Modafinil or armodafinil (ar/mod) augmentation of antipsychotic medication in schizophrenia patients may be considered with a view to reduce negative symptoms associated with the illness or excessive daytime drowsiness due to any cause. The available data suggest that there is no role for ar/mod in reducing negative symptom burden. A recent pharmacokinetic (PK) study suggested that armodafinil (250 mg/d) reduces key PK parameters of risperidone by about 50%, and key PK parameters of 9-hydroxyrisperidone (paliperidone) by about 20%-30%, probably through induction of CYP3A4. Ar/mod augmentation is therefore best avoided in patients receiving risperidone or paliperidone (and most other atypical antipsychotic drugs, as well, because most atypical antipsychotics are metabolized by enzymes that ar/mod induce). If the ar/mod-antipsychotic drug combination is necessary, for whatever reason, then the dose of the atypical antipsychotic drug may need to be appropriately raised. If this is not done, relapse may occur; because the relapse may postdate the introduction of ar/mod by many months, the causal role of a metabolic drug interaction may not be suspected, and physicians may attribute the relapse to the natural course of the illness. Physicians need to be aware that any agent that induces the metabolism of psychotropic drugs that are used in maintenance therapy may, through lowered psychotropic drug levels, result in a delayed drug interaction that is characterized by illness relapse.
- Modafinil and armodafinil (ar/mod) have no role in the treatment of negative symptoms of schizophrenia.
- Armodafinil induces CYP3A4, and modafinil induces CYP1A2 as well as CYP3A4. The long-term use of ar/mod along with antipsychotic drugs that are metabolized by these enzymes will therefore increase the risk of schizophrenia relapse.
- When antipsychotic drug levels fall as a result of enzyme induction, relapse does not occur immediately. The delay in relapse, often by many months or longer, may result in a failure to suspect a relationship between the inducing drug and the relapse.

ABSTRACT
Modafinil or armodafinil (ar/mod) augmentation of antipsychotic medication in schizophrenia patients may be considered with a view to reduce negative symptoms associated with the illness or excessive daytime drowsiness due to any cause. The available data suggest that there is no role for ar/mod in reducing negative symptom burden. A recent pharmacokinetic (PK) study suggested that armodafinil (250 mg/d) reduces key PK parameters of risperidone by about 50%, and key PK parameters of 9-hydroxyrisperidone (paliperidone) by about 20%-30%, probably through induction of CYP3A4. Ar/mod augmentation is therefore best avoided in patients receiving risperidone or paliperidone (and most other atypical antipsychotic drugs, as well, because most atypical antipsychotics are metabolized by enzymes that ar/mod induce). If the ar/mod-antipsychotic drug combination is necessary, for whatever reason, then the dose of the atypical antipsychotic drug may need to be appropriately raised. If this is not done, relapse may occur; because the relapse may postdate the introduction of ar/mod by many months, the causal role of a metabolic drug interaction may not be suspected, and physicians may attribute the relapse to the natural course of the illness. Physicians need to be aware that any agent that induces the metabolism of psychotropic drugs that are used in maintenance therapy may, through lowered psychotropic drug levels, result in a delayed drug interaction that is characterized by illness relapse.
J Clin Psychiatry 2015;76(12):1633-1634
dx.doi.org/10.4088/JCP.15f10514
© Copyright 2015 Physicians Postgraduate Press, Inc.
Clinical Question
A 39-year-old man, diagnosed with schizophrenia, has been stable on risperidone (4 mg/d) for the past year. There are presently no delusions, hallucinations, or other positive symptoms; however, he lacks interest and motivation in work and in leisure, pleasure, and social activities. Observing these and other negative symptoms, and aware that some data have suggested that modafinil may reduce negative symptom burden in schizophrenia, his physician plans to comedicate him with modafinil or armodafinil (ar/mod). What are the potential benefits and risks associated with ar/mod augmentation of risperidone?
Ar/mod Augmentation and Attenuation of Negative Symptoms
Many randomized controlled trials (RCTs) have examined ar/mod augmentation of antipsychotic drugs in patients with schizophrenia.1 Only 1 of these,2 a small (N = 46), short-term (8 weeks) RCT, found that modafinil attenuated negative symptom severity. However, the findings of this study2 are not applicable to the patient under consideration because the study was conducted on acutely ill patients, whereas the patient described has no positive symptoms and is psychiatrically stable. Furthermore, a large (N = 285), intermediate-term (24 weeks) RCT conducted in stable patients preselected for negative symptom burden found no advantage for armodafinil augmentation at any dose (150, 200, 250 mg/d).3 Finally, a meta-analysis1 showed that ar/mod augmentation attenuated negative symptom burden, but the benefit was a negligible 0.27 points on the Positive and Negative Syndrome Scale, and even this benefit disappeared when an outlier study2 was excluded in a sensitivity analysis.
The take-home message here is that there is no justification for the use of ar/mod to augment antipsychotic drugs in schizophrenia if the target is attenuation of negative symptoms and if the patient is otherwise psychiatrically stable.
Ar/mod and Drug Interactions
What if the patient described at the start of this article required ar/mod augmentation for a different reason, such as excessive daytime drowsiness due to any cause? In that case, the treating physician would need to consider potential pharmacokinetic (PK) interactions between ar/mod and risperidone.
In an earlier article in this column,4 ar/mod-related metabolic drug interactions were discussed. To recapitulate an important mechanism highlighted in that article, armodafinil induces cytochrome P450 (CYP)3A4 and modafinil induces both CYP1A2 and CYP3A4. These 2 CYP enzymes are involved in the metabolism of almost all of the atypical antipsychotic drugs available. As a result, it would be imprudent to prescribe ar/mod with antipsychotic drugs that are substrates of these enzymes; the risk is that relapse will occur because of lowered drug levels.
Armodafinil Interaction With Risperidone
Risperidone is predominantly metabolized by CYP2D6, and to a small extent by CYP3A4.5 Is the CYP3A4 induction by ar/mod clinically significant when risperidone is the antipsychotic that is augmented? This question was specifically addressed in a PK study by Darwish et al.6
These authors recruited 36 healthy subjects for their study. The mean age of the sample was about 32 years. The sample was 67% male. The sample was overweight, on average (mean BMI = 26.1). Only 2 subjects were CYP2D6 poor metabolizers, which is in line with estimates of the prevalence of CYP2D6 poor metabolizers in the general population.5
In the first phase of the study, all subjects received a single 2-mg dose of risperidone; this was followed by a 4-day study of risperidone PK. In the second phase, all subjects received armodafinil (uptitrated to 250 mg/d) for 37 days to allow for maximum induction of CYP3A4; risperidone (2 mg) was administered on day 34, and this was followed by 4 days of PK study, as earlier. Geometric mean ratios were calculated for endpoint versus baseline PK variables.
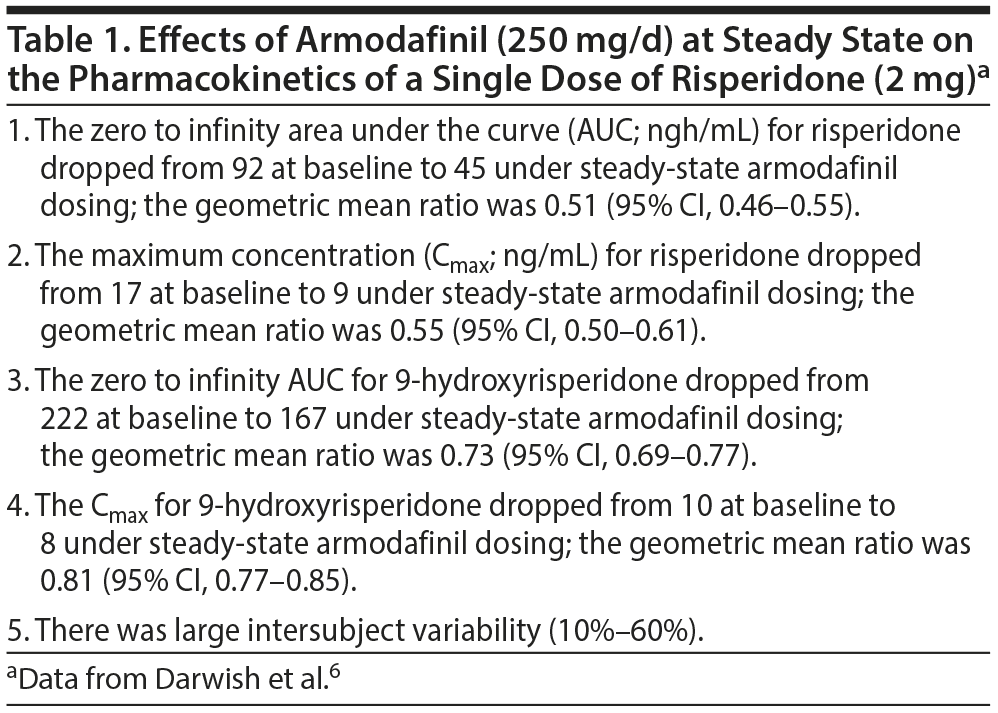
Important findings of the study are presented in Table 1. In summary, at steady state, armodafinil reduced key PK parameters of risperidone by about 50%, and key PK parameters of 9-hydroxyrisperidone (paliperidone) by about 20%-30%. Thus, armodafinil reduces exposure not only to risperidone but also to its active metabolite.
This may not be as alarming as it seems. The half-life of risperidone in CYP2D6 extensive metabolizers (most of the population) is about 3 hours, and the half-life of paliperidone is about 21-24 hours.7 Therefore, in theory, most of the action of risperidone is actually through its active metabolite. So, because the action of armodafinil on paliperidone is small, the drug interaction may not be clinically significant. Some small support for this conjecture comes from the earlier cited RCT of Kane et al,3 which included risperidone-treated patients; this study did not identify a greater risk of relapse with armodafinil relative to placebo.
A limitation of the Darwish et al6 study is that this was a single-dose study of a low dose of risperidone (2 mg) at steady state with high-dose armodafinil (250 mg/d). There is no certainty that the results will accurately describe the effects of lower doses of ar/mod on risperidone steady-state PK when risperidone is clinically dosed.
The above notwithstanding, it is acknowledged that the data on risperidone relapses were not separately presented by Kane et al,3 and the study was not powered to detect differences in relapse-related outcomes. Furthermore, paliperidone is generally stated to be minimally metabolized8; nevertheless, in the Darwish et al6 PK study, armodafinil significantly induced the metabolism of paliperidone, though only to a small extent. This indicates that metabolic drug interactions involving paliperidone cannot be ignored. Prudence therefore dictates that physicians seriously consider the possibility that modafinil or its R-enantiomer armodafinil may lower antipsychotic drug levels, thereby increasing the risk of relapse in risperidone- or paliperidone-treated patients. Because of the large intersubject variability (Table 1), compensatory dose adjustments will need to be individualized.
Ar/mod and a Delayed Risk of Relapse
Many months may pass before relapse occurs in a schizophrenia patient who discontinues antipsychotic medication, and the delay may be even longer if the drug is merely reduced in dose and not discontinued. This is because there are individual-to-individual variations in levels of stress, coping, and social and family support, all of which moderate the risk of relapse. As a result, if ar/mod (or other CYP3A4 inducers) are prescribed to patients receiving risperidone, relapse due to lowered drug levels may not occur for months or longer, at which time the possible causal association with ar/mod initiation will no longer be suspected; rather, physicians may assume that the relapse is part of the natural course of the illness. Physicians should therefore be aware that induction of psychotropic drug metabolism by any drug may result in a delayed drug interaction that manifests as late relapse. Therefore, if comedication between an inducing agent and a maintenance psychotropic agent is required, the dose of the maintenance agent will need to be appropriately raised to maintain efficacy.
Parting Note
Rifampin, which also induces CYP3A4, has a more substantial effect on lowering key PK parameters of risperidone and paliperidone, but this may be through induction of P-glycoprotein, as well.9
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Andrade C, Kisely S, Monteiro I, et al. Antipsychotic augmentation with modafinil or armodafinil for negative symptoms of schizophrenia: systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res. 2015;60(1):14-21. PubMed doi:10.1016/j.jpsychires.2014.09.013
2. Arbabi M, Bagheri M, Rezaei F, et al. A placebo-controlled study of the modafinil added to risperidone in chronic schizophrenia. Psychopharmacology (Berl). 2012;220(3):591-598. PubMed doi:10.1007/s00213-011-2513-z
3. Kane JM, Yang R, Youakim JM. Adjunctive armodafinil for negative symptoms in adults with schizophrenia: a double-blind, placebo-controlled study. Schizophr Res. 2012;135(1-3):116-122. PubMed doi:10.1016/j.schres.2011.11.006
4. Andrade C. Modafinil and armodafinil in schizophrenia. J Clin Psychiatry. 2012;73(8):e1062-e1064. PubMed doi:10.4088/JCP.12f07977
5. Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41(2):89-295. PubMed doi:10.1080/03602530902843483
6. Darwish M, Bond M, Yang R, et al. Evaluation of potential pharmacokinetic drug-drug interaction between armodafinil and risperidone in healthy adults. Clin Drug Investig. 2015;35(11):725-733. PubMed doi:10.1007/s40261-015-0330-6
7. Heykants J, Huang ML, Mannens G, et al. The pharmacokinetics of risperidone in humans: a summary. J Clin Psychiatry. 1994;55(suppl):13-17. PubMed
8. Andrade C. Drugs that escape hepatic metabolism. J Clin Psychiatry. 2012;73(7):e889-e890. PubMed doi:10.4088/JCP.12f07726
9. Kim KA, Park PW, Liu KH, et al. Effect of rifampin, an inducer of CYP3A and P-glycoprotein, on the pharmacokinetics of risperidone. J Clin Pharmacol. 2008;48(1):66-72. PubMed doi:10.1177/0091270007309888
This PDF is free for all visitors!