient clinic for ketamine-induced uropathy in the Netherlands. The median age of the sample was about 25 years, and the sample was approximately two-thirds male.
Important findings from the study are presented in Table 3. In summary, in this retrospective study, uropathy was associated with high-dose (>1 g/d) and long-duration (median, 35 months) abuse of ketamine. In three-quarters (but not all) of patients with ketamine-associated uropathy, stopping ketamine was associated with substantial (but not necessarily complete) reduction of urological symptoms within 3 months. A fifth of patients with ketamine-associated uropathy had severe complaints that required surgical intervention.
As additional information, most subjects who contacted the poisons center used ketamine intranasally, as did all patients in the outpatient clinic; that is, they “snorted” the drug. Data on daily doses were only available from the outpatient clinic, and the minimum self-reported dose associated with uropathy was 1 g/d for 3 months (van Riel, 2025, personal communication).
Using online survey as a method of case ascertainment, Winstock et al15 found that 340 (26.5%) of 1,285 recreational ketamine users had experienced lower urinary tract symptoms during the past 12 months. These symptoms included increased frequency (17.4%), pain in the lower abdomen (11.3%), dysuria (8.1%), urinary incontinence (3.3%), and hematuria (1.5%). There was no comparison group to put these findings in perspective. Among 251 users who provided information, only 51% reported decrease in urinary symptoms after stopping the use of ketamine.
Using pharmacovigilance as a method of case ascertainment, Gandolfo et al16 identified 238 ketamine users in the French National Pharmacovigilance Database for the years 2020–2022. Among these, 20 (8.4%) had urological symptoms.
Ketamine-Associated Urological Symptoms in Clinical Psychiatry
Kerr-Gaffney et al17 described a systematic review of studies that described urological outcomes in adult patients treated with subanesthetic doses of ketamine or its enantiomers, administered by any route, and in the treatment of psychiatric disorders, including substance use disorders. They included all clinical data except case reports.
Of 27 studies that met their search criteria, 24 had been conducted in patients with depressive disorders. Fifteen studies were randomized controlled trials; the remaining 12 studies had no comparator arm. Most studies administered racemic ketamine, and dosing was most commonly intravenous or intranasal. Dosing per occasion ranged from 28 to 100 mg for intranasal administration and from 0.1 to 1.0 mg/kg for intravenous administration. The duration of treatment ranged from 1 day to 243 weeks. Only 13 studies formally assessed adverse events associated with ketamine treatment; the remaining 14 studies merely reported urological adverse events. In these studies, the mean age of patients ranged from 32 to 71 years; the proportion of women ranged from 0% to 91%.
Quantitative analysis was not possible; therefore, only a qualitative review was presented. Important findings from the systematic review are summarized in Table 4. Limitations of the findings of this review, and of the literature, in general, are discussed in the next section.
Limitations of the Literature
Ketamine-associated uropathy has been known since at least 2007.18,19 Despite this, the systematic review by Kerr-Gaffney et al17 could identify only 27 studies that looked for and/or reported urological symptoms in ketamine studies of adults with psychiatric disorders. These authors reported that, surprisingly, many studies of long-term ketamine use did not report the presence or absence of urological symptoms as adverse effects, suggesting that these may not have been considered. When data were available, urological symptoms were not recorded and reported in a manner that could permit pooling in meta-analysis. Clearly, greater awareness is necessary of the urological risks associated with ketamine, especially during long-term maintenance therapy.
As a major limitation, no study in this systematic review17 considered explanations for the urological symptoms identified. Ketamine may have been etiologically responsible, but it is also possible that the symptoms may have been due to comedications, changes in patterns of fluid intake, a Hawthorne effect arising from specific enquiries about urological symptoms, a nocebo effect arising from information provided during the study consenting process, or merely a chance urinary tract infection. These are of especial concern in the 12 studies that were nonblind and that lacked a comparison arm.
Ketamine and Uropathy: The Importance of Pharmacokinetics
A digression on the pharmacokinetics of ketamine is necessary here for a better understanding of how time- and dose-dependent exposure to ketamine and its metabolites may result in cystitis and other urological disorders.
When ketamine is intravenously administered, its bioavailability is 100%. When esketamine is intranasally administered, its bioavailability is 45% to 54%.20–22 When ketamine is orally administered, because of extensive CYP3A4 first pass metabolism in the intestinal lining and liver, its bioavailability is only 17% to 24%. However, with oral dosing, blood levels of the main metabolite norketamine are high, and after correcting for dose, the bioavailability of norketamine is similar to that with intravenous ketamine. This implies that oral ketamine is almost completely bioavailable as the parent drug along with its main metabolite.23 This also implies that, because much higher doses are administered with oral (eg, 150–200 mg) relative to intranasal (eg, 28–84 mg) and intravenous (eg, 30–50 mg) ketamine or esketamine, exposure to norketamine is substantially higher when patients are treated with oral ketamine.
Ketamine is metabolized to norketamine and thence to hydroxynorketamine and dehydroxynorketamine. In minor metabolic pathways, it is converted to hydroxyketamine, phenolketamine, and phenolnorketamine. Most of the metabolites are glucuronidated and excreted in urine.24
In urine, about 2% of ketamine is excreted as the parent drug, about 2% as norketamine, about 16% as dehydronorketamine, and the rest (80%) as glucuronidated conjugates of hydroxylated metabolites.24
The elimination half-life of ketamine is 1.5–5.0 h,25–27 and that of norketamine is 5.3 h25,28; the latter has also been stated to be 12 h, and even 1 day, but the source studies for these higher values could not be traced.
The pharmacokinetics of esketamine are similar to those described for ketamine; that is, ketamine is processed as its S-isomer.20
Ketamine and Uropathy: Effects of Ketamine and Norketamine
The bladder appears to be more sensitive than other organs to toxicity associated with long-term use and especially abuse of ketamine. This suggests a direct effect mediated by the prolonged presence of ketamine and its metabolites in urine in the bladder,13,29 rather than a circulatory effect mediated by ketamine and its metabolites passing through the bladder vasculature. Laboratory studies support this conjecture. Studies conducted on cell lines and tissue cultures in vitro and in animal models in vivo show that direct exposure of bladder epithelial cells to ketamine or norketamine causes barrier damage, inflammation, and cell death at concentrations similar to those found in the urine of people who abuse ketamine.30–32
Although there is substantially more dehydronorketamine in urine than ketamine and norketamine,24 studies have not examined the contribution of dehydronorketamine to ketamine-associated uropathy. Esketamine and its metabolites may be expected to have similar urothelial effects as does ketamine; however, esketamine and its metabolites have not been formally studied in this context, as ketamine has.
Ketamine and Uropathy: Effects on the Bladder
There is a great deal of preclinical and clinical information available about how ketamine and its metabolites may harm the bladder.13,14 A summary is provided below of pathophysiology in the urothelium and in the bladder after high dose, frequent, and long-term exposure to ketamine and norketamine.
The cell organelles involved in the pathophysiological changes include mitochondria and the endoplasmic reticulum; there is oxidative stress. The pathways involved include decreased E-cadherin expression, dysregulated purinergic neurotransmission, Cav1.2 channel blockade, and activation of transforming growth factor-β1. Cellular consequences in the urothelium include apoptosis and urothelial denudation. Tissue changes include increased urothelial permeability and loss of barrier integrity through increased expression of antiproliferative factor and disruption of the glycosaminoglycan layer of the urothelium. There is mast cell infiltration, eosinophilic infiltration, and inflammation of the urothelium. As the end result, changes in the bladder include microvascular damage, urothelial ulceration, and fibrosis.13,14 Other mechanisms have also been suggested.29
These changes result in progressive increase in lower urinary tract symptoms; that is, symptoms such as reduced bladder storage, frequent micturition, urgency, polyuria, dysuria, hematuria, and suprapubic pain. In severe cases, back pressure effects could result in hydronephrosis and renal failure.
Suggestions for Risk Mitigation
Based on what is known from abuse contexts, it is reasonable to assume that, in treatment contexts, the risk of ketamine-associated uropathy increases with higher dose per dosing occasion, higher frequency of use, longer duration of use, and perhaps oral route of administration. Reassuringly, when ketamine is used to treat depression, doses used are far lower and far less frequent than the uropathy-related doses and dosing frequencies reported in recreational use and abuse contexts by authors such as van Riel et al.7 However, this does not rule out the risk of uropathy with lower and less frequent dosing. So, precautions are desirable. This is especially important when the oral route is used for the administration of ketamine because ketamine doses and norketamine levels are highest with oral ketamine.
The simplest precaution is to encourage the patient to be better than well hydrated on the day of the ketamine session, and to void urine frequently. This would dilute the urine as well as reduce exposure of the bladder urothelium to ketamine and its metabolites. This is especially important during and for about an hour after the ketamine session, when ketamine and norketamine are at or around peak levels. As practical suggestions, the patient could drink 1–2 glasses of water before the session, 2 glasses after the session, and several extra glasses across the course of the day. Urine should be voided at least hourly.
Drinking extra water is not expected to reduce the efficacy of ketamine and norketamine in the treatment session. This is because ketamine and norketamine are inactivated by hepatic metabolism, and because urinary excretion of ketamine and norketamine is very small.24
During maintenance therapy, such as when treatment extends across months and longer, and especially in the contexts of oral dosing, use of higher doses, and more frequent dosing, additional precautions are advisable. As examples, fortnightly or monthly urine tests for protein, cells, and blood could alert clinicians to early urothelial damage, if any. Routinely enquiries about the experience of increased urinary frequency, polyuria, urgency, dysuria, and other urinary symptoms are necessary because patients receiving maintenance ketamine, such as for depression or chronic pain, may not recognize the connection between their ketamine treatment and urological symptoms.
Unfortunately, ketamine abusers who do not recognize the warning symptoms may use higher doses of ketamine to manage bladder pain. Eventually, complications can become irreversible and may even necessitate surgical intervention. Early detection is key because if ketamine treatment is immediately stopped, mild urological complaints are usually reversible.
Parting Notes
For further reading about the pathophysiology, clinical features, diagnosis, and management of ketamine-associated cystitis, readers may consult the reviews by Abdelrahman and Belal33 and Hervé et al.14 For additional guidance on the assessment and treatment of ketamine-associated uropathy, readers are referred to the recent French13 and British34 recommendations.
Article Information
Published Online: September 10, 2025. https://doi.org/10.4088/JCP.25f16083
© 2025 Physicians Postgraduate Press, Inc.
To Cite: Andrade C. Ketamine-associated uropathy during therapeutic and nontherapeutic use: prevalence, clinical features, mechanisms, and strategies for risk reduction. J Clin Psychiatry. 2025;86(4):25f16083.
Author Affiliations: Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India; Department of Psychiatry, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, India.
Corresponding Author: Chittaranjan Andrade, MD, Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore 560029, India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India. Please contact Chittaranjan Andrade, MD, at Psychiatrist.com/contact/andrade.
References (34)

- Domino EF, Chodoff P, Corssen G. Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther. 1965;6:279–291. PubMed CrossRef
- Phillips LA, Seruvatu SG, Rika PN, et al. Anaesthesia for the surgeon-anaesthetist in difficult situations: the use of intramuscular 2(O-chlorophenyl)-2 methylamino cyclohexanone HCl (Parke Davis CI-581, Ketamine). Anaesthesia. 1970;25(1):36–45. PubMed CrossRef
- Brown TC, Cole WH, Murray GH. Ketamine: a new anaesthetic agent. Aust N Z J Surg. 1970;39(3):305–310. PubMed CrossRef
- Arditti J, Spadari M, de Haro L, et al. Ketamine - reves et realites. Acta Clin Belg. 2002;57(suppl 1):31–33. PubMed CrossRef
- Andrade C. Ketamine for depression, 3: Does chirality matter? J Clin Psychiatry. 2017;78(6):e674–e677. PubMed CrossRef
- Isba R, Brewster L, Lunn J, et al. Non-prescribed ketamine use is rising in the UK-including among under 16s. BMJ. 2025;390:r1521. PubMed CrossRef
- van Riel AJHP, van der Sanden WMH, de Kort LMO. Increased recreational ketamine use and subsequent outbreak of urological complications in the Netherlands. Clin Toxicol (Phila). 2025;17:1–5.
- Singh B, Pazdernik VK, Kung S, et al. Long-term outcomes in patients with treatment-refractory depression receiving intravenous ketamine and intranasal esketamine: an observational study. J Clin Psychiatry. 2025;86(3):25m15831. PubMed CrossRef
- Zaki N, Chen LN, Lane R, et al. Safety and efficacy with esketamine in treatment-resistant depression: long-term extension study. Int J Neuropsychopharmacol. 2025;28(6):pyaf027. PubMed CrossRef
- Al Shirawi MI, Kennedy SH, Ho KT, et al. Oral ketamine in treatment-resistant depression: a clinical effectiveness case series. J Clin Psychopharmacol. 2017;37(4):464–467. PubMed CrossRef
- Andrade C. Therapeutic compatibility of orally administered racemic ketamine with modafinil, a CYP3A4 enzyme inducer, in treatment-refractory major depressive disorder. Indian J Psychol Med. 2023;45(6):651–653. PubMed CrossRef
- Chan EOT, Chan VWS, Tang TST, et al. Systematic review and meta-analysis of ketamine-associated uropathy. Hong Kong Med J. 2022;28(6):466–474. PubMed CrossRef
- Bourillon A, Cornu JN, Hervé F, et al. Members of the CUROPF; CTMH. Management of ketamine cystitis: national guidelines from the French Association of Urology (CUROPF/CTMH). Fr J Urol. 2024;34(14):102754. PubMed
- Hervé F, Aronsson P, Ochoa DC, et al. Can we better understand, diagnose, and treat ketamine-induced uropathy, and can it be reversed? ICI-RS 2024. Neurourol Urodyn. 2025;44(3):548–557. PubMed
- Winstock AR, Mitcheson L, Gillatt DA, et al. The prevalence and natural history of urinary symptoms among recreational ketamine users. BJU Int. 2012;110(11):1762–1766. PubMed CrossRef
- Gandolfo P, Soeiro T, Jouve É, et al. Patterns of ketamine use among people with substance use disorder in France: multisource analysis of the data from the French Addictovigilance Network. Fundam Clin Pharmacol. 2024;38(5):978–987. PubMed CrossRef
- Kerr-Gaffney J, Tröger A, Caulfield A, et al. Urological symptoms following ketamine treatment for psychiatric disorders: a systematic review. J Psychopharmacol. 2025:2698811251350267. PubMed CrossRef
- Shahani R, Streutker C, Dickson B, et al. Ketamine-associated ulcerative cystitis: a new clinical entity. Urology. 2007;69(5):810–812. PubMed CrossRef
- Chu PS, Kwok SC, Lam KM, et al. “Street ketamine”-associated bladder dysfunction: a report of ten cases. Hong Kong Med J. 2007;13(4):311–313. PubMed
- Bahr R, Lopez A, Rey JA. Intranasal esketamine (SpravatoTM) for use in treatment-resistant depression in conjunction with an oral antidepressant. P T. 2019;44(6):340–375. PubMed
- An D, Wei C, Wang J, et al. Intranasal ketamine for depression in adults: a systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials. Front Psychol. 2021;12:648691. PubMed CrossRef
- Perez-Ruixo C, Rossenu S, Zannikos P, et al. Population pharmacokinetics of esketamine nasal spray and its metabolite noresketamine in healthy subjects and patients with treatment-resistant depression. Clin Pharmacokinet. 2021;60(4):501–516. PubMed CrossRef
- Andrade C. Oral ketamine for depression, 1: pharmacologic considerations and clinical evidence. J Clin Psychiatry. 2019;80(2):19f12820. PubMed CrossRef
- Dinis-Oliveira RJ. Metabolism and metabolomics of ketamine: a toxicological approach. Forensic Sci Res. 2017;2(1):2–10. PubMed CrossRef
- Hijazi Y, Bodonian C, Bolon M, et al. Pharmacokinetics and haemodynamics of ketamine in intensive care patients with brain or spinal cord injury. Br J Anaesth. 2003;90(2):155–160. PubMed CrossRef
- Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–660. PubMed CrossRef
- Schep LJ, Slaughter RJ, Watts M, et al. The clinical toxicology of ketamine. Clin Toxicol. 2023;61(6):415–428. PubMed CrossRef
- Kubota R, Komiyama T, Miwa Y, et al. Pharmacokinetics of ketamine and norketamine after oral administration of a liquid formulation of ketamine. J Curr Surg. 2013;3(2):82–86.
- Anderson DJ, Zhou J, Cao D, et al. Ketamine-induced cystitis: a comprehensive review of the urologic effects of this psychoactive drug. Health Psychol Res. 2022;10(3):38247. PubMed CrossRef
- Shen CH, Wang ST, Lee YR, et al. Biological effect of ketamine in urothelial cell lines and global gene expression analysis in the bladders of ketamine-injected mice. Mol Med Rep. 2015;11(2):887–895. PubMed CrossRef
- Baker SC, Shabir S, Georgopoulos NT, et al. Ketamine-induced apoptosis in normal human urothelial cells: a direct, N-methyl-d-aspartate receptor independent pathway characterized by mitochondrial stress. Am J Pathol. 2016;186(5):1267–1277. PubMed CrossRef
- Lin JW, Lin YC, Liu JM, et al. Norketamine, the main metabolite of ketamine, induces mitochondria-dependent and ER stress-triggered apoptotic death in urothelial cells via a Ca2+-regulated ERK1/2-activating pathway. Int J Mol Sci. 2022;23(9):4666. PubMed CrossRef
- Abdelrahman A, Belal M. Rare but relevant: Ketamine-induced cystitis - an in-depth review for addiction medicine. Addiction. 2025;120(8):1689–1693. PubMed CrossRef
- Belal M, Downey A, Doherty R, et al. British Association of Urological Surgeons Consensus statements on the management of ketamine uropathy. BJU Int. 2024;134(2):148–154. PubMed CrossRef
This PDF is free for all visitors!