Abstract
Breast cancer is the commonest form of cancer among women. Literature suggests that women with schizophrenia are less likely to be screened for breast cancer and that, among women with schizophrenia who develop breast cancer, mortality rates are higher. This article examines recent meta-analyses and recent observational studies on the risk of breast cancer in women with schizophrenia, especially in the context of treatment with first generation and second generation antipsychotic (FGA, SGA) drugs. This article also examines the most recent observational study in detail to help readers better understand how to critically appraise this and, by extension, other studies in the field. In summary, studies in the field suggest that there is a small but statistically significant increase in the risk of breast cancer in women with schizophrenia relative to women without psychiatric disorders as well as relative to women with other psychiatric disorders. Women who appear to be most at risk are those in the perimenopausal age range and those with several years of exposure to FGA or prolactin raising antipsychotics. Whereas the possibility of residual confounding in observational studies precludes ascribing a causal role for antipsychotics, given that FGA and prolactin-raising SGA are associated with other adverse effects, it seems reasonable, wherever possible, to prefer SGA over FGA, and prolactin-sparing over prolactin-raising antipsychotics. Women with schizophrenia, and especially those who use prolactin raising antipsychotics for long periods, should be monitored for the risk of breast cancer as a special part of monitoring general health; and modifiable risk factors for breast cancer should be addressed through appropriate behavioral and pharmacological interventions. Women with schizophrenia comprise a vulnerable population, and their medical health should not be neglected even when caring for their mental health absorbs attention and time. Suggestions are provided for directions for future research.
J Clin Psychiatry 2025;86(3):25f15960
Author affiliations are listed at the end of this article.
Breast cancer is the commonest form of cancer among women.1 The lifetime risk of breast cancer is about 12% among women in the US.2 An earlier article in this column3 examined the risk of breast cancer in the context of schizophrenia and its treatment. In summary, the article3 observed that persons with mental illness are less likely to be screened for cancer in general, and for breast cancer in particular, than persons in the general population; that mammography for breast cancer screening is less frequent among women with schizophrenia than among women without schizophrenia; and that mortality rates for cancer in general, and for breast cancer in particular, are higher in persons with schizophrenia than in controls. The article3 also discussed a Finnish nationwide case-control study4 which found that breast cancer was not related to exposure to prolactin-sparing antipsychotic drugs, regardless of duration of exposure; rather, breast cancer (lobular adenocarcinoma as well as ductal adenocarcinoma) was related to exposure to prolactin-raising antipsychotics, though only when exposure duration was >5 years.
Risk factors for breast cancer include sedentariness, weight gain, and metabolic and endocrinological changes; these risk factors are observed in untreated schizophrenia as well as in association with antipsychotic drug use.3 Prolactin elevation by some antipsychotic drugs is an additional risk factor.4 In patients with schizophrenia, self-care is poor, adherence to healthcare guidance is poor, and medical conditions are neglected. Mental health care tends to become the focus of attention at the expense of health screening and general medical care. As a result, women with schizophrenia may be at increased risk not only of breast cancer but also of late detection of breast cancer; in consequence, prognosis may be poorer.
There have been many studies in the field; these were pooled in at least 4 meta-analyses between 2022 and 20255–8; all 4 meta-analyses had limitations. Several large observational studies have also been published during recent years. In this narrative critical review, findings from 1 meta-analysis and 4 of the observational studies9–12 are summarized, and a fifth, very recent, study13 is discussed in detail; the latter, with a view to help the reader not only receive a take-home message but also understand how to critically read and draw conclusions from research in the field.
Antipsychotic Drugs and Breast Cancer: Meta-Analysis5
Leung et al5 described a systematic review and random effects meta-analysis of observational studies of the association between antipsychotic use (vs nonuse) and new-onset breast cancer. These authors searched 3 electronic databases and reference lists for relevant cohort and case-control studies. They identified 7 studies (pooled N = 1,557,013) that met their search criteria for quantitative analysis. Three out of the 4 cohort studies and 2 out of the 3 case-control studies found a significant association between antipsychotic drug exposure and breast cancer.
In this meta-analysis,5 the pooled hazard ratio (HR) in the 4 cohort studies was 1.39 (95% confidence interval [CI], 1.11–1.73; I 2, 75%) and the pooled odds ratio (OR) in the 3 case-control studies was 1.37 (95% CI, 0.90–2.09; I 2, 93%). The association between antipsychotic treatment and breast cancer was statistically significant only in the cohort studies. Heterogeneity was high to very high in both analyses. A limitation of this meta-analysis is that it did not pool other outcomes as some of the other meta-analyses did (see below).
Meta-Analyses Excluded From Review6-8
Three other meta-analyses have recently been published but are not reviewed in detail here for reasons that are explained. One6 identified 11 relevant studies but pooled data from only 5 without explaining why the other 6 were excluded. Of these 5 studies, 3 were case control studies and 2 were cohort studies. Pooling data from case-control and cohort studies in the same forest plot is poor practice and is discouraged for reasons explained elsewhere.14
A second meta-analysis7 also identified 11 relevant studies and provided breast cancer risks across several contexts: in antipsychotic exposed vs unexposed subjects, for atypical antipsychotics vs typical antipsychotics, for prolactin-raising antipsychotics vs prolactin-sparing antipsychotics, and for antipsychotics at maximum vs minimum doses. Data were also provided for mortality as an outcome related to antipsychotic exposure. However, this meta-analysis also pooled cohort and case-control study data in the same forest plots, making the validity of its conclusions problematic.14
The third and most recently published meta analysis8 identified 15 relevant studies. This was a single-author, unregistered study that searched only 1 database. It is more usual for a systematic review and meta-analysis to be a multiauthor effort with different investigators playing different roles. The many investigators not only distribute roles to handle the large effort necessary but also duplicate roles (in screening titles, abstracts, and full texts, and in data extraction) to identify and eliminate errors during data compilation. Registration of the study protocol at the PROSPERO website requires investigators to choose study outcomes and to outline study procedures in advance; this promotes transparency and reduces the likelihood that investigators may “exercise discretion” in a manner that favors their interests during data extraction, data analysis, and outcome reporting. Searching more than 1 database (recommended, at least 3 databases) is desirable because a single database may not contain all the studies on the subject and because a search may not necessarily identify all the relevant studies in that database; the missed studies may be picked up in searches of other databases. Finally, and also of concern, was that the author pooled data from cohort and case-control studies in the same forest plot; as already stated, this is problematic and is discouraged.14 Separately, this meta-analysis8 presented data from 2 studies that were not referenced.
Cohort Study, South Korea, 20229
Joo et al9 described a cohort study with data for 2008–2018, extracted from the Health Insurance Review Agency database in South Korea. The exposed group (n= 498,970) comprised women who had received a second generation antipsychotic (SGA) drug for at least 30 days. The comparison (unexposed) group (n= 997,940) comprised women, age-matched with exposed women in a 2:1 ratio, who did not receive an SGA. As an aside, it is not clear whether the comparison group excluded women who received first generation antipsychotics (FGAs). The outcome was new-onset breast cancer.
Selected findings from the study are presented in Table 1; all findings are not presented because the women in this sample would have very substantially overlapped with the women in the study by Yang et al,13 which is discussed in detail later in this article. In summary, the mean age of the sample was 53 years. The sample was followed for a mean of approximately 5.5 years. SGA drugs were stratified by prolactin-raising potential, and all 3 strata were associated with a statistically significantly increased risk of breast cancer. The risk was numerically greater in drug strata that were associated with higher likelihood of prolactin elevation.
There are two noteworthy points. One is that the authors presented only crude hazard ratios (HRs); analyses were not adjusted for covariates and confounds. The other is that the authors explicitly stated matching only for age, not for index date. The crude HRs are appropriate for understanding real world risks but not for improving the understanding of risks unique to antipsychotic exposure. And, if matching was not done for date of first antipsychotic prescription, immortal time bias may have favored women in the antipsychotic group, resulting in an underestimation of antipsychotic related risk.
Cohort Study, US, 202410
Kern et al10 described a cohort study with data for 2006–2021, extracted from the MarketScan Medicaid database, US. Exposed groups comprised women initiating treatment with antipsychotic drugs with high and with moderate potential to raise prolactin levels. The comparison group, matched for date of treatment initiation, comprised women initiating treatment with antipsychotic drugs with no or low potential to raise prolactin. There were 16 unique plans of analysis based on risk window end dates, groups for comparison, propensity score matching methods, and outcome definitions; of these, only 5 passed statistical diagnostics. Sample size in the largest analysis was <13,000 women among whom only 80 patients developed the outcome, new-onset breast cancer. Follow up time averaged about 4 years. All analyses were adjusted for a large number of covariates.
In summary, in no analysis was a statistically significant association found between incident breast cancer and antipsychotics with high vs low potential to raise prolactin levels. The HRs in these analyses ranged from 0.96 (95% CI, 0.62–1.48) to 1.28 (95% CI, 0.40–4.07).
Case-Control Study, Sweden, 202411
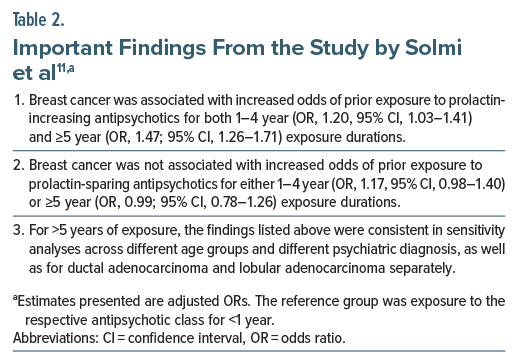
Solmi et al11 described a nested case-control study using data extracted from Swedish nationwide registers for 2010–2021. The sample comprised 1,642 women with major mental illness who developed malignant breast cancer. The mean age of these women was 63 years. These women were matched 1:5 with 8,173 cancer-free control women. Matching was based on age, and also on psychiatric diagnosis and illness duration; the latter helped control for confounding by indication. Analyses were adjusted for medical comorbidities and use of other medications.
Important findings from the study are presented in Table 2. In summary, exposure to prolactin-sparing antipsychotics (clozapine, quetiapine, aripiprazole, brexpiprazole, cariprazine) was not associated with increased risk of malignant breast cancer regardless of duration of exposure. However, exposure to prolactin raising antipsychotics (all other antipsychotics) for >1 year was associated with an increased risk.
Case-Control Study, Taiwan, 202512
Li et al12 described a nested case-control study using data extracted from the Taiwan National Health Insurance Research Database. The sample comprised 1,281 women with major depressive disorder and 283 women with bipolar disorder, all of whom had breast cancer, matched 1:10 with women who did not have breast cancer. Matching was based on age, diagnosis, duration of illness, and duration of follow-up; thus, control for confounding by indication was good. Analyses adjusted for sociodemographic variables, comorbidities, and healthcare utilization.
In different regressions that compared varying durations of exposure with <30 days history of exposure, breast cancer was surprisingly associated with a reduced odds of 1–6 months exposure to SGA as a group (OR, 0.58; 95% CI, 0.44–0.75) and, among all antipsychotic drugs, to olanzapine, risperidone, and chlorpromazine in particular; ORs were not statistically significant for 1–6 months exposure to other SGA, including amisulpride, aripiprazole, clozapine, and quetiapine. ORs were also not statistically significant for FGA as a group and for clotiapine, flupentixol, fluphenazine, haloperidol, loxapine, and sulpiride in particular. For both SGA and FGA, ORs were not statistically significant for 6–12 months and >12 months exposures. Ziprasidone stood out as an exception; the OR was hugely elevated for 6–12 months of exposure (OR, 4.70; 95% CI, 1.47–15.07).
This may be the only study in literature to suggest that antipsychotic exposure is protective against breast cancer, and it is hard to know what to make of the findings of the study (because if exposure to SGAs was truly protective, then exposure for >6 months should also have been protective). Three possibilities are suggested, any or all of which may apply.
The first possibility is that the sample was small and atypical. A second possibility is that the authors presented several dozen analyses without correcting for a false discovery rate. A third possibility is that the lower risks emerged in analyses of only 1–6 month exposures and no protective effect was evident with longer exposure; so, perhaps there was a spike in detection of breast cancer associated with <30 days of exposure, when women were acutely ill and under close medical care. This may have been followed by a trough in detection at 1–6 months partly because of higher detection during the previous interval and lower detection during the current interval (when screening for medical conditions may have dropped as a health priority).
Of note, in this same sample, exposure to valproate, fluoxetine, sertraline, and citalopram during various time intervals was also associated with lower risk of breast cancer. The findings were not statistically significant for other mood stabilizers and antidepressant drugs, including gabapentin, lithium, lamotrigine, carbamazepine, oxcarbazepine, topiramate, bupropion, duloxetine, fluvoxamine, paroxetine, milnacipran, mirtazapine, and venlafaxine. No pattern in exposure duration was evident in the findings.
Miscellaneous Studies15,16
In a small case-control study from Hong Kong,15 563 women with schizophrenia and 109 women with bipolar disorder, all of whom had breast cancer, were matched by age and diagnosis with up to 10 control women without breast cancer. In women with schizophrenia, relative to antipsychotic nonusers, breast cancer was associated with increased odds of FGA exposure only for exposure >5 years; and breast cancer was not associated with increased odds of SGA exposure regardless of the duration of exposure. In women with bipolar disorder, relative to antipsychotic nonusers, breast cancer was associated with increased odds of antipsychotic exposure only for exposure of 1–4 years; this finding was true for FGA and SGA separately.15
A nationwide cohort study in Japan16 found that, among 12,479 antipsychotic-treated patients with schizophrenia, use of progestogens was associated with a markedly elevated risk of breast cancer (HR, 4.47; 95% CI, 1.04–19.18).
Cohort Study, South Korea, 202513
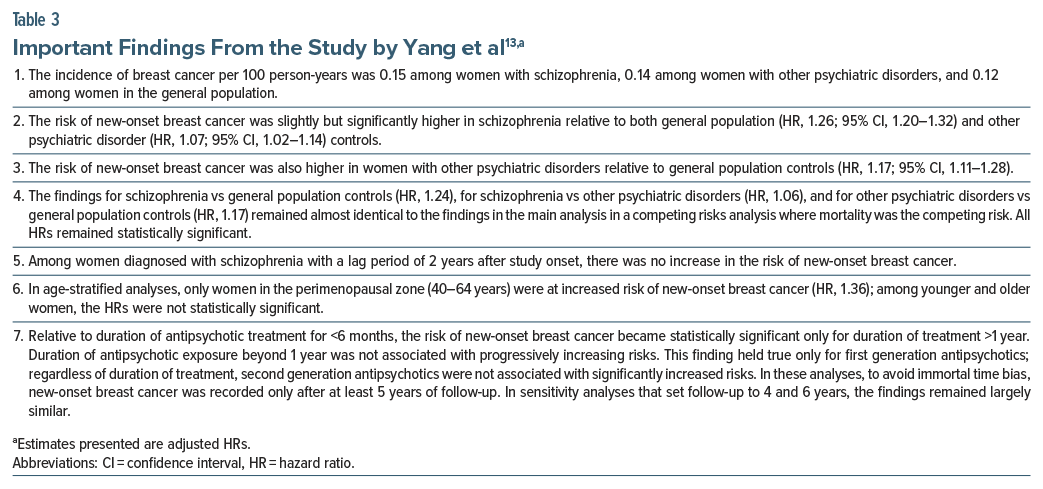
The most recent study was published by Yang et al.13 These authors described a South Korean population based cohort study that examined the risk of breast cancer in women with schizophrenia. The data were drawn from the Korean National Health Information Database for the years 2007–2018. Eligible women were aged 18–80 years. Women with existing or new onset schizophrenia (n = 224,612) were matched 1:1:2 and compared with women with other psychiatric disorders (n = 224,612) and women in the general population who did not have psychiatric disorders (n = 449,224). Matching was based on date of diagnosis of schizophrenia and subject age.
The mean age of the sample was 48 years. Women with schizophrenia had lower socioeconomic status and more medical comorbidity. Breast cancer was operationalized as a new ICD-10 diagnosis of invasive breast cancer or ductal carcinoma in situ. Analyses were adjusted using proxies for socioeconomic status, medical comorbidities, and healthcare utilization. Women in the 3 groups were followed for a mean of about 7 years.
Important findings from the study 13 are presented in Table 3. In summary, this observational study found that, across approximately 7 years of follow-up, there was a small but statistically significant increase in the risk of new-onset breast cancer in women with schizophrenia relative to women without psychiatric disorders (in the general population) as well as relative to women with other psychiatric disorders. The risk was significantly elevated only in women aged 40–64 years and only in connection with use of FGA drugs for a period of 1 year or longer. The risk was not elevated with SGA, regardless of duration of exposure.
Comments on the Cohort Study, South Korea, 202513
The study by Yang et al13 had many strengths but also some limitations. These are discussed in some detail to provide readers with a perspective on how to critically appraise this and, by extension, other studies in the field.
The risk of new-onset breast cancer was elevated in women with schizophrenia relative to women with other psychiatric disorders; this comparison group is important because it helps reduce confounding by indication from unmeasured and unknown sources. Confounding by indication could have been further reduced by comparing women with schizophrenia and women with, specifically, other major mental illness; however, this was not done.
The authors13 did not adjust for other risk factors for breast cancer, such as sedentariness, obesity, smoking, and use of alcohol. However, many of these risk factors are also associated with schizophrenia and the treatment thereof and, in such contexts, these risk factors may be mediators of breast cancer risk in schizophrenia. So, not adjusting for these risk factors could be considered appropriate because we do not adjust for mediators in regression if we wish to examine the total effect of the risk factor of interest (the diagnosis of schizophrenia). As a contrary argument, it could certainly be meaningful to adjust for these mediators for at least two reasons. One is that women could smoke, drink, be physically inactive, and be overweight regardless of the presence of psychiatric disorder; so, adjusting for these risk factors could make sense. The other is that by adjusting for these risk factors, an idea can be obtained whether, after taking into account the contributions of these risk factors, schizophrenia and its treatment with antipsychotic drugs continue to significantly increase the cancer risk through other mediators, including raised serum prolactin. The bottom line is that the authors missed the opportunity to present 3 models: an unadjusted model, a partially adjusted model (what they actually did), and a fully adjusted model (also adjusting for the mediators).
Interestingly, the risk of breast cancer was not increased among women diagnosed with schizophrenia with a lag period of 2 years after study onset. Perhaps this was because these women may have been younger, and because exposures to schizophrenia-related risk factors and risk mediators might therefore have been fewer in number and shorter in duration.
Antipsychotics are used for disorders other than schizophrenia; so, might the validity of the analysis of antipsychotic exposure have been compromised by the use of these drugs in women with other diagnoses? Perhaps not to a meaningful extent because, in the “other psychiatric disorders” group, <10% of women received antipsychotics, and these were mostly SGA drugs.
Women with schizophrenia may have been exposed to antipsychotic drugs for longer than recognized in this study. The authors13 included women who already had schizophrenia on the study start date, and these women would surely have been on treatment. Data on the duration of treatment before the study start date may not have been available for inclusion in the analyses. This is problematic because it could compromise the internal validity of the analyses.
It is surprising that, in the duration of antipsychotic exposure analysis, the authors13 were able to identify a large comparison group of women who had been exposed to antipsychotics for 6 months or less. What treatment might these women have received for a further 4.5 years to render them eligible for analysis in a model that controlled for immortal time bias by setting 5 years of follow-up as the eligibility threshold? The authors provided no explanation.
For reasons related to safeguarding of personal information, the authors13 did not have access to data on individual antipsychotics. However, they did have access to even more personal data, including age, diagnosis, comorbidities, and duration of antipsychotic treatment. What material aspect of privacy would the name of antipsychotic drug violate that class of antipsychotic treatment (FGA vs SGA) did not? Absence of information about specific drug exposure resulted in the inability to analyze the effects of defined daily doses and cumulative dose received on breast cancer risk. Also, effects of specific SGA that raise (eg, risperidone, paliperidone) or possibly lower (eg, aripiprazole, brexpiprazole, cariprazine) prolactin could have been examined.
The authors13 presented pooled but not separate results for invasive breast cancer and ductal carcinoma in situ; although the latter does require intervention, being noninvasive, it is classified as Stage 0 and carries a far better prognosis. Readers would have wanted to see a sensitivity analysis that excluded ductal carcinoma in situ as the outcome.
As a final note, the authors13 presented several dozen adjusted HRs in the main paper and supplementary materials. They did not adjust for a false discovery rate. Given that only a few of the HRs were statistically significant, and given that many of the HRs were close to 1.00, one wonders how many may have been false positives related to multiple hypothesis testing.
Breast Cancer Outcomes Associated With Schizophrenia and Antipsychotic Drugs
One study17 found that, among patients who underwent breast cancer surgery, relative to patients without psychiatric disorders, patients with schizophrenia (n = 3,660) had higher in-hospital morbidity (OR, 1.37; 95% CI, 1.21–1.55), more postoperative bleeding (OR, 1.34; 95% CI, 1.05–1.71), more surgical site infections (OR, 1.22; 95% CI, 1.04–1.43), and more sepsis (OR, 1.20; 95% CI, 1.03–1.41). Another study18 found that, among patients who underwent radiotherapy for breast cancer, relative to patients who did not use antipsychotic drugs, patients who did use antipsychotic drugs (n = 3,361) had markedly higher mortality (HR, 11.07; 95% CI, 10.42–11.77). In this study, in a subcohort that excluded patients with metastases and other cancers, relative to patients who did not use antipsychotic drugs, patients who did use antipsychotic drugs also had higher mortality (HR, 5.83; 95% CI, 5.21–6.52). In both studies,17,18 analyses were adjusted for potential confounders, but sources of residual confounding could not be ruled out.
Among 91,531 women with breast cancer who had been exposed to antipsychotic drugs during the previous year, relative to an equal number of propensity score matched women with breast cancer who had never been exposed to antipsychotic drugs, antipsychotic exposure was associated with worse 5-year outcomes for overall mortality (18% vs 13%; OR, 1.49; 95% CI, 1.45–1.53), cancer recurrence (23% vs 13%; OR, 1.89; 95% CI, 1.84–1.93), chemotherapy requirement (48% vs 41%; OR, 1.34; 95% CI, 1.32–1.37), and lymphedema (12% vs 8%; OR, 1.56; 95% CI, 1.51–1.61).19
Clinical Messages
Findings from meta-analysis and subsequent observational studies suggest that there is a small but statistically significant increase in the risk of new-onset breast cancer in women with schizophrenia relative to women without psychiatric disorders (in the general population) as well as relative to women with other psychiatric disorders. The risk appears to be significantly elevated mainly in women aged 40–64 years and mainly in connection with use of FGA drugs for a period of several years.
These findings, though of concern, do not ring loud alarms. There are several reasons. In the many studies, the elevation of risk was small, with ORs or HRs around 1.30 and below. The statistical significances emerged mainly with FGAs or prolactin-raising antipsychotics and with longer durations of exposure, such as >5 years. Significant findings were surrounded by many other HRs that were not statistically significant; so, it is unclear to what extent the findings resulted from Type 1 errors because authors did not correct for false discovery rates.
With especial reference to the role of antipsychotic drugs, cause-effect relationships cannot be established in the observational studies that comprise the only evidence that is available in the field. The possibility remains that statistical significances associated with antipsychotic exposure arise from residual confounding. As an example, most studies did not adjust for obesity and smoking, both of which are common in schizophrenia and both of which increase the risk of breast cancer.
The above notwithstanding, there does seem to be a signal for a small increase in the risk of new-onset breast cancer in women with schizophrenia, and especially in those women who receive FGA or prolactin-raising antipsychotics for longer durations. Given that FGA and prolactin-raising SGA are associated with other adverse effects, it seems reasonable, wherever possible, to prefer SGA over FGA, and prolactin-sparing over prolactin-raising antipsychotics. This is because breast cancer is the commonest form of cancer among women1 and because even a small increase in risk can translate into a large number of women affected in the population. It must also be remembered that statistically significant risks clearly emerged with >5 years of antipsychotic exposure, and that women with schizophrenia are likely to require antipsychotic medication not just for 5 years but for life.
The results of studies in the field strongly recommend that women with schizophrenia, and especially those who require prolactin-raising antipsychotic for extended periods, need to be regularly screened or otherwise monitored for the risk of breast cancer. These women comprise a vulnerable population, and their medical health should not be neglected because caring for their mental health absorbs attention and time.
Finally, because prevention is better than early detection and cure, effort should be made to address modifiable breast cancer risks in women with schizophrenia. This would include discouraging sedentariness and encouraging physical exercise, promoting weight loss, and tackling smoking and drinking, as applicable, through behavioral and pharmacological interventions.
Directions for Future Research
Future research should examine the clinical stage of breast cancer at the time that it is diagnosed in schizophrenia relative to controls; more advanced breast cancer at the time of detection is a red flag that signals the need for earlier and better screening in this vulnerable population. Because management and prognosis differ, risks should be presented separately for invasive breast cancer and noninvasive breast cancer, including ductal carcinoma in situ.
Analyses should be conducted for prolactin-raising drugs with prolactin-sparing drugs as the comparison group; such analyses would best control for confounding by indication. Because study quality is compromised by inadequate adjustment for covariates and confounds, effort should be made to obtain information on and adjust for known risk factors for breast cancer. Examples of variables that need to be measured and included in adjustments are sedentariness and obesity, smoking and drinking, and use of hormonal treatments. Where variables adjusted for could be independently associated with schizophrenia as well as mediators of antipsychotic related risks, analyses can be presented in steps, as in unadjusted, partially adjusted, and fully adjusted models.
Finally, most patients with schizophrenia require lifelong antipsychotic treatment; therefore, risks should be examined in women with longer durations of follow up, such as beyond 10 years. This would provide a perspective on, for example, whether the risks stabilize after an exposure threshold or whether the risks are cumulative across time. In these analyses, sample sizes for affected vs unaffected subjects in exposed vs unexposed groups should be presented so that the reader obtains an understanding of whether the analyses at different time points were adequately powered.
Parting Note
On an unrelated note, olanzapine may be useful to prevent or treat nausea and vomiting associated with chemotherapy in breast cancer20 and to treat cachexia associated with breast cancer.21 These potential benefits with olanzapine are applicable to cancer in general and are not limited to the context of breast cancer, alone.
Article Information
Published Online: June 16, 2025. https://doi.org/10.4088/JCP.25f15960
© 2025 Physicians Postgraduate Press, Inc.
To Cite: Andrade C. Schizophrenia, antipsychotic drugs, and risk of breast cancer. J Clin Psychiatry 2025;86(3):25f15960.
Author Affiliations: Department of Psychiatry, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, India; Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India.
Corresponding Author: Chittaranjan Andrade, MD, Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore 560029, India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India. Please contact Chittaranjan Andrade, MD, at Psychiatrist.com/contact/andrade.
References (21)

- Cai Y, Dai F, Ye Y, et al. The global burden of breast cancer among women of reproductive age: a comprehensive analysis. Sci Rep. 2025;15(1):9347. PubMed CrossRef
- Practice Bulletin No. 179 Summary: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130(1):241–243.
- Andrade C. Understanding the limitations of the odds ratio in a case-control study of the association between breast cancer and exposure to antipsychotic drugs. J Clin Psychiatry. 2021;82(6):21f14319. PubMed CrossRef
- Taipale H, Solmi M, Lähteenvuo M, et al. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry. 2021;8(10):883–891. PubMed CrossRef
- Leung JCN, Ng DWY, Chu RYK, et al. Association of antipsychotic use with breast cancer: a systematic review and meta-analysis of observational studies with over 2 million individuals. Epidemiol Psychiatr Sci. 2022;31:e61. PubMed CrossRef
- Indrakusuma AABP, Sadeva IGKA, Kusuma IGNAW, et al. The risk of antipsychotic drugs on breast cancer: a systematic review and meta-analysis. Oman Med J. 2022;37(6):e453. PubMed CrossRef
- Gao Z, Xi Y, Shi H, et al. Antipsychotic exposure is an independent risk factor for breast cancer: a systematic review of epidemiological evidence. Front Oncol. 2022;12:993367. PubMed CrossRef
- Bird SB. Antipsychotic-induced hyperprolactinemia: toxicologic mechanism and the increased breast cancer risk. Toxicol Rep. 2025;14:101927. PubMed CrossRef
- Joo SW, Lee BC, Lee J, et al. Risk of breast cancer in association with the use of second-generation antipsychotics. Clin Psychopharmacol Neurosci. 2022;20(4):675–684. PubMed CrossRef
- Kern DM, Shoaibi A, Shearer D, et al. Association between prolactin increasing antipsychotic use and the risk of breast cancer: a retrospective observational cohort study in a United States Medicaid population. Front Oncol. 2024;14:1356640. PubMed CrossRef
- Solmi M, Lähteenvuo M, Tanskanen A, et al. Antipsychotic use and risk of breast cancer in women with severe mental illness: replication of a nationwide nested case-control database study. Schizophr Bull. 2024;50(6):1471–1481. PubMed CrossRef
- Li DJ, Tsai SJ, Chen TJ, et al. Exposure to psychotropic drugs and breast cancer risk in patients with bipolar disorder and major depressive disorder: a nested case-control study. Eur Arch Psychiatry Clin Neurosci. 2025;275(2):533–543. PubMed CrossRef
- Yang JS, Kang S, Kim K, et al. Breast cancer risk among women with schizophrenia and association with duration of antipsychotic use: population-based cohort study in South Korea. Br J Psychiatry. 2025;226(4):206–212. PubMed CrossRef
- Andrade C. Towards a further understanding of meta-analysis using gestational exposure to cannabis and birth defects as a case in point. J Clin Psychiatry. 2024;85(4):24f15673. PubMed CrossRef
- Chu RYK, Wei Y, Osborn DP, et al. Breast cancer risks following antipsychotic use in women with bipolar disorder versus schizophrenia: a territory-wide nested case-control study spanning two decades. Psychiatry Res. 2023;326:115287. PubMed CrossRef
- Ota R, Hirata A, Hata T, et al. Breast cancer risk of hormone replacement therapy in Japanese women with schizophrenia on antipsychotic treatment: a retrospective cohort study. J Psychiatr Res. 2025;185:67–73. PubMed CrossRef
- Konishi T, Fujiogi M, Michihata N, et al. Breast cancer surgery in patients with schizophrenia: short-term outcomes from a nationwide cohort. Br J Surg. 2021;108(2):168–173. PubMed CrossRef
- Hwang IG, Kim SM, Kang DR, et al. Effects of antipsychotic drugs during radiotherapy in breast cancer in South Korea: a retrospective cohort study. Sci Rep. 2024;14(1):27138. PubMed CrossRef
- Den J, Nelson N, Klimberg VS. Analysis of the incidence and outcomes of breast cancer in women with schizophrenia. Am J Surg. 2025;239:116050. PubMed CrossRef
- Sakai H, Tsurutani J, Ozaki Y, et al. A randomized, double-blind, placebo controlled phase II study of olanzapine-based prophylactic antiemetic therapy for delayed and persistent nausea and vomiting in patients with HER2-positive or HER2-low breast cancer treated with trastuzumab deruxtecan: ERICA study (WJOG14320B). Ann Oncol. 2025;36(1):31–42. PubMed CrossRef
- Kawai T, Momo K, Ota K. Olanzapine improves cachexia in a breast cancer patient under home hospice care. J Palliat Med. 2022;25(1):4–5. PubMed CrossRef
This PDF is free for all visitors!