Background: Delirium is common and dangerous, yet underdetected and undertreated. Current screening questionnaires are subjective and ineffectively implemented in busy hospital workflows. Electroencephalography (EEG) can objectively detect the diffuse slowing characteristic of delirium, but it is not suitable for high-throughput screening due to size, cost, and the expertise required for lead placement and interpretation. This study hypothesized that an efficient and reliable point-of-care EEG device for high-throughput screening could be developed.
Methods: This prospective study, which measured bispectral EEG (BSEEG) from elderly inpatients to assess their outcomes, was conducted at the University of Iowa Hospitals and Clinics from January 2016 to October 2017. A BSEEG score was defined based on the distribution of 2,938 EEG recordings from the 428 subjects who were assessed for delirium; primary outcomes measured were hospital length of stay, discharge disposition, and mortality.
Results: A total of 274 patients had BSEEG score data available for analysis. Delirium and BSEEG score had a significant association (P < .001). Higher BSEEG scores were significantly correlated with length of stay (P < .001 unadjusted, P = .001 adjusted for age, sex, and Charlson Comorbidity Index [CCI] score) as well as with discharge not to home (P < .01). Hazard ratio for survival controlling for age, sex, CCI score, and delirium status was 1.35 (95% CI,1.04 to 1.76; P = .025).
Conclusions: In BSEEG, an efficient and reliable device that provides an objective measurement of delirium status was developed. The BSEEG score is significantly associated with pertinent clinical outcomes of mortality, hospital length of stay, and discharge disposition. The BSEEG score better predicts mortality than does clinical delirium status. This study identified a previously unrecognized subpopulation of patients without clinical features of delirium who are at increased mortality risk.
Identification of Patients With High Mortality Risk and Prediction of Outcomes in Delirium by Bispectral EEG
ABSTRACT
Background: Delirium is common and dangerous, yet underdetected and undertreated. Current screening questionnaires are subjective and ineffectively implemented in busy hospital workflows. Electroencephalography (EEG) can objectively detect the diffuse slowing characteristic of delirium, but it is not suitable for high-throughput screening due to size, cost, and the expertise required for lead placement and interpretation. This study hypothesized that an efficient and reliable point-of-care EEG device for high-throughput screening could be developed.
Methods: This prospective study, which measured bispectral EEG (BSEEG) from elderly inpatients to assess their outcomes, was conducted at the University of Iowa Hospitals and Clinics from January 2016 to October 2017. A BSEEG score was defined based on the distribution of 2,938 EEG recordings from the 428 subjects who were assessed for delirium; primary outcomes measured were hospital length of stay, discharge disposition, and mortality.
Results: A total of 274 patients had BSEEG score data available for analysis. Delirium and BSEEG score had a significant association (P < .001). Higher BSEEG scores were significantly correlated with length of stay (P < .001 unadjusted, P = .001 adjusted for age, sex, and Charlson Comorbidity Index [CCI] score) as well as with discharge not to home (P < .01). Hazard ratio for survival controlling for age, sex, CCI score, and delirium status was 1.35 (95% CI,1.04 to 1.76; P = .025).
Conclusions: In BSEEG, an efficient and reliable device that provides an objective measurement of delirium status was developed. The BSEEG score is significantly associated with pertinent clinical outcomes of mortality, hospital length of stay, and discharge disposition. The BSEEG score better predicts mortality than does clinical delirium status. This study identified a previously unrecognized subpopulation of patients without clinical features of delirium who are at increased mortality risk.
J Clin Psychiatry 2019;80(5):19m12749
To cite: Shinozaki G, Bormann NL, Chan AC, et al. Identification of patients with high mortality risk and prediction of outcomes in delirium by bispectral EEG. J Clin Psychiatry. 2019;80(5):19m12749.
To share: https://doi.org/10.4088/JCP.19m12749
© Copyright 2019 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of Iowa Carver College of Medicine, Iowa City, Iowa
bDepartment of Neurosurgery, University of Iowa Carver College of Medicine, Iowa City, Iowa
cIowa Neuroscience Institute, University of Iowa, Iowa City, Iowa
dInterdisciplinary Graduate Program for Neuroscience, University of Iowa, Iowa City, Iowa
eDepartment of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa
fDepartment of Family Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa
gDepartment of Neurology, University of Iowa Carver College of Medicine, Iowa City, Iowa
hDepartment of Orthopedic Surgery, University of Iowa Carver College of Medicine, Iowa City, Iowa
iCollege of Public Health, University of Iowa, Iowa City, Iowa
jDepartment of Emergency Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa
kDepartment of Surgery, University of Iowa Carver College of Medicine, Iowa City, Iowa
*Corresponding author: Gen Shinozaki, MD, Department of Psychiatry, University of Iowa Carver College of Medicine, University of Iowa Hospitals and Clinics, 25 S Grand Ave, Medical Laboratories B002, Iowa City, IA, 52246 ([email protected]).
Delirium is common and dangerous in hospitalized elderly patients.1-3 It is also underdiagnosed and therefore undertreated.4-6 It is estimated there are at least 2-3 million cases of delirium per year in the United States alone.1,7 Delirium is associated with increased mortality,8-11 rate of complications, hospital length of stay (LOS), and institutionalization after discharge.1-3 Patients with delirium also have a high risk of long-term cognitive impairment.12 Delirium can add over $60,000 in health care costs per patient per year, or more than $150 billion in health care costs in the United States per year.3 Early identification of risk and immediate intervention are keys to reducing poor outcomes associated with delirium.13-16
Extensive efforts have been made to identify efficient methods for screening for delirium, including the Confusion Assessment Method (CAM),16,17 the CAM for the Intensive Care Unit (CAM-ICU),18,19 and the Delirium Rating Scale-Revised-98 (DRS-R-98).20 Despite these tools, delirium remains underdiagnosed.4,6 Although studies3,16,21 show that 30%-40% of delirium cases can be prevented using low-technology interventions, these are often challenging to apply because they need to be administered frequently by busy hospital personnel. The questionnaires’ subjective nature makes monitoring change over time difficult, particularly when administered by different evaluators, as is often the case. As a result, these tools have been reported to have suboptimal sensitivity (approximately 38%-47%) in busy clinical environments such as the ICU.22,23 These considerations highlight the critical need for more objective detection of delirium.
Electroencephalography (EEG) has been a useful modality in detecting brain waves characteristic of delirium.24,25 Several limitations, however, prevent its routine use for screening large volumes of inpatients. First, a standard 20-lead EEG machine is typically large and expensive; thus, patient access to such machines is limited. Second, an experienced technician must spend significant time correctly positioning the 20 leads and obtaining the tracings, and a specialized neurologist must interpret the data. These requirements often result in significant delays in initiating appropriate treatment.
Objective
Electrophysiologic signals characteristic of delirium are often reported as “diffuse slowing.” The term implies that brain waves across most channels are of a reduced frequency. The emergence of low-frequency waves indicates potential occurrence of delirium. That all channels are able to detect the same reduction in frequency suggests only a small number of channels would be sufficient to obtain the relevant data. Bispectral EEG (BSEEG) utilizes only 2 channels and, when combined with appropriate signal analysis algorithms, may be easily applied by non-experts, thus greatly facilitating its use as a screening tool. Due to the objective nature of BSEEG, interrater reliability does not affect it, and it can be more strongly correlated with patient outcomes. Previously, our team showed that BSEEG signals have significant correlation with delirium in general hospital26 as well as in emergency department27 settings. Thus, we conducted an expanded study to determine whether BSEEG signals are associated with delirium and whether they predict patient outcomes, including mortality.
METHODS
Study Design and Oversight
This prospective cohort study sought to determine an EEG signal feature associated with delirium. Initially, we aimed to establish the association between BSEEG signals and clinical delirium. Also, to test the usefulness of this approach on patient care, the association of BSEEG scores from this algorithm and patient outcomes was investigated. This study protocol was reviewed and approved by the University of Iowa Institutional Review Board prior to enrollment and data collection. Written informed consent was received from participants prior to their inclusion in the study.
Setting and Participants
For the purpose of this study, we recruited patients 18-99 years old from the University of Iowa Hospitals and Clinics (UIHC) from January 2016 to October 2017. The study team screened and reviewed potential subjects admitted to UIHC. Eligible patients were assessed for capacity to provide consent and participate. If the subject was found not to have capacity to provide consent, consent was obtained from his or her legally authorized representative. If the subject’s capacity to provide consent was intact, consent was obtained from the subject directly. Subjects aged 18-99 years were included. Exclusion criteria were patients with droplet or contact precautions or whose goals of care were comfort measures only. All participants or their legally authorized representative provided written informed consent. Given our aim to investigate the potential role of brain wave signals associated with delirium and patient outcomes, our analysis focused on individuals aged 55 years or older, who are more vulnerable to delirium.

- Diagnosing delirium is difficult in busy clinical settings, and delirium remains underdiagnosed and undertreated, leading to poor patient outcomes, including mortality.
- This study aimed to investigate whether bispectral electroencephalography (BSEEG) can detect delirium and predict patient outcomes, including mortality.
- BSEEG score was significantly associated with clinical presence of delirium as well as patient outcomes, including hospital length of stay, discharge disposition, and mortality.
Variables and Data Sources
Questionnaire instruments and delirium definition. Baseline medical and surgical history and demographic information were obtained from medical records and patient interviews. For measurement of clinical symptoms of delirium, the CAM-ICU,18,19 the DRS-R-98,20 and Delirium Observation Screening Scale (DOSS)28,29 were used. For the assessment of baseline cognitive function, the Montreal Cognitive Assessment (MoCA)30 was used. The CAM-ICU and DRS-R-98 were administered to each subject twice daily unless the subject declined an instance of assessment. DOSS score was tabulated by clinical nursing staff during their routine care and was obtained from review of the medical record. Delirium was defined based on any questionnaire screening positive (ie, CAM-ICU positive, DRS-R-98 score ≥ 19, DOSS score ≥ 3) or clinical documentation of altered mental status or confusion consistent with delirium from the medical record.31 Each case was reviewed by a weekly research meeting led by a board-certified consultation-liaison psychiatrist (G.S.).
BSEEG data collection. A hand-held, 2-channel EEG device (CMS2100, CONTEC, Qinhuangdao, Hebei, China) was used for brain wave recording. BSEEG data were collected twice daily unless the subject declined an instance of assessment. The trained research assistant cleaned the patient’s forehead with an alcohol swab and placed 1 electrode on the center of forehead as a ground, 2 electrodes on the left and right sides of the forehead (Fp), and 2 electrodes on both sides of the earlobe as references to obtain BSEEG signals for 10 minutes. The 10-minute duration was chosen a priori as an amount of time that allowed for collection of adequate EEG data without sacrificing efficiency and throughput, which are essential features of a screening test. The recording was obtained while the patient was at his or her highest level of consciousness, as we roused the patient so that they were as awake and alert as their clinical status allowed. Patients were instructed to keep their eyes closed and jaw relaxed and to remain quiet and still as much as possible. The obtained BSEEG data were converted into spectral density plots, and the signal-processing algorithm was used to produce a BSEEG score.
Spectral density analysis and BSEEG score. Raw EEG signal from each channel was subjected to power spectral density analysis to determine relative presence of “high” and “low” frequency components. Through an iterative approach, a score reflecting the relative presence of high- and low-frequency activity was developed. From 2,938 recordings of EEG scores from all 428 study participants, a mean value and standard deviation (SD) were calculated (Supplementary Figure 1). The BSEEG score is defined as the number of SDs from the study population mean.
Outcome measures. We measured 3 patient outcomes as follows: (1) hospital LOS; (2) discharge not to home, which included death during hospitalization; and (3) mortality at the time of study conclusion. LOS, discharge outcome, and mortality status data were obtained through each subject’s hospital record. Mortality was also assessed by a follow-up phone call interview and obituary record.
Statistical Methods and Analysis
Regression analyses were used to illustrate how the proposed BSEEG score is associated with clinical delirium and patient outcomes such as hospital LOS, discharge not to home, and mortality. Specifically, logistic regression was conducted by treating delirium and discharge not to home as binary response variables, while linear regression was used to evaluate the relationship between hospital LOS and BSEEG score. In addition, the hazard ratio32 for mortality was computed through Cox proportional hazards regression analyses. Age, sex, and severity of illness (as quantified with the Charlson Comorbidity Index [CCI]33) were controlled in regression analyses. The association between mortality and BSEEG scores was further illustrated by comparing 2 nonparametric survival functions for BSEEG-positive and BSEEG-negative groups. The R package survminer (version 0.3.1) was utilized to determine a cut point that maximizes the difference of mortality between 2 groups. The survival function is a series of the Kaplan-Meier estimators obtained from the number of deaths and the total number of individuals at risk at the time. The log rank test was conducted to determine whether the 2 survival functions differ. Two-sided P values of .05 or less were considered to indicate statistical significance. All analyses were performed with R software, version 3.4.3.
RESULTS
Participants, Descriptive Data, and Outcome Data
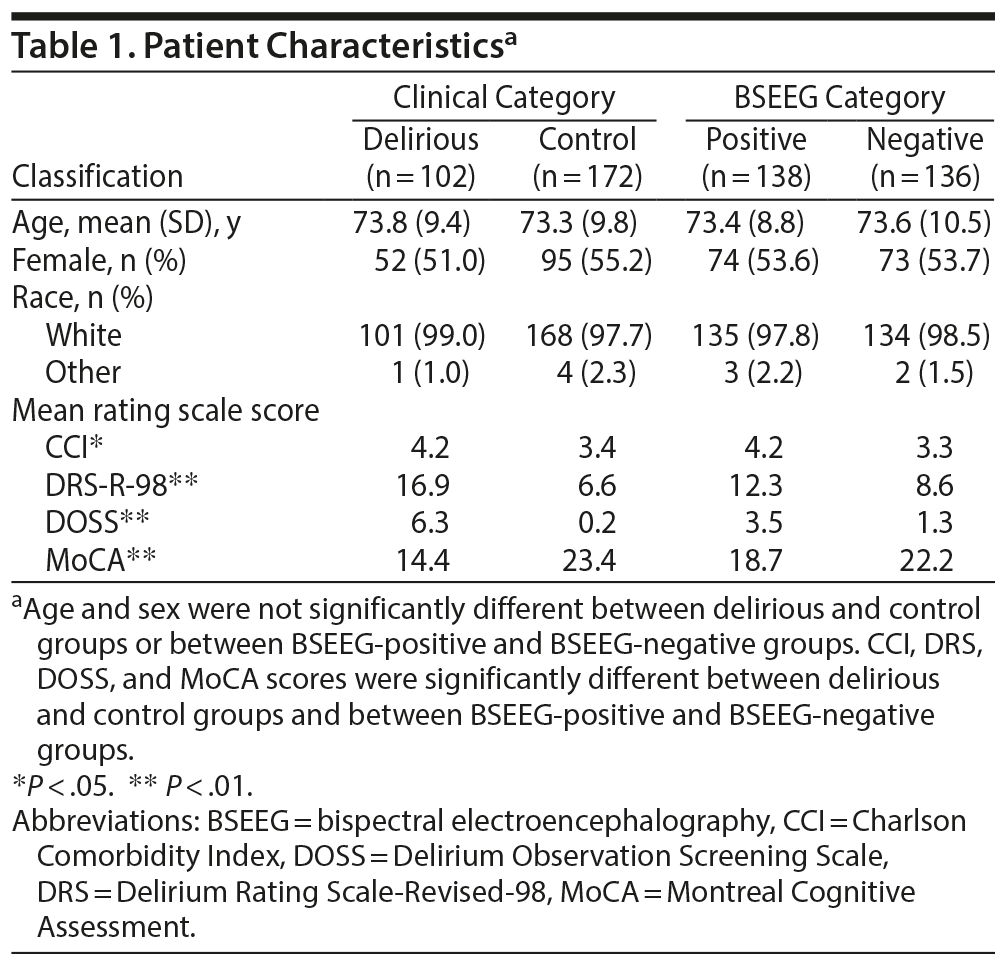
From January 30, 2016, to October 30, 2017, 820 patients were approached and a total of 428 patients were enrolled in the study. We decided to focus on patients aged 55 years or above, as older age is associated with increased susceptibility to delirium. Overall, 337 of 428 patients were aged 55 years or older, and 274 of 337 had BSEEG scores available for analysis. In some cases, BSEEG scores could not be calculated due to poor signal quality of raw EEG data. In the group aged 55 years or older, 37.2% of patients were categorized as delirious per screening by questionnaire such as the CAM-ICU, DRS-R-98, or DOSS or clinical documentation. The study population was also independently divided into 2 groups—BSEEG-positive, with higher BSEEG scores indicative of more low-frequency components in their brain waves, and BSEEG-negative, with lower BSEEG scores indicative of fewer low-frequency components in their brain waves—based on a threshold to differentiate patient outcomes as described in the following section (Supplementary Figure 2). Otherwise, these cohorts were balanced with respect to overall baseline characteristics (Table 1).
Main Results
Association between BSEEG score and clinical delirium. Data from 274 subjects were analyzed to establish association between BSEEG score and clinical delirium. Logistic regression showed significant association between delirium category and BSEEG score (P = 6.39 ×— 10−6, unadjusted; P = 1.22 ×— 10−5, adjusted for age, sex, and CCI score).
BSEEG score and patient outcomes. To test the usefulness of the BSEEG score in predicting patient outcomes, we used outcome data available from 274 subjects who were aged 55 years or older to investigate the association of BSEEG scores obtained at the time of study enrollment with patient outcomes commonly affected by delirium. Specifically, we assessed hospital LOS, discharge disposition, and mortality.
First, LOS and BSEEG scores were significantly associated (P = .00099, unadjusted; P = .0014, adjusted for age, sex, and CCI score). A higher BSEEG score coincides with an increase in a patient’s LOS.
Second, we compared the discharge outcome and BSEEG score. When BSEEG was compared between those who were discharged to their home and those discharged not to home, including death during hospitalization, a higher BSEEG score was significantly associated with discharge not to home (P = .0038, unadjusted; P = .0090, adjusted for age, sex, and CCI score).
Third, when we analyzed subject mortality controlling for age, sex, and CCI score, the hazard ratio32 based on 1 SD change in BSEEG score was 1.44 (95% CI, 1.12 to 1.84; P = .004). Even after control for clinical delirium status in addition to age, sex, and CCI, the hazard ratio based on BSEEG score remained significant at 1.35 (95% CI, 1.04 to 1.76; P = .025).
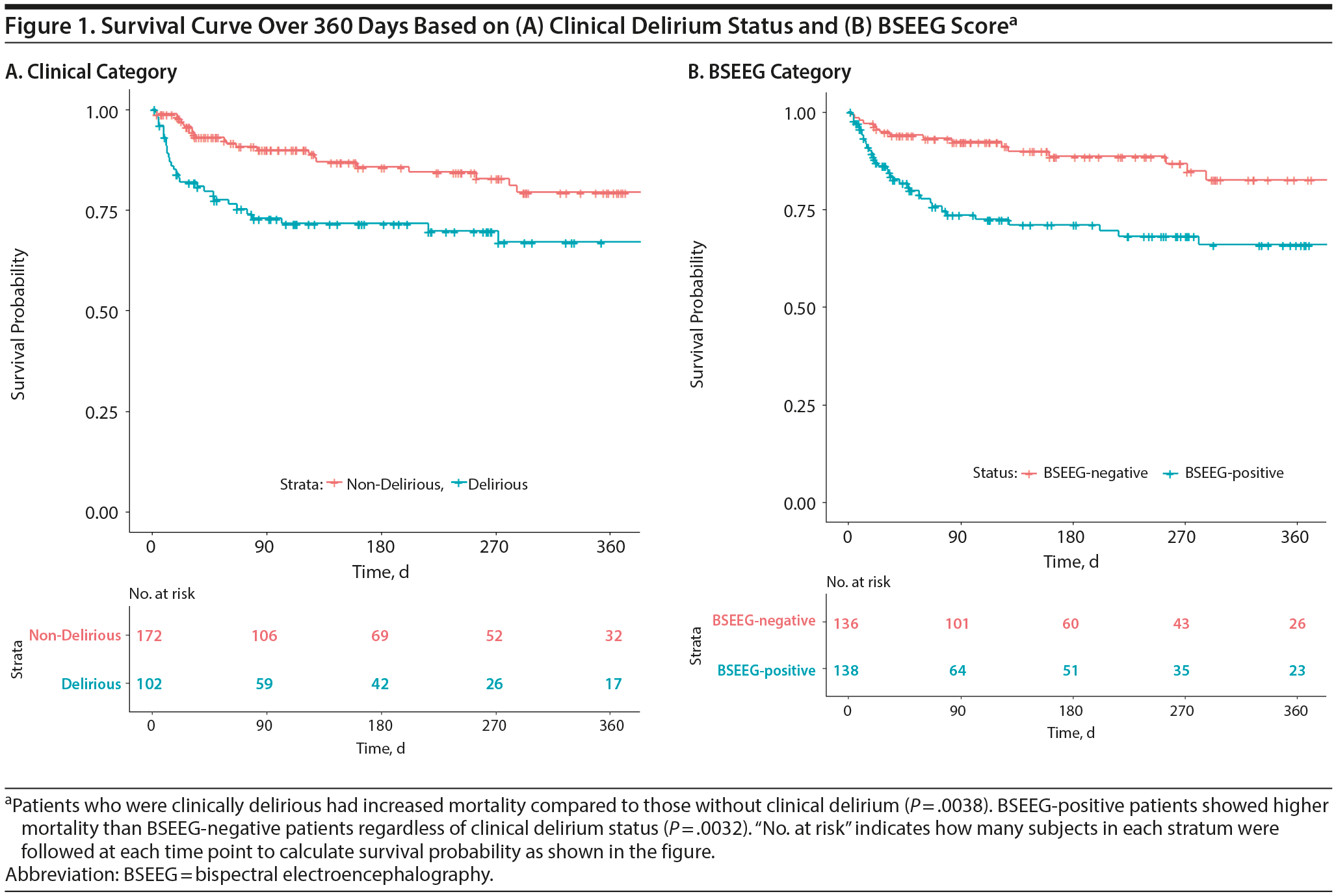
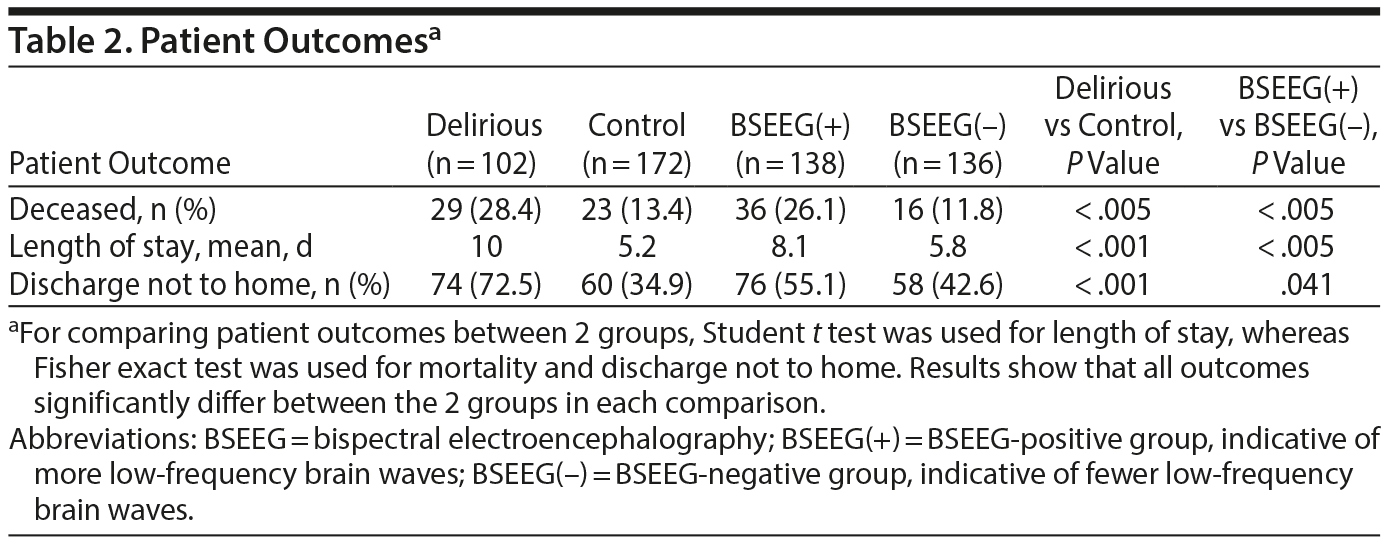
Besides mathematical association, we wanted to determine if the BSEEG could be useful as a measure to assess risk for poor patient outcomes. We divided the study population into a BSEEG-positive group and a BSEEG-negative group, as mentioned previously. Then, we assessed if there was also a correlation between groups based on BSEEG scores and all-cause mortality at the end of our study period in patients in our dataset, because association between delirium and mortality has been shown previously in the literature.8,9 We first assessed overall survival rates among our study participants to confirm that our clinical categorization of delirium is valid enough to replicate well-established association between delirium and higher mortality. Our result showed differences in mortality between those with and without clinical delirium (P = .0038) (Figure 1). Second, we tested a group difference based on a BSEEG cutoff score and confirmed that the BSEEG-positive group showed worse survival compared to the BSEEG-negative group (P = .0032) (Figure 1). This categorization also differentiated other outcomes, including LOS and discharge disposition, significantly (Table 2).
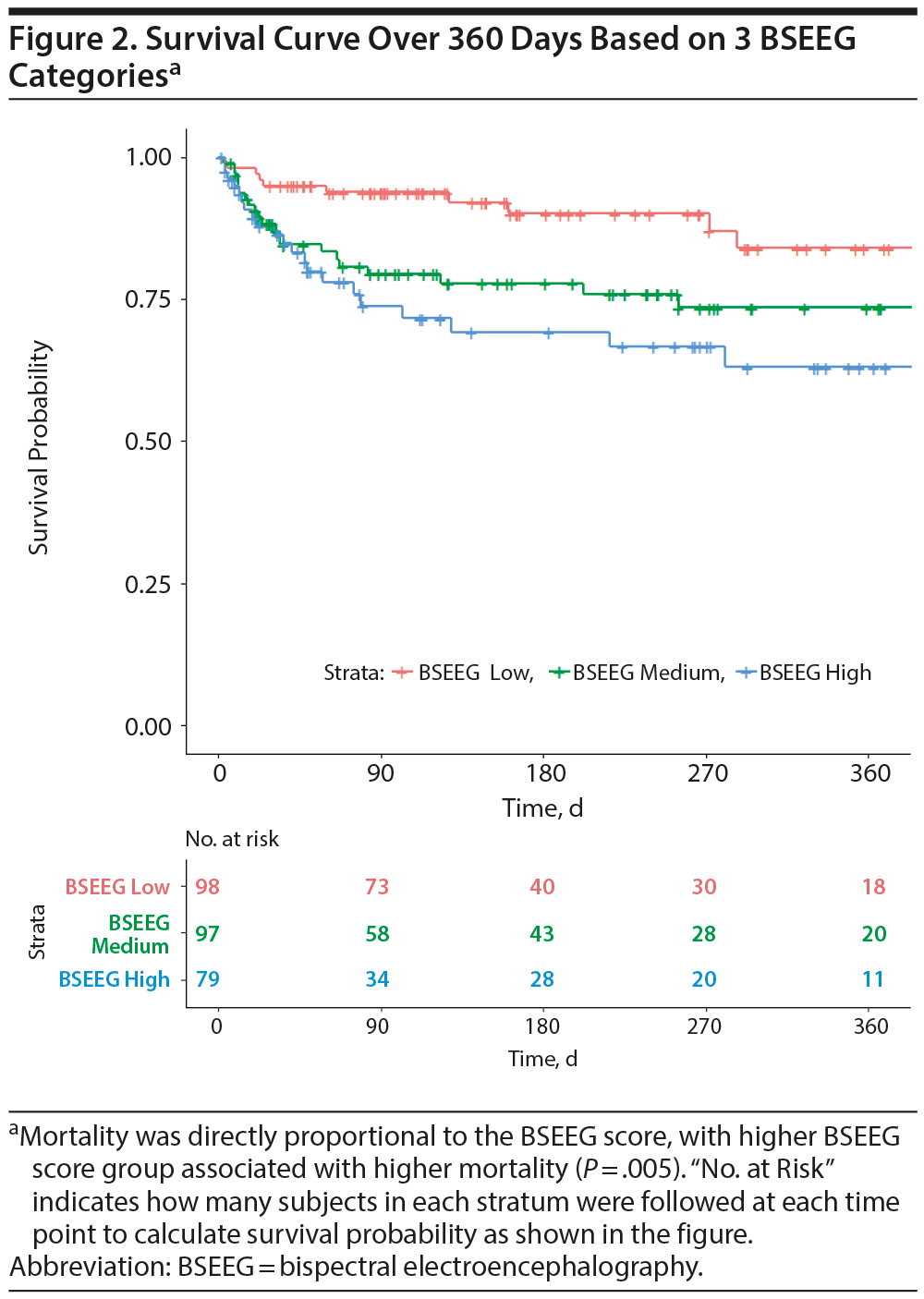
We also hypothesized that BSEEG score not only measures the presence of delirium but also represents delirium severity. We therefore divided subjects in 3 groups of approximately equal sample size based on BSEEG score: BSEEG high, BSEEG intermediate, and BSEEG low. The survival curve showed a “dose-dependent” relationship of increasing mortality with increasing BSEEG score (Figure 2, P = .005), suggesting a strong relationship between BSEEG score and mortality.
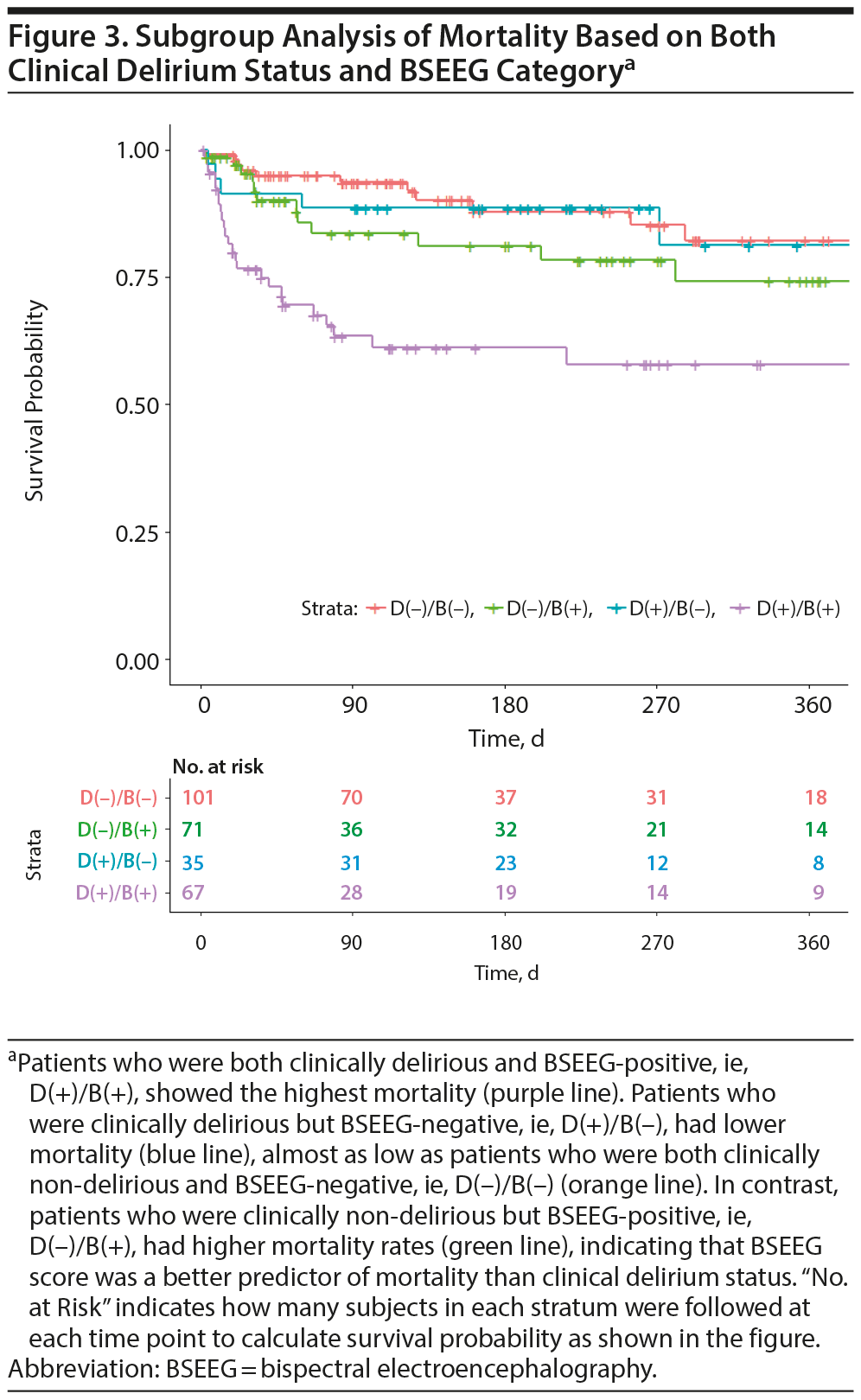
We wondered about outcomes of subjects in whom clinical assessment and BSEEG score were discordant. We therefore divided the cohort into 4 groups based on clinical delirium diagnosis and BSEEG. Clinically delirious subjects with positive BSEEG scores showed the highest mortality. In contrast, those patients categorized as clinically delirious but with a negative BSEEG score had lower mortality, similar to that of non-delirious subjects with negative BSEEG scores. Moreover, those thought to be non-delirious subjects based on results of clinical assessment but with positive BSEEG scores had a higher mortality, even compared to those patients with clinical delirium but with a negative BSEEG score (Figure 3).
DISCUSSION
Key Results and Interpretation
The data presented here show that the BSEEG score was significantly associated with the clinical presence of delirium, even after control for age, sex, and CCI score. More importantly, our results indicate that the BSEEG score was strongly associated with patient outcomes, including hospital LOS, discharge disposition, and mortality among hospitalized patients. Importantly, this association was based on a BSEEG score obtained at the time of enrollment, often within 24 hours of admission. These results suggest that a single BSEEG score obtained at the beginning of hospitalization can predict patient outcomes. This result also indicates that among patients who cannot be clinically identified as delirious, a subset is at high risk of death that is distinguishable by differences in brain wave activity as detected by BSEEG. We have termed this state as subclinical brain failure (SBF), a finding that warrants further investigation and more attention. Thus, identification of this population with the BSEEG method could lead to early intervention and potentially improved survival rates.
The search for EEG-based biomarkers of delirium has a long history involving several research groups. Since the 1940s, it has been reported that EEG patterns detected from only 2 channels can be used to distinguish marked delirium from normal awareness.24 However, currently available technologies using a small number of EEG leads are not “tuned” for delirium screening and lack a form factor appropriate for mass screening. In the area of anesthesiology in the last 2 decades, new devices utilizing EEG signals obtained from a few leads attached to a patient’s forehead have been used to monitor the depth of anesthesia. This approach has gained much popularity and is commonly used during surgical operations.34-36 Although this approach is similar to the one proposed here, the algorithm is trained only to measure depth of anesthesia and the equipment used is heavy and embedded in a large anesthesia machine; thus, the cost is quite high and it is unsuitable for high-throughput screening of the general hospitalized population. Two-channel brain wave monitoring has also been used in psychiatric treatment involving electroconvulsive therapy (ECT).37 The ECT machine includes an EEG function, with 2 leads to monitor seizure activity. However, it also lacks delirium screening technology and has an inappropriate form factor for mass point-of-care screening use. Nevertheless, these applications demonstrate that obtaining EEG signals from a limited number of leads is established technology. The usefulness of an EEG obtained using a limited number of electrodes for detecting delirium has only recently been demonstrated; one study38 confirmed that the sensitivity and specificity of limited EEG leads are excellent and comparable to those for machines with the traditional 20 leads for this purpose. However, that report did not provide information about the relationship of limited-lead EEG screening for delirium with patient outcomes.
Although previous literature supports the notion that EEG is useful for detecting delirium, no investigation has determined the effects of using a point-of-care BSEEG device to identify biomarkers of delirium on a large number of patients, nor has any study tested the association of such a screening method with patient outcomes such as hospital LOS, discharge disposition, and mortality or the effect of intervention on these outcomes. Only a few studies32,39,40 have investigated the association of conventional EEG data and mortality, although, in general, those EEG recordings were limited to those subjects who were thought to need EEG examination for another medical indication such as altered mental status, and thus a broader range of general inpatients has never been studied.
Our data demonstrated the usefulness of the BSEEG score in identifying patients at risk for delirium and in predicting patient outcomes such as hospital LOS, discharge disposition, and mortality among elderly hospitalized patients. This study is the first to use a point-of-care EEG device to demonstrate the association between BSEEG biomarker signals and their association with delirium and patient outcomes. BSEEG was used not only for patients with obvious mental status change but also for a broader cohort of inpatients. Because the BSEEG method would not require extensive training for lead placement or interpretation by a neurology specialist, application for screening large numbers of patients even without notable mental status change becomes possible.
With additional clinical validation, such BSEEG-based biomarkers will enable early intervention and will potentially improve the current practice of medicine and surgery for patients at risk of delirium. For example, BSEEG analysis may be an important factor in the decision to perform elective surgery or be used for heightened monitoring after surgery. When high-risk patients are identified through BSEEG analysis, it is then possible to direct hospital resources more efficiently and effectively compared to the current standard of care.
BSEEG monitoring may also be applicable in additional settings such as the primary care clinic, emergency department, and nursing home or home-care settings. Delirium is particularly dangerous when patients experience it outside of hospitals because of the lack of recognition and resources to manage it. The simple, noninvasive nature of this test makes it ideal for routine screening. BSEEG has the potential to be a fast, easy, noninvasive, and objective assessment tool, similar to the measurement of vital signs that can be used to monitor or screen for delirium in appropriate populations. A positive result would raise an early alarm and trigger more comprehensive workup for an acute illness. The BSEEG may be more clinically relevant than and would provide an objective and quantitative replacement for currently used criteria for “altered mental status” in other prognostic models such as the Sequential Organ Failure Assessment (SOFA).41 As the aging population is expanding rapidly, efficient modalities for delirium screening such as BSEEG are predicted to be in high demand.
Limitations
We introduced constraints into this study by limiting electrode placement to the forehead, which was intentional due to our ultimate goal of developing a user-friendly screening method for hospital staff. Other lead placement conformations may result in better performance, but could hinder broad usability. The usefulness of BSEEG score for delirium screening depends on the assumption that EEG changes are generalized or diffuse. Therefore, focal change, either by slowing or seizure activity or a structural brain abnormality, may confound results. Another limitation is that we do not have information about several patient characteristics that could potentially affect risk for delirium and thus patient outcomes. Those characteristics include rates of dementia or mild cognitive impairment, past history of delirium, admission diagnosis, habitual use of alcohol or benzodiazepine receptor agonists, and other prescribed medications such as opioids and corticosteroids. An additional limitation of this study is that it was conducted at a single institution in the Midwest region of the United States, where most of the patient population consists of non-Hispanic white individuals. Thus, generalizability needs to be tested with a future multicenter study based on the presented results. Nevertheless, we have developed a useful algorithm to differentiate delirium and associate the BSEEG score with patient outcomes.
Our future investigations will determine whether certain BSEEG-triggered preemptive interventions improve outcomes. For example, new studies have shown that both ramelteon42 and suvorexant43 can decrease the risk of delirium. Thus, exploring the effect of these treatments in conjunction with BSEEG monitoring will allow us to determine the impact of certain medications on BSEEG score and outcomes. These provide new avenues to explore better treatments for delirium and, ultimately, improve patient outcomes.
Implications for Practice
In conclusion, we demonstrate that simple, noninvasive, point-of-care EEG collection combined with BSEEG scoring was able to predict adverse patient outcomes, including mortality, in an elderly population. Importantly, we identified a certain patient population who cannot be identified by current clinical assessments but is at high risk for mortality that warrants further investigation to see if early detection and intervention can modify their mortality.
Submitted: January 18, 2019; accepted May 1, 2019.
Published online: September 3, 2019.
Author contributions: Dr G. Shinozaki contributed to the overall design of the study, data acquisition, data analysis, interpretation, and manuscript drafting. Dr Cromwell contributed to the development of the original signal processing algorithm, which was used for acquisition of raw data. Drs Bormann and Chan, Mr Zarei, and Dr Cho contributed to data analysis and data interpretation. Ms Gaul, Mr Klisares, Ms Jellison, Dr Heinzman, Mr Dahlstrom, Ms Duncan, Mr Sparr, Dr Wanzek, Ms Cramer, and Ms Wimmel contributed to data collection. Ms Sabbagh and Dr Yuki contributed to data collection and data management. Drs Weckmann, Yamada, Karam, Noiseux, E. Shinozaki, and Lee contributed to data interpretation and manuscript drafting. All authors approved the final manuscript.
Potential conflicts of interest: Drs G. Shinozaki and Cromwell are co-founders of Predelix Medical LLC and have the patent “Non-Invasive Device for Predicting and Screening Delirium” under the Patent Cooperation Treaty Application No. PCT/US2016/064937, and in US Provisional Patent No. 62/263,325 pending. All other authors have declared that no conflict of interest exists.
Funding/support: This study was supported by the University of Iowa Research Foundation GAP funding award for Drs G. Shinozaki and Cromwell. Dr G. Shinozaki received grant support from the National Science Foundation (award no. 1664364). Dr Chan was supported by the National Institute of Mental Health (award no. T32MH019113).
Role of the sponsor: The supporters had no role in the design, analysis, interpretation, or publication of this study.
Acknowledgments: The authors thank patients who participated to this study.
Supplementary material: See accompanying pages.
REFERENCES
1.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165. PubMed CrossRef
2.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. PubMed CrossRef
3.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. PubMed CrossRef
4.Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004;32(1):106-112. PubMed CrossRef
5.Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. PubMed CrossRef
6.Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280. PubMed CrossRef
7.Pisani MA, McNicoll L, Inouye SK. Cognitive impairment in the intensive care unit. Clin Chest Med. 2003;24(4):727-737. PubMed CrossRef
8.McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. PubMed CrossRef
9.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. PubMed CrossRef
10.Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66-73. PubMed CrossRef
11.Kakuma R, du Fort GG, Arsenault L, et al. Delirium in older emergency department patients discharged home: effect on survival. J Am Geriatr Soc. 2003;51(4):443-450. PubMed CrossRef
12.Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316. PubMed CrossRef
13.González M, Mart×nez G, Calderón J, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics. 2009;50(3):234-238. PubMed CrossRef
14.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419-427. PubMed CrossRef
15.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. PubMed CrossRef
16.Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. PubMed CrossRef
17.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the Confusion Assessment Method: a new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. PubMed CrossRef
18.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. PubMed CrossRef
19.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379. PubMed CrossRef
20.Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242. PubMed CrossRef
21.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. PubMed CrossRef
22.van Eijk MM, van den Boogaard M, van Marum RJ, et al. Routine use of the Confusion Assessment Method for the Intensive Care Unit: a multicenter study. Am J Respir Crit Care Med. 2011;184(3):340-344. PubMed CrossRef
23.Nishimura K, Yokoyama K, Yamauchi N, et al; TMAD investigators. Sensitivity and specificity of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and the Intensive Care Delirium Screening Checklist (ICDSC) for detecting post-cardiac surgery delirium: a single-center study in Japan. Heart Lung. 2016;45(1):15-20. PubMed CrossRef
24.Romano J, Engel GL. Delirium: I, electroencephalographic data. Arch Neurol Psychiatry. 1944;51(4):356-377. CrossRef
25.Jacobson SA, Leuchter AF, Walter DO. Conventional and quantitative EEG in the diagnosis of delirium among the elderly. J Neurol Neurosurg Psychiatry. 1993;56(2):153-158. PubMed CrossRef
26.Shinozaki G, Chan AC, Sparr NA, et al. Delirium detection by a novel bispectral electroencephalography device in general hospital. Psychiatry Clin Neurosci. 2018;72(12):856-863. PubMed CrossRef
27.Lee S, Yuki K, Chan A, et al. The point-of-care EEG for delirium detection in the emergency department. Am J Emerg Med. 2019;37(5):995-996. PubMed CrossRef
28.Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31-50. PubMed CrossRef
29.Gavinski K, Carnahan R, Weckmann M. Validation of the Delirium Observation Screening Scale in a hospitalized older population. J Hosp Med. 2016;11(7):494-497. PubMed CrossRef
30.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. PubMed CrossRef
31.Inouye SK, Leo-Summers L, Zhang Y, et al. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312-318. PubMed CrossRef
32.Azabou E, Magalhaes E, Braconnier A, et al; Groupe d’ Explorations Neurologiques en Réanimation (GENER). Early Standard Electroencephalogram abnormalities predict mortality in septic intensive care unit patients. PLoS One. 2015;10(10):e0139969. PubMed CrossRef
33.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251. PubMed CrossRef
34.Liu J, Singh H, White PF. Electroencephalographic bispectral index correlates with intraoperative recall and depth of propofol-induced sedation. Anesth Analg. 1997;84(1):185-189. PubMed CrossRef
35.Schmidlin D, Hager P, Schmid ER. Monitoring level of sedation with bispectral EEG analysis: comparison between hypothermic and normothermic cardiopulmonary bypass. Br J Anaesth. 2001;86(6):769-776. PubMed CrossRef
36.Powers KS, Nazarian EB, Tapyrik SA, et al. Bispectral index as a guide for titration of propofol during procedural sedation among children. Pediatrics. 2005;115(6):1666-1674. PubMed CrossRef
37.Scott AI. Monitoring electroconvulsive therapy by electroencephalogram: an update for ECT practitioners. Adv Psychiatr Treat. 2007;13(4):298-304. CrossRef
38.van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101. PubMed CrossRef
39.Stecker MM. The EEG as an independent indicator of mortality and healthcare utilization. Clin Neurophysiol. 2009;120(10):1777-1781. PubMed CrossRef
40.Gilmore EJ, Gaspard N, Choi HA, et al. Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med. 2015;41(4):686-694. PubMed CrossRef
41.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707-710. PubMed CrossRef
42.Hatta K, Kishi Y, Wada K, et al; DELIRIA-J Group. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71(4):397-403. PubMed CrossRef
43.Hatta K, Kishi Y, Wada K, et al; DELIRIA-J Group. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78(8):e970-e979. PubMed CrossRef
This PDF is free for all visitors!