Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Three years ago, we reported on a successful case of deep-brain stimulation (DBS), targeted at the nucleus accumbens, in a 30-year-old man with obsessive-compulsive disorder (OCD). Here we provide the follow-up to that case.
Â
Case report. At presentation, our patient had had a 5-year history of OCD (DSM-IV)Â with contamination obsessions and washing compulsions.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Long-Term Deep-Brain Stimulation Treatment for Obsessive-Compulsive Disorder
To the Editor: Three years ago, we reported1 on a successful case of deep-brain stimulation (DBS), targeted at the nucleus accumbens, in a 30-year-old man with obsessive-compulsive disorder (OCD). Here we provide the follow-up to that case.
Case report. At presentation, our patient had had a 5-year history of OCD (DSM-IV) with contamination obsessions and washing compulsions. The OCD had resulted in severe functional impairment, including dropping out of school and an inability to leave the home. He had undergone adequate trials of most selective serotonin reuptake inhibitors (SSRIs) (paroxetine, fluoxetine, fluvoxamine, escitalopram, and sertraline), both as monotherapy and in combination with several pharmacologic augmentation strategies (diazepam, topiramate, buspirone, olanzapine, aripiprazole, risperidone, memantine). In addition, he underwent cognitive-behavioral therapy using exposure response prevention on a weekly basis for 20 weeks. At the time of surgery, his Yale-Brown Obsessive Compulsive Scale2 (Y-BOCS) score was 32, consistent with extreme illness severity. After ethical review board approval and providing informed consent, he underwent bilateral implantation of electrodes targeting the nucleus accumbens.
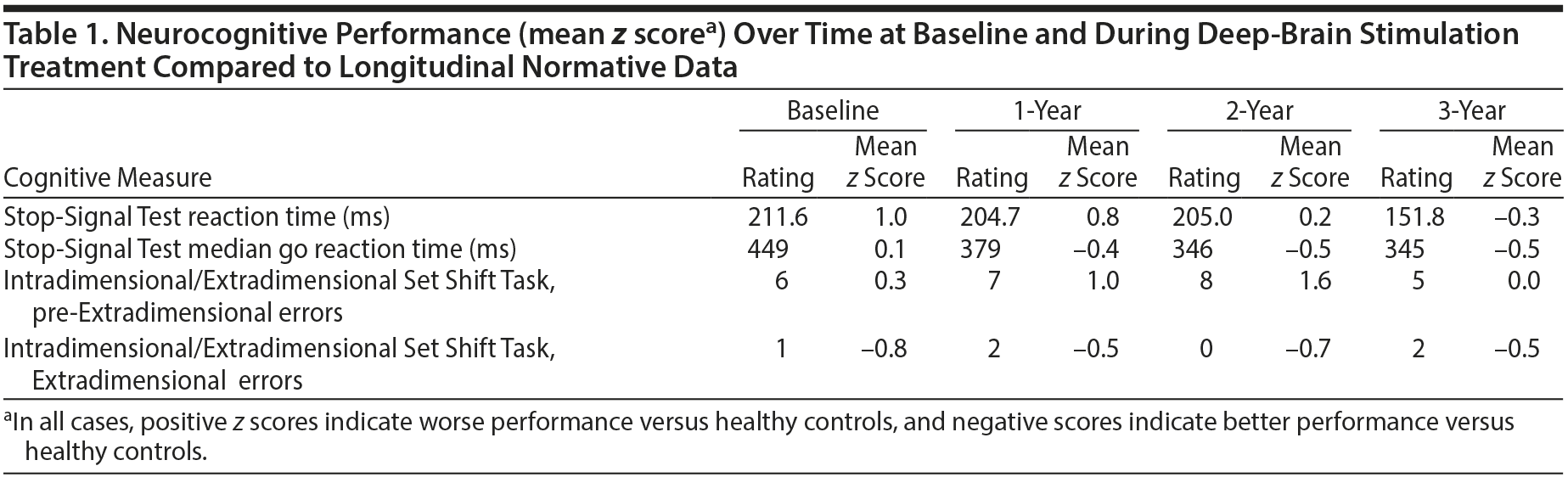
We performed cognitive assessments at baseline (prestimulation), 8 months after DBS began, and then yearly for 3 years. Cognitive tasks were taken from the Cambridge Neuropsychological Test Automated Battery (www.camcog.com) and included the Stop-Signal Test (assessing ability to suppress prepotent motor responses) and the Intradimensional/Extradimensional Set Shift Task (examining rule learning and behavioral flexibility).
Prior research3 in a sample of OCD patients, their first-degree relatives, and unrelated healthy volunteers demonstrated that variation in motor inhibitory control (Stop-Signal Test) was correlated with gray matter density changes in an extensive system comprising orbitofrontal, cingulate, and parietal cortical areas, as well as striatal and other subcortical regions, and that these inhibition-related brain systems might be considered a neurocognitive endophenotype for OCD. Such cognitive assessments conducted over time have the potential to help researchers and clinicians better understand whether symptom improvement coincides with cognitive processing improvement several years after surgical intervention. To control for potential practice effects, cognitive tasks were also performed at the same yearly intervals in age- and gender-matched controls with no current or lifetime psychiatric disorders based on a structured clinical interview by a board-certified psychiatrist.3
At the time of surgery, our patient was taking the following medications (stable doses for at least 3 years): clomipramine 250 mg/d; ziprasidone 120 mg/d; and clonazepam 1 mg 3 times daily. These medications continued unchanged throughout the study. DBS was not interrupted for battery change over the course of the study, and the DBS settings were held constant after an initial period of adjustment. He experienced symptom improvement 8 months after surgery (Y-BOCS score of 10), and over the course of the 3 years, his Y-BOCS scores were consistently 8-10. In addition to OCD symptom improvement, the patient exhibited a dramatic improvement in social and occupational health. He obtained and maintained a job and has re-developed meaningful social relationships (including dating). His motor inhibitory performance (ie, the candidate cognitive endophenotype for OCD) was impaired at baseline and did not improve to the levels of controls until the third year after surgery (Table 1).
Despite OCD-symptom improvement almost immediately after surgery, our results suggest that response inhibition did not improve until several years later. Previous research4 suggests that stop-signal impairment in OCD persists despite first-line intervention with SSRIs. Acute dopamine manipulation of the nucleus accumbens core had no effect on response inhibition in rodents,5 while the accumbens shell does appear to be involved in aspects of inhibitory control.6 We speculate that chronic DBS of the accumbens core led to indirect upstream effects on other neural regions (eg, accumbens shell or subthalamic nucleus), which in turn were responsible for the cognitive improvement reported.
These findings highlight that certain cognitive deficits in OCD, refractory to usual interventions, may resolve with prolonged DBS treatment in OCD and be temporally dissociable from symptom improvement. Further research is needed to confirm these findings, rule out nonspecific contributing factors (eg, improvement in social functioning itself resulting in cognitive change), and characterize the neural mechanisms involved. The results also lead to an important question: once cognitive deficits normalize, could stimulation for DBS in OCD be turned off without deficits and symptoms recurring?
References
1. Grant JE, Odlaug BL, Chamberlain SR. Neurocognitive response to deep brain stimulation for obsessive-compulsive disorder: a case report. Am J Psychiatry. 2011;168(12):1338-1339. PubMed doi:10.1176/appi.ajp.2011.11071108
2. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011. PubMed doi:10.1001/archpsyc.1989.01810110048007
3. Menzies L, Achard S, Chamberlain SR, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130(pt 12):3223-3236. PubMed doi:10.1093/brain/awm205
4. Chamberlain SR, Fineberg NA, Blackwell AD, et al. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry. 2006;163(7):1282-1284. PubMed doi:10.1176/appi.ajp.163.7.1282
5. Eagle DM, Wong JC, Allan ME, et al. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. J Neurosci. 2011;31(20):7349-7356. PubMed doi:10.1523/JNEUROSCI.6182-10.2011
6. Feja M, Koch M. Frontostriatal systems comprising connections between ventral medial prefrontal cortex and nucleus accumbens subregions differentially regulate motor impulse control in rats. Psychopharmacology (Berl). 2015;232(7):1291-1302. PubMed doi:10.1007/s00213-014-3763-3
aDepartment of Psychiatry and Behavioral Neuroscience, University of Chicago, Illinois
bDepartment of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
cDepartment of Psychiatry, University of Cambridge; and Cambridge and Peterborough NHS Foundation Trust (CPFT), Cambridge, United Kingdom
Potential conflicts of interest: Dr Grant has received research grants from the National Institute of Mental Health, National Center for Responsible Gaming, Forest, and Roche. He also receives yearly compensation from Springer Publishing for acting as Editor-in-Chief of the Journal of Gambling Studies and has received royalties from Oxford University Press, American Psychiatric Publishing, Norton Press, and McGraw Hill. Mr Odlaug has received a research grant from the Trichotillomania Learning Center, consults for H. Lundbeck A/S, and has received royalties from Oxford University Press. Dr Chamberlain’s involvement in this research was supported by a grant from the Academy of Medical Sciences, UK. He also consults for Cambridge Cognition.
Funding/support: None reported.
J Clin Psychiatry 2016;77(1):132-133
dx.doi.org/10.4088/JCP.15cr09931
© Copyright 2016 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!