Abstract
Objective: Vagus nerve stimulation (VNS) therapy is a long-term intervention for treatment-resistant major depression (TRD) adjunctive to treatment as usual (TAU). To enhance clinical decision- making, we identified subgroups that respond especially well or poorly with active VNS vs no stimulation sham VNS (prognostic predictors) and subgroups that specifically benefit from active VNS vs sham VNS (prescriptive predictors).
Methods: In the RECOVER trial, patients with marked TRD (N=493) were randomized to either active VNS (N=249) or sham VNS (N=244); both groups continued TAU. Baseline demographic, clinical, and treatment history characteristics were evaluated as potential prognostic and/or prescriptive outcome predictors. Outcome assessment was based on a tripartite measure that combined depressive symptoms (Quick Inventory of Depressive Symptomatology—Clinician), psychosocial function (Work Productivity and Activity Impairment Questionnaire item 6), and quality of life (Mini-Quality of Life Enjoyment and Satisfaction Questionnaire). Generalized linear mixed models were employed to identify both prognostic and prescriptive predictors of tripartite outcomes.
Results: Several baseline features predicted outcomes across the entire sample and within the sham VNS group (prognostic prediction). History of treatment with electroconvulsive therapy (ECT; lifetime and current episode) or transcranial magnetic stimulation (TMS; current episode) was associated with poorer prognosis. However, these same features were associated with greater benefit from active VNS vs sham VNS. The presence of comorbid anxiety disorders was predictive of a better prognosis overall, but smaller benefit from active VNS vs sham VNS.
Conclusions: Marked TRD patients with a history of ECT or TMS had especially poorer outcomes when receiving sham VNS plus TAU for 1 year than those without this history. These same subgroups showed significant differential benefit with active VNS than with sham VNS (positive prescriptive effect). The absence of a comorbid anxiety disorder was linked to superior benefit from active VNS vs sham VNS. These predictors may inform clinical decision-making when considering VNS.
Trial Registration: ClinicalTrials.gov identifier: NCT03887715.
J Clin Psychiatry 2025;86(3):25m15850
Author affiliations are listed at the end of this article.
Major depressive disorder (MDD) is a common, often chronic or recurrent, condition that typically responds well to initial treatments with psychotherapy or medications. However, 15%–30% of patients with MDD do not have sufficient symptomatic benefit or cannot sustain it after multiple treatment trials.1–3
Patients with treatment-resistant depression (TRD)—defined as having had 2 or more failed adequate antidepressant treatments3,4—have greater morbidity, mortality, and considerably higher mental and physical health services costs than those who symptomatically respond to initial treatments.4–10
Vagus nerve stimulation (VNS Therapy), an adjunctive treatment for patients with TRD, has been shown to result in meaningful symptomatic improvement in 30%–40% of patients over 1 year.11,12 The recently completed randomized phase of the RECOVER trial13,14 found that adjunctive VNS, delivered over 1 year, exceeded the effects of adjunctive sham VNS in selective measures of depressive symptoms, psychosocial function, and quality of life (QoL), 3 recognized primary outcome domains for depression treatment.13–16 Nonetheless, the selection and management of optimal candidates for VNS are challenging. Treatment entails outpatient surgery with associated adverse effects, while the therapeutic effects of VNS may not occur for months following device activation.13,14,16 Clinicians and patients need the ability to predict which patients are likely to benefit from VNS and, once selected, what outcomes to expect. These questions may be addressed by identifying prescriptive and prognostic predictors.17–20 These predictors provide distinct information that can inform clinical decision- making and care delivery. Prognostic predictors are baseline patient features linked to clinical outcomes, regardless of treatment, and specific to each treatment, in this case, active or sham VNS. Prescriptive predictors are baseline patient features associated with differential outcomes dependent on which intervention is provided, ie, active vs sham VNS. Prognostic associations should inform outcomes in both treatment groups: adjunctive VNS plus treatment as usual (TAU) or sham VNS plus TAU. Prescriptive associations reveal which patient subgroups benefit from adjunctive active VNS more than adjunctive sham VNS.21
This report focuses on RECOVER trial participants with markedly treatment-resistant MDD (n=493) for whom symptomatic, daily function, and quality of life outcomes have been reported.13,16 We use the qualifier “markedly” as an adverb and “marked” as an adjective to emphasize the extreme degree of treatment resistance previously documented in this sample based on the number of failed treatment trials in both the current episode and lifetime.
RECOVER is a prospective, multicenter, sham- controlled, blinded trial designed to evaluate the safety and effectiveness of adjunctive VNS Therapy in patients with marked TRD. Patients were randomized to receive either active or sham VNS (no stimulation) group, while continuing TAU over 12 months.
To develop prognostic and prescriptive predictors for active VNS, we chose a priori a composite metric that combines depressive symptoms, psychosocial function, and QoL outcomes to provide a more complete picture of potential benefit to the patient,22 rather than focusing on depressive symptoms alone. Previously, we found that the active VNS group had clinically meaningful improvements significantly beyond that obtained in the sham VNS group in measures of function and QoL, in addition to the difference in symptom improvement.13,16 The composite metric was more sensitive and applied to more patients than symptoms alone in detecting a difference between the randomized groups in extent of clinically meaningful benefit.
Here, we present analyses of data from the 12-month, randomized-controlled period. These analyses examine which participants with multiple antidepressant and interventional treatment failures experienced the greatest comparative benefit. These patients, therefore, should most likely be recommended for VNS implantation.
METHODS
In the RECOVER study, patients with MDD were randomized to receive active VNS or sham VNS for 12 months. After completing the 12-month participation visit, the sham VNS group received active VNS treatment, and all patients regardless of their original randomization group entered long-term follow-up. Examination of the outcomes in the second year of the trial is not yet complete.
The exploratory analyses in this report were conducted with a database derived from the RECOVER trial. Details on the study rationale, design, and primary and secondary outcomes are provided elsewhere.13,14 Participants were enrolled at 84 clinical sites in the United States. Ethics committee approval was obtained at each study site, as applicable, and in accordance with the Declaration of Helsinki guidelines. This report is based on data collected in the MDD study arm between October 2019 and April 2024. All participants provided written informed consent before any study-related medical record review or procedure. RECOVER is being conducted under “coverage with evidence development” from the US Centers for Medicare & Medicaid Services. This study was registered with ClinicalTrials.gov (identifier: NCT03887715).
Trial Entry Criteria
Eligible participants were at least 18 years old with a current diagnosis of MDD according to DSM-5 criteria and documentation of either chronic or recurrent course.23 Chronic MDD was defined as the current episode lasting ≥2 years; recurrent MDD was defined as 4 or more lifetime episodes with each episode separated by at least 2 months without meeting DSM-5 criteria for MDD. The diagnosis was confirmed by Mini-International Neuropsychiatric Interview (MINI) at the site24,25 and an independent psychiatric medical record review.
Eligibility criteria included insufficient benefit (eg, lack of response) from at least 4 adequate antidepressant treatments in the current episode using the clinician- rated Antidepressant Treatment History Form–Short Form.26 Eligible patients were at least moderately depressed at baseline with a score ≥22 on the Montgomery-Asberg Depression Rating Scale (MADRS) that was administered on 2 occasions 14–16 days apart.27 The absolute difference between the 2 MADRS scores could not exceed 25%.
Individuals at immediate risk of suicide requiring hospitalization based on clinical judgment and history, and those with a suicide attempt within the past 6 months prior to baseline, were excluded from the study. Additional study exclusion criteria included careful review of the last 2 years of psychiatric medical history and clinical evaluation of borderline or other personality disorders sufficiently severe to interfere with trial participation, any history of psychotic symptoms, or a primary diagnosis of obsessive-compulsive disorder, eating disorder, posttraumatic stress disorder, dementia, or other major neurocognitive disorder (based on a review of the medical history and clinical assessment by the site investigators). Participants with a DSM-5 defined substance use disorder without sustained remission for at least the past 12 months were also ineligible. Participants with bipolar depression were included in RECOVER and randomized in a separate cohort.
A Study Eligibility Committee, led by a psychiatrist (author CRC) specializing in TRD and a registered nurse (author CLK) with extensive psychiatric experience, reviewed medical records documenting psychiatric and treatment history to ensure study eligibility criteria were met in individuals already screened by the enrolling sites.
Blinding
Per the clinical study protocol, unblinding was defined as receiving identification of the subject’s randomization assignment. The investigators, interviewers, central raters, other study staff, and participants remained blinded by turning OFF the VNS devices in all patients at the start of the clinic visits. Speculation about the randomization assignment by blinded individuals was not considered unblinding (eg, perceived device stimulation or lack of, side effects perceived as possibly related to VNS Therapy, or improvement or lack of changes in depression status).
As we previously presented,28 participants were blinded to treatment assignment, with modest differences in guess accuracy: 67.5% in the active VNS + TAU group guessed their treatment assignment correctly, compared to 53.3% in the sham VNS + TAU group—closer to chance. These findings suggest partial blinding success.
Study Assessments
The assessment scales employed in the RECOVER trial have been described elsewhere.13,14 This report focuses on the Quick Inventory of Depressive Symptomatology—Clinician (QIDS-C) completed by trained, off-site, blinded raters masked to the protocol and treatment groups,29–32 the patient-rated Mini-Quality of Life Enjoyment and Satisfaction Questionnaire (Mini-Q-LES-Q),33,34 and the Work Productivity and Activity Impairment Questionnaire (WPAI).35–37 Thresholds for minimal clinically important difference (MCID) in each outcome domain were established based on prior publications.38–40
The QIDS-C assessed 9 depressive symptom domains (each rated 0–3) that define a major depressive episode based on the DSM-5 over the previous week. Partial response was defined as a ≥30% reduction in the baseline total score based on independent studies that established this threshold as clinically meaningful.41–43
The 7-item Mini-Q-LES-Q assessed QoL over the previous week,34 using a subset of the 14 Q-LES-Q items found most sensitive to change during treatment of MDD (ie, work, household activities, social relationships, family relationships, leisure time activities, ability to function in daily life, and overall sense of well-being). Each item is rated from 1 (very poor) to 5 (very good). To calculate an MCID,38 we converted to percent maximum scores by subtracting 7 from the raw total score (range, 7–35) and dividing by 28. The MCID for the Mini-Q-LES-Q was estimated a priori to be ≥11.89% increase from baseline based on previous evidence from the 14-item Q-LES-Q.38
Item 6 of the WPAI assessed daily functioning. The WPAI measures absenteeism, presenteeism, and productivity for working participants (items 2–5). However, most participants (74.6%) were not employed. Regardless of work status, item 6 assesses the effect of depression (from 0 for “no effect” to 10 for “massive effect”) on regular daily activities (work around the house, shopping, childcare, exercising, studying, etc.) over the prior week.35–37 A MCID on item 6 was a reduction of ≥2 points.39,40
In addition to baseline assessments before randomization, depressive symptoms were assessed monthly from months 3 through 12, while QoL and function were assessed quarterly during the 12 months following randomization.
Statistical Analyses
The data were pooled across study sites. The average of the last 2 MADRS assessments before device implantation was used as the baseline MADRS value. For the other assessments, the baseline was defined as the latest nonmissing assessment prior to device implantation. The tripartite outcome metric used 4 measurement occasions from assessments at months 3, 6, 9, and 12 following implantation for the prognostic assessment and outcomes only at month 12 for both prognostic and prescriptive analyses. The tripartite outcome measure determined at each measurement occasion whether there was a clinically meaningful improvement from baseline in each of 3 outcome domains: depressive symptoms (QIDS-C), psychosocial function (WPAI item 6), and QoL (Mini- Q-LES-Q). An improvement of ≥30% in the QIDS-C PR, ≥11.89% in the Mini-Q-LES-Q, and ≥2 in WPAI Item 6 were considered a priori as denoting minimal clinically meaningful benefits. Thus, for each scale and at each of the 4 measurement occasions, we determined whether a minimal clinical meaningful benefit was achieved. At each measurement occasion, patients were assigned a score of 0 or 1, depending on whether they demonstrated clinically meaningful benefit in any domain. Missing data at any measurement point were considered as indicating no benefit at that time.
Generalized linear mixed models (GLMM) were employed to identify both prognostic and prescriptive predictors of tripartite outcomes, utilizing the binary indicator (0 or 1) of clinically meaningful benefit in any domain at each measurement occasion. Two sets of analyses examined potential prognostic predictors. One goal of this trial was to identify patient features predictive of outcome in this marked TRD population regardless of treatment with VNS. A series of GLMMs were performed on the total sample of active VNS and sham VNS patients (N = 493). These models were applied to the binary tripartite outcome, first analyzed separately at month 12 and subsequently using repeated measures at months 3, 6, 9, and 12. Each model incorporated a random intercept and a logit link function, adjusting for covariates including visit month, age group (<65 and ≥65), and baseline MADRS score group (<34 and ≥34). These GLMMs sequentially incorporated individual patient demographic, clinical, and treatment history characteristics as covariates to identify prognostic predictors. Additionally, similar analyses were conducted separately within each treatment group (active VNS and sham VNS).
To identify prescriptive predictors, the GLMM analyses used to determine prognostic factors in the total sample at month 12 were repeated, now incorporating treatment group and the treatment group-by-time interaction as model terms. These models sequentially tested outcome differences between the active VNS and sham VNS groups at each level of the potential prescriptive predictors. These analyses produced odds ratios (ORs) and 95% confidence intervals (CIs), quantifying degree of outcome differentiation between active VNS and sham VNS across subgroups defined by potential predictive patient characteristics (eg, history of electroconvulsive therapy [ECT]). Subgroups with a lower bound of the 95% CI >1 were considered to exhibit statistically significant separation favoring active VNS over sham. Additionally, subgroups with an OR point estimate >1.87 demonstrated a numerically greater observed treatment effect compared to the overall sample. In other words, these analyses identified patient subgroups that consistently demonstrated greater benefit with active VNS compared to sham VNS, as indicated by a lower bound of the 95% CI >1. Moreover, the magnitude of this effect was equal to or greater than that observed across the full sample. Notably, subgroup identification did not require a statistically significant difference between the identified subgroup and its counterpart without the patient feature (eg, with vs without history of ECT exposure).
The number needed to treat (NNT) was calculated for the prescriptive analyses. The observed proportions of patients achieving a positive outcome on the tripartite metric were recorded separately for the active VNS and sham VNS groups. The NNT was derived as the reciprocal of the absolute difference in event rates between the two groups.
The 33 variables examined as potential predictors are listed in Supplementary Table 1. If the baseline value of the variable was significantly associated with the composite end point, it was identified as a prognostic variable. If the baseline value of a variable was significantly associated with a differential probability of response to the composite end point, favoring active vs sham VNS, it was identified as a prescriptive variable. Each of the 33 variables could fall into one of following categories: neither prognostic nor prescriptive, both prognostic and prescriptive, only prognostic, or only prescriptive.
Variables such as ethnicity were excluded from the analysis if one level comprised more than 90% of the sample, leaving fewer than 50 patients in another level. A large number of prespecified baseline variables were analyzed to identify factors associated with treatment response, consistent with the trial’s stated objectives. As no correction for multiple comparisons was applied, findings should be interpreted with appropriate caution given the number of statistical tests conducted. Two- tailed P values <0.05 were considered statistically significant. Analyses were conducted using SAS 9.4 (Cary, NC).
RESULTS
The report presents findings from all 493 patients with MDD who were randomized, including 249 patients in the active VNS group and 244 patients in the sham VNS group.
Prognostic Predictors of Outcome
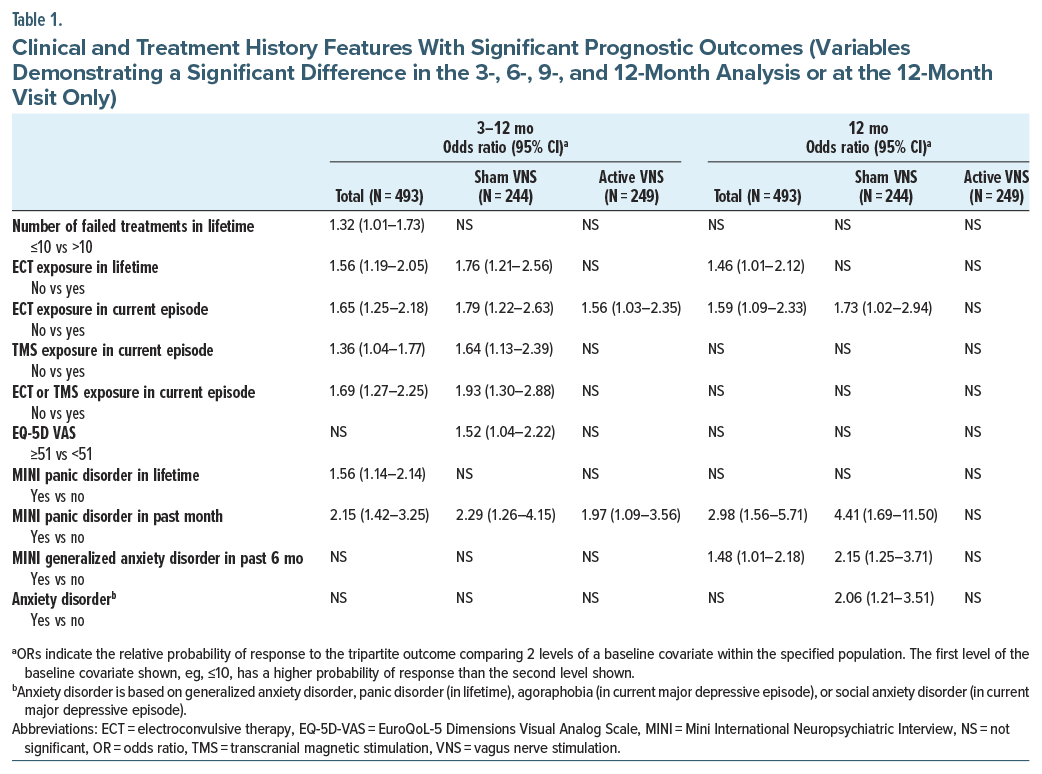
Table 1 presents the baseline variables that were significantly associated with benefit in the GLMM analyses, as defined by the tripartite metric using months 3–12 or only month 12. These variables are a subset of the 33 potential predictors examined (Supplementary Table 1 and Supplementary Table 2).
Multiple variables related to treatment history demonstrated consistent prognostic predictive power. Despite the sample being marked TRD, patients with more than 10 failed antidepressant treatments or a lifetime history of ECT or transcranial magnetic stimulation (TMS) in the current episode had poorer outcomes. Among psychiatric comorbidities, a current or historical panic disorder and generalized anxiety disorder were predictors of better outcome.
Prescriptive Predictors of Outcome
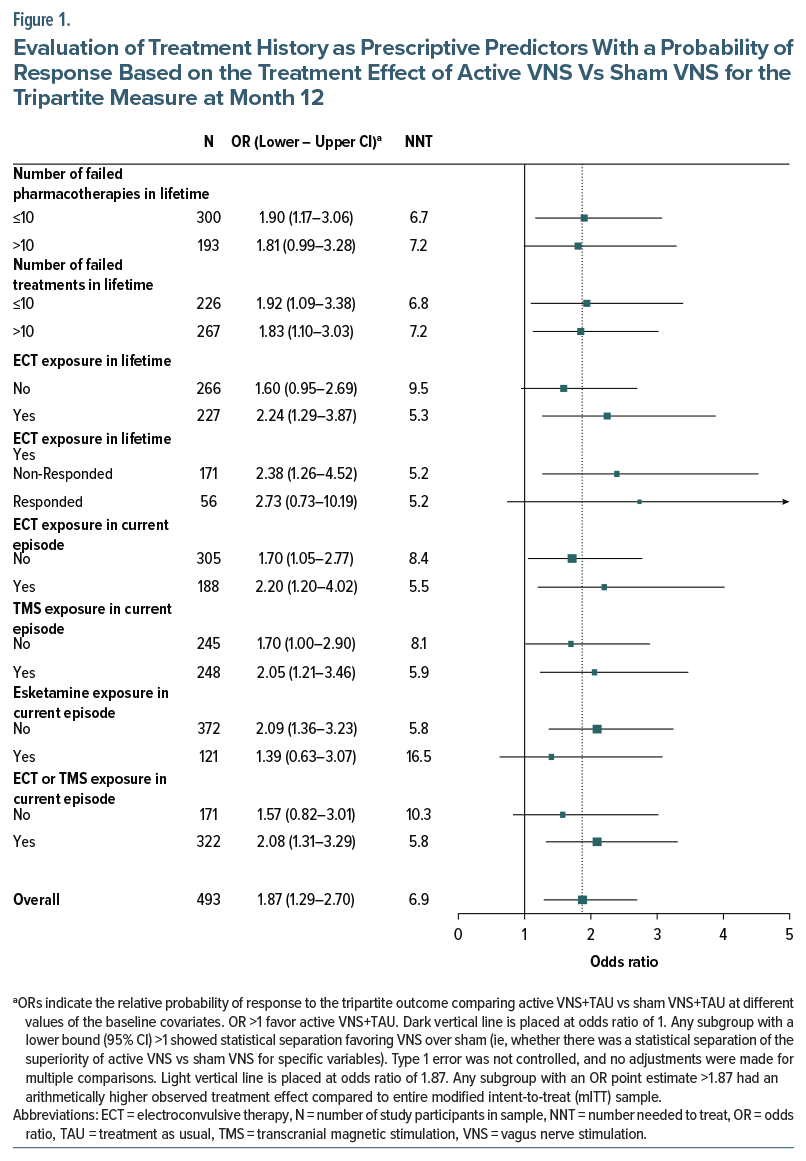
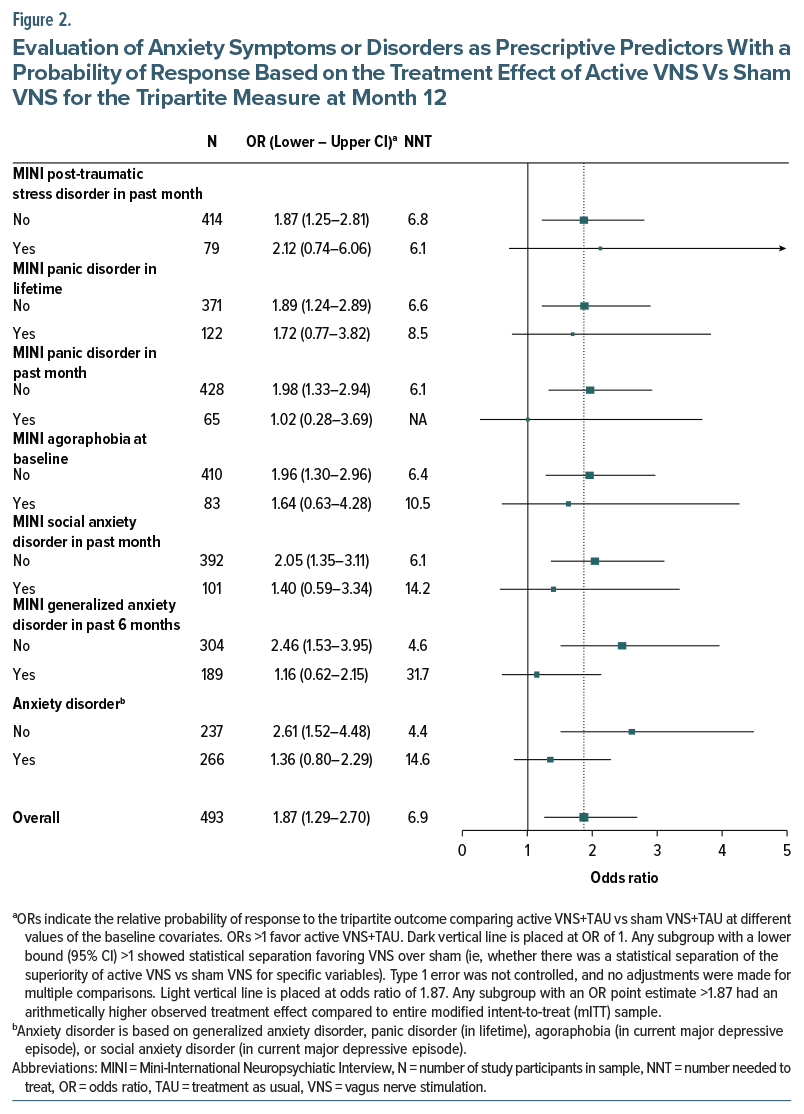
Across the 66 levels of the 33 tested variables, all 66 subgroups (100%) had an OR >1, indicating that greater benefit in the active VNS group vs the sham VNS group was generally seen in this sample for the tripartite outcome (Supplementary Table 3). Figure 1 and Figure 2 show the relationship between the presence or absence of selected treatment history features (Figure 1) or selected anxiety disorders based on the MINI (Figure 2) and the magnitude of the treatment group difference (active VNS vs sham VNS) at the month 12 visit. The differential treatment effect is represented by the OR within each subgroup.
Use of ECT, whether lifetime or in the current episode, was associated with a greater benefit with active vs sham VNS (Figure 1). This effect was observed both in patients with positive and negative response to ECT, as lack of exposure to ECT was linked to a less positive separation. Similarly, treatment with TMS in the current episode was associated with a greater benefit from active vs sham VNS. Thus, exposure to either ECT or TMS was associated with greater differential benefit of active over sham VNS (OR 2.08, 95% CI, 1.31–3.29, NNT=5.8). In contrast, the use of esketamine (current episode; approximately 25% of the sample) was associated with less of a differential benefit with active vs sham VNS.
Why Are Some Variables Both Prognostic and Prescriptive?
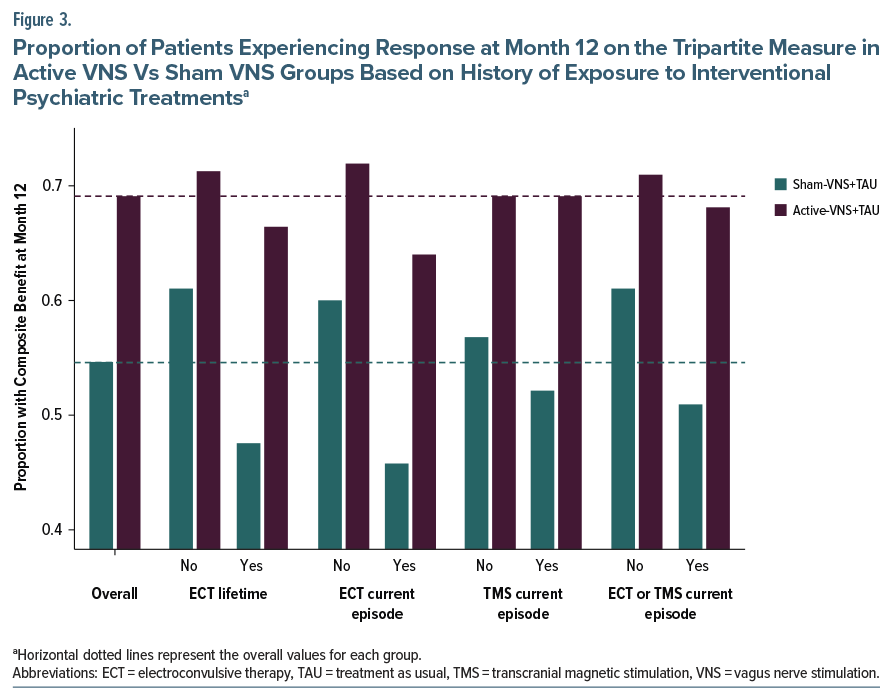
Figure 3 presents the proportion of patients at month 12 with a positive outcome on any dimension of the composite tripartite measure, separately for the active VNS and sham VNS groups, and as a function of exposure to ECT, TMS, or either of these interventional procedures. Both treatment groups showed the same relationship, with exposure to interventional treatments associated with poorer outcomes. However, this effect was especially pronounced in the sham VNS group, so that the treatment group differences were greatest for those with poorer prognosis, ie, the patients with exposure to these interventions. For example, patients in the active VNS group had similar rates of positive outcome regardless of exposure to TMS or ECT, whereas the sham VNS group with prior interventional treatment had an especially poor outcome.
DISCUSSION
This sample of MDD patients was among the most chronically ill, treatment-resistant, and functionally impaired ever studied with any intervention, averaging 18 years duration for the current depressive episode, history of an average of 13 failed lifetime antidepressant treatments, and with 75% unemployed. These marked TRD patients, as a group, were unexposed to relatively few available treatment options, other than VNS at study entry. In this marked TRD sample, we sought to identify the baseline patient features that predicted course of the illness with or without treatment with adjunctive VNS (prognostic predictors) and the patient features that specifically predicted greater benefit from active vs sham VNS (prescriptive predictors).
Treatment history features had consistent and robust prognostic and prescriptive associations with clinical outcome despite requiring a minimum of 4 failed antidepressant trials in the current episode for study entry. Specifically, patients with previous ECT or TMS (interventional psychiatry) treatments had poorer outcomes in the overall population and especially in the sham VNS group (Table 1) (prognostic prediction). The mechanisms underlying this pattern are not known. However, in patients with TRD, ECT, and TMS each exert greater efficacy than further medication trials.44–46 These treatment failures likely represent a greater degree of or different types of treatment resistance that ordinarily portend poor prognosis that can be ameliorated with VNS. These same patients, however, had superior benefit with active vs sham VNS (Figure 1) (prescriptive prediction). In essence, receipt of these interventional psychiatry treatments identified a subgroup especially likely to show lower levels of improvement on the tripartite outcome metric during the trial. However, this poorer outcome as a function of treatment history, while pronounced in the sham VNS group, was largely absent in the active VNS group (Figure 3). Essentially, active VNS appeared to mitigate the adverse/negative prognostic effects of history of treatment with ECT or TMS. Thus, the patient subgroups manifesting the poorest clinical outcome during this trial, such as those with a lifetime history of ECT exposure or failure of TMS or ECT in the current episode, were also the same subgroups that reliably benefited more from active VNS than sham VNS, with NNTs of about 5 to 6.
Another set of patient features showed both prognostic and prescriptive associations. The presence of DSM-5-TR classified anxiety disorders (including panic disorder and generalized anxiety disorder) at baseline was linked to superior clinical outcomes during the trial (Table 1). Additionally, the absence of a comorbid anxiety disorder was prescriptive of superior outcome with active vs sham VNS. These findings are consistent with a large body of research indicating inferior clinical outcomes with pharmacologic treatments of MDD among patients with comorbid anxiety disorders.47,48 The prognostic associations, linking these comorbid conditions to superior outcomes independent of the VNS intervention, suggest that these subgroups, at least in the context of marked TRD, indicate greater nonspecific therapeutic change than patients without a comorbid anxiety disorder.
It was noteworthy that, of the 66 variables examined, no subgroup demonstrated better outcomes in the VNS-sham group. Furthermore, we restricted the identification of prescriptive variables to those having significant prognostic value. As seen in Supplementary Table 3, several variables that lacked prognostic significance had prescriptive value, particularly those indicative of more severe illness at baseline, such as higher depression rating scores and a history of a suicide attempt or suicidal ideation. This suggests that adjunctive VNS therapy may have particularly better efficacy with more severe illness.
This study used a novel tripartite metric to classify clinical outcomes using conjoint measures of depressive symptoms, psychosocial function, and QoL. We previously reported that the 3 components (QIDS-C PR, Mini Q-LES-Q, and WPAI Item 6) in the tripartite measure each showed significant separation of the active VNS vs sham VNS groups.16,28,49 These findings supported the rationale for constructing a composite measure using the 3 components simultaneously. We showed that this composite identified the largest group of patients as having meaningful benefit and strongly covaries with clinician global judgment.22
This patient-centric metric has been validated against blinded clinician ratings based on the Clinical Global Impression-Improvement, and is sensitive to between- treatment group differences in the trial.22 An analogous tripartite metric based on symptoms, function, and quality of life, when applied to the data from the Sequenced Treatment Alternatives to Relieve Depression (STAR-D) study,1 was a better predictor of relapse during continuation phase treatment with citalopram than any single domain.50 By capturing improvement in any of 3 critical domains, an advantage of the tripartite metric is that it classifies a larger number of patients as obtaining positive clinical outcome than any single domain measures. The tripartite metric may be advantageous in marked TRD patients in whom small, but clinically meaningful changes, may be more reasonable outcome measures.3,4 Furthermore, this method may be more reflective of the benefit patients perceive since improvements in function and QoL are often prioritized by patients over symptom change.51
This report revealed that the tripartite measure was useful in identifying patient subgroups with the best long- term course and those most likely to benefit from VNS.
Limitations
This study was exploratory. It screened 66 demographic, diagnostic, symptomatic, and treatment history features to identify prognostic and prescriptive predictors. No correction for multiple comparisons was applied to the data, yet the findings were largely consistent with identifying 2 main sets of variables—treatment history and comorbid anxiety disorders—as having consistent prognostic and prescriptive associations with outcomes. The analyses tested the statistical significance of each patient feature in prognostic prediction (Table 1). However, we did not require a significant interaction between treatment
group and the levels of the patient feature covariate when identifying prescriptive predictors. The aim was to identify the subgroups that showed reliably greater benefit from active vs sham VNS. This does not entail that the different levels of a feature (eg, positive vs negative history of ECT) differed in terms of outcomes. The prognostic and prescriptive predictors we identified derive from treatment of marked TRD patients and may not apply to other samples.
CONCLUSIONS
In a marked TRD sample, patients with a history of treatment with either ECT or TMS had especially poor outcomes when receiving sham VNS with TAU for 1 year. Active VNS appeared to mitigate this negative prognostic effect, as these same subgroups showed significant differential benefit with adjunctive active VNS relative to sham VNS. The absence of a comorbid anxiety disorder was also linked to superior benefit in the active VNS group vs sham VNS group. These prognostic and prescriptive predictors may help guide clinical decision- making when considering VNS.
Article Information
Published Online: July 14, 2025. https://doi.org/10.4088/JCP.25m15850
© 2025 Physicians Postgraduate Press, Inc.
Submitted: March 5, 2025; accepted May 14, 2025.
To Cite: Aaronson ST, Conway CR, Gordon C, et al. Prognostic and prescriptive predictors of treatment response to adjunctive VNS therapy in major depressive disorder: A RECOVER trial report. J Clin Psychiatry 2025;86(3):25m15850.
Author Affiliations: Institute for Advanced Diagnostics and Therapeutics, Sheppard Pratt Health System, Baltimore, Maryland (Aaronson); Department of Psychiatry, Washington University in St. Louis, St. Louis, Missouri (Conway, Hristidis, Brown, Kriedt); LivaNova PLC (or a subsidiary), London, Great Britain, United Kingdom (Gordon, Lee, Tran, Bunker); Brain Stimulation Division, Department of Psychiatry, Medical University of South Carolina, Charleston, South Carolina (George); Ralph H. Johnson VA Medical Center, Charleston, South Carolina (George); Department of Psychiatry and Behavioral Sciences, Rush University Medical Center, Chicago, Illinois (Zajecka); Psychiatric Medicine Associates, LLC, Skokie, Illinois (Zajecka); Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia (Riva-Posse); Center for Anxiety and Depression, Mercer Island, Washington (Dunner); Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, Washington (Dunner); UAB Depression and Suicide Center, Department of Psychiatry and Behavioral Neurobiology, Heersink School of Medicine, The University of Alabama at Birmingham, Birmingham, Alabama (Macaluso); Department of Psychiatry and Health Behavior, Medical College of Georgia at Augusta University, Augusta, Georgia (Rosenquist); Department of Psychiatry, University Neuropsychiatric Institute, University of Utah, Salt Lake City, Utah (Mickey); Department of Anesthesiology, University of Utah, Salt Lake City, Utah (Mickey); Department of Psychiatry, University of Michigan, Ann Arbor, Michigan (Mickey); Center for Neuromodulation in Depression and Stress, Department of Psychiatry and Behavioral Sciences, Department of Radiology, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania (Sheline); Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, South Carolina (Sackeim); Duke-NUS Medical School, Singapore (Rush); Curbstone Consultant LLC, Dallas, Texas (Rush).
Corresponding Author: Scott T. Aaronson, MD, Institute for Advanced Diagnostics and Therapeutics, Sheppard Pratt Health System, 6501 N. Charles St, Baltimore, MD 21204 ([email protected]).
Relevant Financial Relationships: Dr. Aaronson is a consultant to Genomind, LivaNova, Janssen, Neuronetics, and Sage Therapeutics and has received research support from Compass Pathways and Neuronetics. Dr. Conway has received research support from the American Foundation for Suicide Prevention, Assurex Health, August Busch IV Foundation, Barnes-Jewish Hospital Foundation, LivaNova, National Institute of Mental Health, and the Taylor Family Institute for Innovative Psychiatric Research; consulted for LivaNova and Sage Therapeutics; and was a part-time employee at the John Cochran VA Medical Center in St. Louis. Mr. Gordon, Ms. Lee, and Ms. Tran own stocks and are employees of LivaNova. Dr. George was a paid consultant for Abbott (Boston Scientific), Sooma, and Neurolief; was an unpaid consultant for Brainsway and Magnus Medical; and received research grants for MUSC from LivaNova and Magnus Medical. Dr. Zajecka receives research support from Boehringer-Ingelheim, Compass Pathways, Hoffman-LaRoche, Johnson and Johnson (Janssen), LivaNova, Otsuka, Neurocrine Bioscience, and Sage Therapeutics and received consulting fees from Alphasigma USA and Johnson and Johnson (Janssen). Dr. Riva-Posse has received consulting fees from LivaNova, Abbott Neuromodulation, Johnson & Johnson, and Motif, and received honoraria from Elsevier. Dr. Dunner receives payment for clinical services for a former research patient from LivaNova; has been a speaker for Janssen (esketamine nasal spray); and conducts forensic consultations, independent medical evaluations, and legal testimony for various firms. Dr. Macaluso received grant support from Alto, Boehringer- Ingelheim, LivaNova, Janssen, Merck, Neurocrine, Otsuka, SAGEl, PCORI, and NIH/ NIMH with all clinical trial payments made to the University of Alabama at Birmingham; served as a paid advisor with UAB’s permission with CME Institute, NuSachi Labs, PharmaTher, edYOU, Residents Medical, Tactical Mind Solutions, and the University of Missouri; from April 2019 to June 2020. Dr. Macaluso was a member of the speaker bureau for Janssen (Spravato/esketamine) and has also received royalties from American Psychiatric Association Publishing and Springer Nature for textbooks published. Dr. Rosenquist has received research support in the conduct of the RECOVER trial and has received honoraria for participation in a LivaNova medical advisory board meeting. Dr. Mickey has received research support from the National Institutes of Health, National Science Foundation, Wellcome Leap, Patient Centered Outcomes Research Institute, Health Rhythms, LivaNova, Compass Pathways, and Abbott; and consulting fees from Inside Edge, VML, Atheneum, Guidepoint, Kx Advisors, and S2N Health. Dr. Sheline has received research support from the National Institutes of Mental Health and the Milken Foundation and honoraria from the Brain and Behavior Research Foundation. Dr. Hristidis received partial research support from NIMH Grant NIH R25 MH112473-01 and is a PGY4 resident at Washington University/Barnes Jewish Hospital Consortium. Dr. Bunker is a former employee and a current consultant of LivaNova. Dr. Sackeim serves as a scientific adviser and receives consulting fees from Cerebral Therapeutics, Holmusk Technologies, LivaNova, MECTA Corporation, NeuroInsights, Neurolief, Neuronetics, Parow Entheobiosciences, and SigmaStim; receives honoraria and royalties from Elsevier and Oxford University Press; is the inventor of nonremunerative US patents for Focal Electrically Administered Seizure Therapy (FEAST), titration in the current domain in ECT, and the adjustment of current in ECT devices, each held by the SigmaStim Corporation; and is also the originator of magnetic seizure therapy (MST). Dr. Rush has received consulting fees from Compass, Curbstone Consultant, Emmes, Evecxia Therapeutics, Holmusk Technologies, ICON, Johnson and Johnson (Janssen), LivaNova, MindStreet, Neurocrine Biosciences, Otsuka-US; speaking fees from LivaNova, Johnson and Johnson (Janssen), and Wolters Kluwer Health; royalties from Guilford Press and UT Southwestern Medical Center (for the Inventory of Depressive Symptoms and its derivatives); named coinventor on US Patent 7,795,033 (Methods to Predict the Outcome of Treatment with Antidepressant Medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, Paddock S, Wilson AS) and US Patent 7,906,283 (Methods to Identify Patients at Risk of Developing Adverse Events During Treatment with Antidepressant Medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, Paddock S). Mr. Brown and Mr. Kriedt have no conflicts of interest to declare.
Funding/Support: This article reports findings from assessments obtained in a clinical study conducted by LivaNova PLC, the developer and manufacturer of the Vagus Nerve Stimulation (VNS) therapy system.
Role of the Sponsor: Data analysis and drafting of the report were supported by LivaNova. While the staff at LivaNova provided comments on the manuscript, the final approval of the manuscript’s content and the decision to submit were solely determined by the authors.
Members of the RECOVER study group: Alivation Research LLC, Lincoln, NE (Walter Duffy); AMR-Baber Research Inc, Naperville, IL (Riaz Baber); APG Research, LLC, Orlando, FL (Morteza Nadjafi); ATP Clinical Research, Inc, Costa Mesa, CA (Gustavo Alva); Beacon Medical Group Behavioral Health, South Bend, IN (Suhayl Nasr); Carilion Clinic, Roanoke, VA (Anita Kablinger); Center for Anxiety and Depression, Mercer Island, WA (David L. Dunner); The Center for Neuropsychiatry and Brain Stimulation (CNBS), ARC Health, Durham, NC (Sandeep Vaishnavi); Charak Center for Health and Wellness, Garfield Heights, OH (Rakesh Ranjan); DTMS Center, LLC, West Palm Beach, FL (Aron Tendler); Dent Neurological Institute, Amherst, NY (Horacio Capote); Emory University School of Medicine, Atlanta, GA (Patricio Riva-Posse); Florida Behavioral Medicine, Largo, FL (Ashok Patel); Florida Center for TMS, Orlando, FL (Todd Broder); Florida Center for TMS, St. Augustine, FL (Heather Luing); Galiz Research LLC, Hialeah, FL (Jose Gamez); Hapworth Research, Inc, New York, NY (William Hapworth); Healthy Perspectives, Nashua, NH (Hisham Hafez); Icahn School of Medicine at Mount Sinai, New York, NY (James Murrough); Kaizen Brain Center, La Jolla, CA (Mohammed Ahmed); Marshall Psychiatry, Huntington, WV (Suzanne Holroyd); Keck School ofMedicine, University of California Los Angeles, Los Angeles, CA (Ashraf Elmashat); Massachusetts General Hospital, Boston, MA (Cristina Cusin); Medical College of Georgia at Augusta University, Augusta, GA (Peter Rosenquist); Medical University of South Carolina, Charleston, SC (Mark George); Michigan Clinical Research Institute, Ann Arbor, MI (Rajaprabhakaran Rajarethinam); Mindful Behavioral Health PLLC, Boca Raton, FL (Ivan Cichowicz); Neuroscience and TMS Treatment Center, Brentwood, TN (Jonathan Becker); Neuropsychiatric Associates at Woodstock Research Center, Woodstock, VT (Susan Smiga); Nova Psychiatry Inc., Orlando, FL (David Medina); The Ohio State University, Columbus, OH (Kevin Reeves); OU Physicians, Tulsa, OK (Ondria Gleason); Precise Research Centers, Flowood, MS (Joseph Kwentus); Psych Atlanta, Marietta, GA (Michael Banov); PsychCare Consultants Research, St. Louis, MO (Mohd Malik); Psychiatry Care and Research Center, O’Fallon, MO (John Canale); Rush University Medical Center, Chicago, IL and Psychiatric Medicine Associates, Skokie, IL (John Zajecka); Offices of Psychiatry and Counseling Services, Moosic, PA (Matthew Berger); Seattle Neuropsychiatric Treatment Center, Seattle, WA (Rebecca Allen); SF- Care, Inc, San Rafael, CA (Jason Bermak); Sheppard Pratt Health System Inc, Baltimore, MD (Scott T. Aaronson); Signature Research Associates, Inc, Fairlawn, OH (Anand Chaturvedi); Southern Illinois University School of Medicine, Springfield, IL (Jeffrey Bennett); Stedman Clinical Trials LLC, Tampa, FL (Mary Stedman); Stony Brook University Hospital, Stony Brook, NY (Lucian Manu); Syrentis Clinical Research, Santa Ana, CA (John Duffy); Texas Tech University Health Science Center, El Paso, TX (Peter Thompson); Trinity Medical, Lewiston, NY (Alfred Belen III); University of Alabama at Birmingham, Birmingham, AL (Matthew Macaluso); University of Alabama Huntsville Regional Medical Center, Huntsville, AL (Richard Shelton); University of California San Diego, San Diego, CA (Mounir Soliman); University of Minnesota, Minneapolis, MN (Ziad Nahas); University of Missouri, Columbia, MO (Muaid Ithman); University of Pennsylvania Perelman School of Medicine, Philadelphia, PA (Yvette Sheline); University of Texas Health Austin, Mulva Clinic for the Neurosciences, Austin, TX (Julie Farrington); The University of Texas Health Science Center, Houston, TX (João de Quevedo), The University of Utah Neuropsychiatric Institute, Salt Lake City, UT (Brian Mickey); University of Wisconsin, Madison, WI (Steven Garlow); Washington School of Medicine, St. Louis, MO (Donald Bohnenkamp).
Acknowledgment: The authors are grateful to the patients and their families who participated in the RECOVER study and to the US Centers for Medicare & Medicaid Services (CMS) for providing financial support for VNS Therapy devices and implantation surgeries. The authors also thank Karishma L. Manzur, PhD of Lenimen Consulting, LLC, for providing medical writing assistance.
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- Baseline participant outcomes from the randomized vagus nerve stimulation (VNS) RECOVER trial combining symptom, quality of life, and function were analyzed to determine prognostic and prescriptive outcome predictors.
- History of electroconvulsive therapy (ECT) use in lifetime and current episode or transcranial magnetic stimulation (TMS) use in current episode was prognostic of poorer outcomes across the total sample and superior benefit from active vs sham VNS.
- Adjunctive VNS may be especially effective in marked TRD patients who have not benefitted from ECT or TMS.
References (51)

- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Zhdanava M, Pilon D, Ghelerter I, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. 2021;82(2):20m13699. PubMed CrossRef
- McIntyre RS, Alsuwaidan M, Baune BT, et al. Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry. 2023;22(3):394–412. PubMed CrossRef
- McAllister-Williams RH, Arango C, Blier P, et al. The identification, assessment and management of difficult-to-treat depression: an international consensus statement. J Affect Disord. 2020;267:264–282. PubMed CrossRef
- Amital D, Fostick L, Silberman A, et al. Physical co-morbidity among treatment resistant vs. treatment responsive patients with major depressive disorder. Eur Neuropsychopharmacol. 2013;23(8):895–901. PubMed CrossRef
- Amos TB, Tandon N, Lefebvre P, et al. Direct and Indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a US commercial claims database. J Clin Psychiatry. 2018;79(2):17m11725. PubMed CrossRef
- Bergfeld IO, Mantione M, Figee M, et al. Treatment-resistant depression and suicidality. J Affect Disord. 2018;235:362–367. PubMed CrossRef
- Huang SS, Chen HH, Wang J, et al. Investigation of early and lifetime clinical features and comorbidities for the risk of developing treatment-resistant depression in a 13-year nationwide cohort study. BMC Psychiatry. 2020;20(1):541. PubMed CrossRef
- Rush AJ, Aaronson ST, Demyttenaere K. Difficult-to-treat depression: a clinical and research roadmap for when remission is elusive. Aust N Z J Psychiatry. 2019;53(2):109–118. PubMed CrossRef
- Lundberg J, Cars T, Lööv SÅ, et al. Association of treatment-resistant depression with patient outcomes and health care resource utilization in a population-wide study. JAMA Psychiatry. 2023;80(2):167–175. PubMed CrossRef
- Rush AJ, Sackeim HA, Marangell LB, et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58(5):355–363. PubMed CrossRef
- Dunner DL, Rush AJ, Russell JM, et al. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry. 2006;67(5):688–695. PubMed CrossRef
- Conway CR, Aaronson ST, Sackeim HA, et al. Clinical characteristics and treatment exposure of patients with marked treatment-resistant unipolar major depressive disorder: a RECOVER trial report. Brain Stimul. 2024;17(2):448–459. PubMed CrossRef
- Conway CR, Olin B, Aaronson ST, et al. A prospective, multi-center randomized, controlled, blinded trial of vagus nerve stimulation for difficult to treat depression: a novel design for a novel treatment. Contemp Clin Trials. 2020;95:106066. PubMed CrossRef
- Rush AJ, Thase ME. Improving depression outcome by patient-centered medical management. Am J Psychiatry. 2018;175(12):1187–1198. PubMed CrossRef
- Rush AJ, Conway CR, Aaronson ST, et al. Effects of vagus nerve stimulation on daily function and quality of life in markedly treatment-resistant major depression: findings from a one-year, randomized, sham-controlled trial. Brain Stimul. 2025;18(3):690–700. PubMed CrossRef
- Rush AJ, Wisniewski SR, Warden D, et al. Selecting among second-step antidepressant medication monotherapies: predictive value of clinical, demographic, or first-step treatment features. Arch Gen Psychiatry. 2008;65(8):870–880. PubMed CrossRef
- Haskins R, Rivett DA, Osmotherly PG. Clinical prediction rules in the physiotherapy management of low back pain: a systematic review. Man Ther. 2012;17(1):9–21. PubMed CrossRef
- Cook CE, Learman KE, O’Halloran BJ, et al. Which prognostic factors for low back pain are generic predictors of outcome across a range of recovery domains? Phys Ther. 2013;93(1):32–40. PubMed
- Zeeck A, von Wietersheim J, Weiss H, et al. Prognostic and prescriptive predictors of improvement in a naturalistic study on inpatient and day hospital treatment of depression. J Affect Disord. 2016;197:205–214. PubMed CrossRef
- Fournier JC, DeRubeis RJ, Shelton RC, et al. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol. 2009;77(4):775–787. PubMed CrossRef
- Conway CR, Rush AJ, Gordon C, et al. An examination of symptoms, function and quality of life as conjoint clinical outcome domains for treatment-resistant depression. J Anxiety Disord. 2025;10:100121. CrossRef
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
- Hergueta T, Weiller E. Evaluating depressive symptoms in hypomanic and manic episodes using a structured diagnostic tool: validation of a new Mini International Neuropsychiatric Interview (M.I.N.I.) module for the DSM-5 ’With Mixed Features’ specifier. Int J Bipolar Disord. 2013;1:21. PubMed CrossRef
- Sheehan, DV, Lecrubier, Y, Sheehan, KH, et al, The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–57;quiz 34–57. PubMed
- Sackeim HA, Aaronson ST, Bunker MT, et al. The assessment of resistance to antidepressant treatment: rationale for the Antidepressant Treatment History Form: Short Form (ATHF-SF). J Psychiatr Res. 2019;113:125–136. PubMed CrossRef
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. PubMed CrossRef
- Conway CR, Aaronson ST, Sackeim HA, et al. Vagus nerve stimulation in treatment-resistant depression: a one-year, randomized, sham-controlled trial. Brain Stimul. 2025;18(3):676–689. PubMed CrossRef
- Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. PubMed CrossRef
- Rush AJ, Carmody TJ, Reimitz P. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and Self-Report (IDS-SR) Ratings of Depressive Symptoms. Int J Methods Psychiatric Res. 2000;9:45–95.
- IDS-QIDS. Inventory of Depressive Symptomatology (IDS) and Quick Inventory of Depressive Symptomatology (QIDS). 2022. Accessed 20 November 2022. http://ids-qids.org/administration.html
- Cameron IM, Crawford JR, Cardy AH, et al. Psychometric properties of the Quick Inventory of Depressive Symptomatology (QIDS-SR) in UK primary care. J Psychiatr Res. 2013;47(5):592–598. PubMed CrossRef
- Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326. PubMed
- Rush AJ, South CC, Jha MK, et al. Toward a very brief quality of life enjoyment and Satisfaction Questionnaire. J Affect Disord. 2019;242:87–95. PubMed CrossRef
- Jha MK, Minhajuddin A, Greer TL, et al. Early improvement in work productivity predicts future clinical course in depressed outpatients: findings from the CO-MED Trial. Am J Psychiatry. 2016;173(12):1196–1204. PubMed CrossRef
- Reilly MC. Work Productivity and Activity Impairment Questionnaire (WPAI). Reilly Associates Health Outcomes Research; 2019. http://www.reillyassociates.net.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. PubMed CrossRef
- Endicott J, Rajagopalan K, Minkwitz M, et al. A randomized, double-blind, placebo-controlled study of quetiapine in the treatment of bipolar I and II depression: improvements in quality of life. Int Clin Psychopharmacol. 2007;22(1):29–37. PubMed CrossRef
- Ford JH, Ye W, Ayer DW, et al. Validation and meaningful within-patient change in Work Productivity and Activity Impairment Questionnaire (WPAI) for episodic or chronic migraine. J Patient-Rep Outcomes. 2023;7(1):34. PubMed CrossRef
- Tillett W, Lin CY, Zbrozek A, et al. A threshold of meaning for work disability improvement in psoriatic arthritis measured by the Work Productivity and Activity Impairment Questionnaire. Rheumatol Ther. 2019;6(3):379–391. PubMed CrossRef
- Dunlop BW, Kelley ME, Aponte-Rivera V, et al. Effects of patient preferences on outcomes in the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study. Am J Psychiatry. 2017;174(6):546–556. PubMed CrossRef
- Zhang C, Virani S, Mayes T, et al. Toward a definition of “No meaningful benefit” from antidepressant treatment: an equipercentile analysis with cross-trial validation across multiple rating scales. J Clin Psychiatry. 2022;83(4):21m14239. PubMed CrossRef
- Conway CR, Kumar A, Xiong W, et al. Chronic vagus nerve stimulation significantly improves quality of life in treatment-resistant major depression. J Clin Psychiatry. 2018;79(5):18m12178. PubMed CrossRef
- Papakostas GI, Trivedi MH, Shelton RC, et al. Comparative effectiveness research trial for antidepressant incomplete and non-responders with treatment resistant depression (ASCERTAIN-TRD) a randomized clinical trial. Mol Psychiatry. 2024;29(8):2287–2295. PubMed CrossRef
- Dalhuisen I, van Oostrom I, Spijker J, et al. rTMS as a next step in antidepressant nonresponders: a randomized comparison with current antidepressant treatment approaches. Am J Psychiatry. 2024;181(9):806–814. PubMed CrossRef
- Pagnin D, de Queiroz V, Pini S, et al. Efficacy of ECT in depression: a meta-analytic review. J ECT. 2004;20(1):13–20. PubMed CrossRef
- Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–351. PubMed CrossRef
- Dold M, Bartova L, Souery D, et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders - results from a European multicenter study. J Psychiatr Res. 2017;91:1–13. PubMed CrossRef
- Sackeim HA, Conway CR, Aaronson ST, et al. Characterizing the effects of vagus nerve stimulation on symptom improvement in markedly treatment- resistant major depressive disorder: a RECOVER trial report. J Affect Disord. 2025;380:135–145. PubMed CrossRef
- Ishak WW, Greenberg JM, Cohen RM. Predicting relapse in major depressive disorder using patient-reported outcomes of depressive symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression (IBI-D). J Affect Disord. 2013;151(1):59–65. PubMed CrossRef
- Zimmerman M, McGlinchey JB, Posternak MA, et al. How should remission from depression be defined? The depressed patient’s perspective. Am J Psychiatry. 2006;163(1):148–150. PubMed CrossRef
This PDF is free for all visitors!