Minocycline Augmentation of Pharmacotherapy in Obsessive-Compulsive Disorder: An Open-Label Trial
To the Editor: Most obsessive-compulsive disorder (OCD) patients treated with serotonin reuptake inhibitors (SRIs) show only partial reduction of symptoms.1 Data suggest that OCD may be caused in part by glutamatergic dysfunction in orbitofrontal/basal ganglia brain circuits,2,3 and prior trials of glutamate modulators (eg, riluzole, memantine) given as augmentation to SRIs suggest that from 30% to 54% of OCD patients respond.4-8
We conducted a 12-week, prospective, open-label study to assess whether SRI augmentation with minocycline, a tetracycline derivative with putative glutamate-modulating activity (ie, enhancing glial glutamate transport)9 in addition to its antibiotic properties, would improve OCD symptoms.
Advantages of minocycline include low cost (riluzole is expensive) and US Food and Drug Administration (FDA) approval in adults and children > 12 years (memantine is FDA approved only in adults). Minocycline is the most widely prescribed antibiotic for chronic acne because its antibiotic resistance is lower than those of other tetracyclines and antimicrobials.10 It has an excellent side effect profile, even chronically: one study11 showed that minocycline taken for 2 years was well tolerated, with no serious adverse effects.
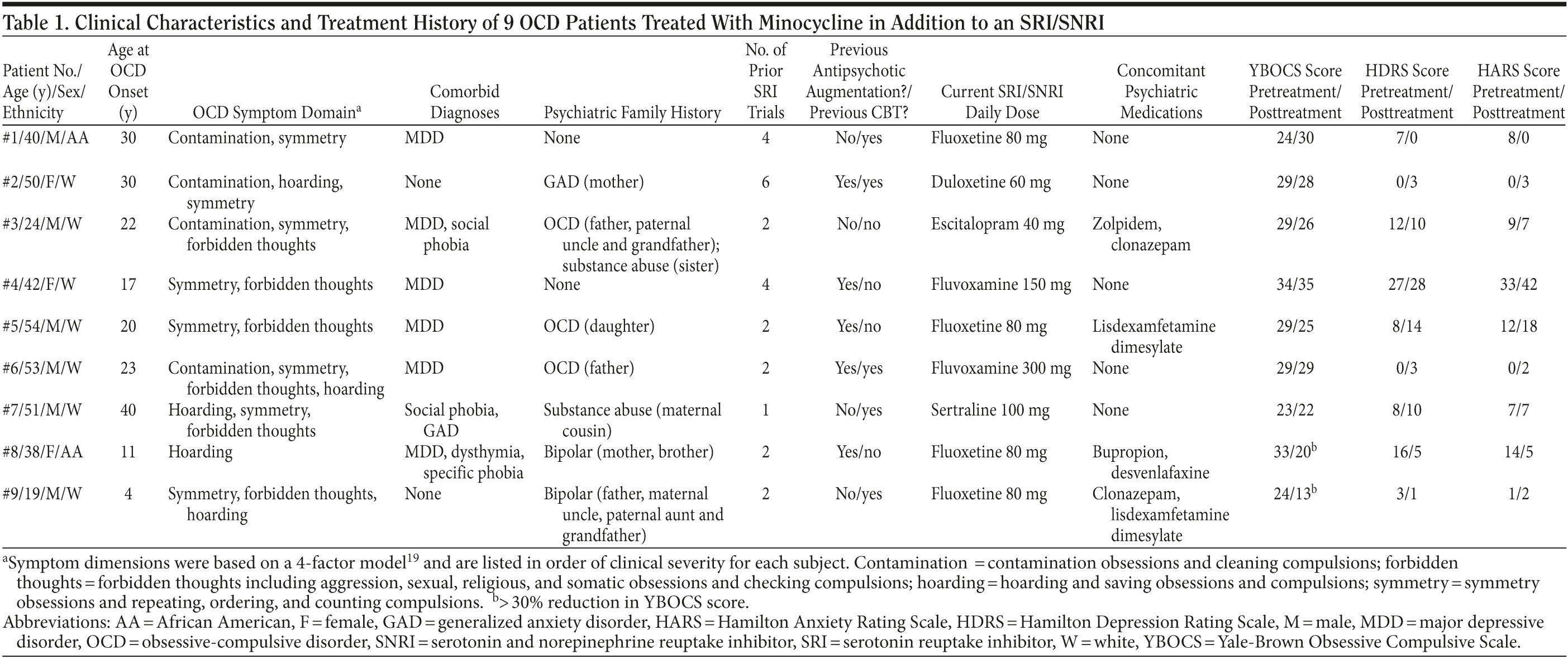
Method. Adult outpatients (N = 9) aged 18 to 65 years who met DSM-IV criteria for OCD and had a Yale-Brown Obsessive Compulsive Scale (YBOCS)12 score of ≥ 16 despite a therapeutic SRI dose were recruited from the community between July 2008 and July 2009 and gave informed consent after the study procedures were fully explained. Institutional review board approval was obtained for the study. Subjects’ SRI dose was stable for at least 12 weeks (and concomitant psychotropic medications, for at least 4 weeks) prior to study entry. Subjects were excluded for current cognitive-behavioral therapy, comorbid psychiatric or medical conditions that made participation unsafe, or use of medications that reduced the bioavailability of minocycline. Patients were assessed by an independent rater who administered the YBOCS (primary outcome measure), Hamilton Depression Rating Scale (HDRS, 17-item),13 and Hamilton Anxiety Rating Scale (HARS)14 every 2 weeks. Response was defined as at least a 30% reduction on the YBOCS.15
Subjects received minocycline at 50 mg bid for 3 days to ensure no allergic reaction, then at the FDA-approved adult dosing of 100 mg bid for 12 weeks in addition to their SRI. This dosing is expected to produce brain minocycline concentrations in the range that antagonize glutamate effects on neurons.16,17 All subjects completed the study, supporting the fact that minocycline was well tolerated. Outcome was analyzed using mixed-effects regression to model symptoms as a function of time.18
Results. Patient clinical characteristics are shown in Table 1. OCD severity was moderate: mean (SD) YBOCS score at baseline was 28.2 (3.9), and illness duration was 18.2 (10.4) years. Subjects were treatment-resistant: the mean number of prior SRI trials was 2.8 (1.6), 56% (5/9) had failed at least 1 adequate trial of antipsychotic augmentation, and 56% had failed an adequate trial of cognitive-behavioral therapy. They had a range of OCD symptoms; 1 subject had hoarding as the primary symptom domain.
As a group, patients showed no significant differences in YBOCS, HDRS, or HARS rate of improvement over time (mixed-effects regression: YBOCS, z = −1.14, P = .25; HDRS, z = 0.60, P = .55; HARS, z = 0.12, P = .90). However, 2 of 9 patients (22%) met and exceeded treatment response criteria (40% and 46% YBOCS reductions). Both of these patients reported early onset of their OCD symptoms. One had primary hoarding, and 1 no longer met criteria for OCD at study end. Both chose to continue minocycline treatment after study end.
These data suggest that minocycline augmentation of SRI pharmacotherapy may not improve OCD symptoms in all adult OCD patients, but may improve symptoms in those with early-onset OCD and those with primary hoarding. The robust response of 2 of 9 patients in this study coupled with the response of 2 other subjects (aged 16 and 17 years) with early-onset OCD in an identical parallel study of minocycline in adolescents (M.R., unpublished data, 2008) suggests that minocycline warrants further study. Early-onset OCD differs from later-onset OCD in phenomenology, genetic risk, and SRI response.20 Future studies should target early-onset OCD and focus on minocycline’s mechanism of action.21
References
1. Pigott TA, Seay SM. A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J Clin Psychiatry. 1999;60(2):101-106. PubMed
2. Pittenger C, Krystal JH, Coric V. Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. NeuroRx. 2006;3(1):69-81. PubMed doi:10.1016/j.nurx.2005.12.006
3. Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28(2):343-347. PubMed doi:10.1016/S0896-6273(00)00113-6
4. Coric V, Taskiran S, Pittenger C, et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry. 2005;58(5):424-428. PubMed doi:10.1016/j.biopsych.2005.04.043
5. Aboujaoude E, Barry JJ, Gamel N. Memantine augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. J Clin Psychopharmacol. 2009;29(1):51-55. PubMed doi:10.1097/JCP.0b013e318192e9a4
6. Lafleur DL, Pittenger C, Kelmendi B, et al. N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl). 2006;184(2):254-256. PubMed doi:10.1007/s00213-005-0246-6
7. Stewart SE, Jenike EA, Hezel DM, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol. 2010;30(1):34-39. PubMed doi:10.1097/JCP.0b013e3181c856de
8. Feusner JD, Kerwin L, Saxena S, et al. Differential efficacy of memantine for obsessive-compulsive disorder vs generalized anxiety disorder: an open-label trial. Psychopharmacol Bull. 2009;42(1):81-93. PubMed
9. Darman J, Backovic S, Dike S, et al. Viral-induced spinal motor neuron death is non-cell-autonomous and involves glutamate excitotoxicity. J Neurosci. 2004;24(34):7566-7575. PubMed doi:10.1523/JNEUROSCI.2002-04.2004
10. Seukeran DC, Eady EA, Cunliffe WJ. Benefit-risk assessment of acne therapies. Lancet. 1997;349(9060):1251-1252. PubMed doi:10.1016/S0140-6736(05)62445-2
11. Bonelli RM, Hödl AK, Hofmann P, et al. Neuroprotection in Huntington’s disease: a 2-year study on minocycline. Int Clin Psychopharmacol. 2004;19(6):337-342. PubMed doi:10.1097/00004850-200411000-00004
12. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011. PubMed
13. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
14. Hamilton M. The assessment of anxiety states by rating. Br Med J Psychol. 1959;32:50-55.
15. Tolin DF, Abramowitz JS, Diefenbach GJ. Defining response in clinical trials for obsessive-compulsive disorder: a signal detection analysis of the Yale-Brown Obsessive Compulsive Scale. J Clin Psychiatry. 2005;66(12):1549-1557. PubMed doi:10.4088/JCP.v66n1209
16. Fagan SC, Edwards DJ, Borlongan CV, et al. Optimal delivery of minocycline to the brain: implication for human studies of acute neuroprotection. Exp Neurol. 2004;186(2):248-251. PubMed doi:10.1016/j.expneurol.2003.12.006
17. Baptiste DC, Hartwick AT, Jollimore CA, et al. An investigation of the neuroprotective effects of tetracycline derivatives in experimental models of retinal cell death. Mol Pharmacol. 2004;66(5):1113-1122. PubMed doi:10.1124/mol.104.001081
18. Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry. 1993;50(9):739-750. PubMed
19. Bloch MH, Landeros-Weisenberger A, Rosario MC, et al. Meta-analysis of the symptom structure of obsessive-compulsive disorder. Am J Psychiatry. 2008;165(12):1532-1542. PubMed doi:10.1176/appi.ajp.2008.08020320
20. Rosario-Campos MC, Leckman JF, Mercadante MT, et al. Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry. 2001;158(11):1899-1903. PubMed doi:10.1176/appi.ajp.158.11.1899
21. Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-d-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166(12):7527-7533. PubMed
Author affiliations: New York State Psychiatric Institute (Drs Rodriguez, Bender, Marcus, Rynn, and Simpson) and Department of Psychiatry, College of Physicians and Surgeons, Columbia University (Drs Rodriguez, Rynn, and Simpson), New York, New York; and Neuropharm Ltd, Surrey, United Kingdom (Dr Snape). Potential conflicts of interest: Dr Snape is a stock shareholder in, director of, and legal officer of Neuropharm. Dr Rynn receives research support from Boehringer Ingelheim, Wyeth, and Neuropharm; is a consultant for Wyeth; and has received royalties from American Psychiatric Publishing Inc. Dr Simpson has been a consultant or scientific advisor for Jazz (September 2007-September 2008) and Pfizer (September 2009), receives medication at no cost for a National Institute of Mental Health-funded study from Janssen, received an unrestricted research grant from Neuropharm to explore novel medications for OCD, and is a stock shareholder in Pfizer. Drs Rodriguez, Bender, and Marcus report no additional financial or other relationships relevant to the subject of this letter. Funding/support: This investigation was supported by the NARSAD Joan Grandlund Investigator Award (to Dr Rodriguez) and by Neuropharm Ltd (to Drs Rynn and Simpson). Previous presentation: Presented at the 49th Annual Meeting of NCDEU; June 30, 2009; Hollywood, Florida, and the 48th Annual Meeting of the American College of Neuropsychopharmacology; December 8, 2009; Hollywood, Florida. Acknowledgment: The authors acknowledge Andrew Schmidt, PhD, for data management and Kim Glazier, MA, for data analysis. Dr Schmidt and Ms Glazier report no financial or other relationship relevant to the subject of this letter. Trial registration: www.clinicaltrials.gov Identifier: NCT00728923
doi:10.4088/JCP.09l05805blu
© Copyright 2010 Physicians Postgraduate Press, Inc.