Objective: This study examined the association between benzodiazepine use alone or in combination with antipsychotics and risk of mortality in patients with schizophrenia.
Methods: A retrospective longitudinal analysis was performed using Medicaid claims data merged with death certificate data for 18,953 patients (aged 18-58 years) with ICD-9-diagnosed schizophrenia followed from July 1, 2006, to December 31, 2013. Cox proportional hazard analyses were used to estimate the risk of all-cause mortality associated with benzodiazepine use; adjustment was made for a wide array of fixed and time-varying confounders, including demographics, psychiatric and medical comorbidities, and other psychotropic medications.
Results: Of the 18,953 patients diagnosed with schizophrenia, 13,741 (72.5%) were not prescribed a benzodiazepine, 3,476 (18.3%) were prescribed benzodiazepines in the absence of antipsychotic medication, and 1,736 (9.2%) were prescribed benzodiazepines in combination with antipsychotics. Controlling for a wide array of demographic and clinical variables, the hazard of mortality was 208% higher for patients prescribed benzodiazepines without an antipsychotic (HR = 3.08; 95% CI, 2.63-3.61; P < .001) and 48% higher for patients prescribed benzodiazepines in combination with antipsychotics (HR = 1.48; 95% CI, 1.15-1.91; P = .002). Benzodiazepine-prescribed patients were at greater risk of death by suicide and accidental poisoning as well as from natural causes.
Conclusions: Benzodiazepine use is associated with increased mortality risk in patients with schizophrenia after adjusting for a wide range of potential confounders. Given unproven efficacy, physicians should exercise caution in prescribing benzodiazepines to schizophrenic patients.
See free commentary by Rosenheck
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

Benzodiazepine Use and Risk of Mortality Among Patients With Schizophrenia:
A Retrospective Longitudinal Study
ABSTRACT
Objective: This study examined the association between benzodiazepine use alone or in combination with antipsychotics and risk of mortality in patients with schizophrenia.
Methods: A retrospective longitudinal analysis was performed using Medicaid claims data merged with death certificate data for 18,953 patients (aged 18-58 years) with ICD-9-diagnosed schizophrenia followed from July 1, 2006, to December 31, 2013. Cox proportional hazard analyses were used to estimate the risk of all-cause mortality associated with benzodiazepine use; adjustment was made for a wide array of fixed and time-varying confounders, including demographics, psychiatric and medical comorbidities, and other psychotropic medications.
Results: Of the 18,953 patients diagnosed with schizophrenia, 13,741 (72.5%) were not prescribed a benzodiazepine, 3,476 (18.3%) were prescribed benzodiazepines in the absence of antipsychotic medication, and 1,736 (9.2%) were prescribed benzodiazepines in combination with antipsychotics. Controlling for a wide array of demographic and clinical variables, the hazard of mortality was 208% higher for patients prescribed benzodiazepines without an antipsychotic (HR = 3.08; 95% CI, 2.63-3.61; P < .001) and 48% higher for patients prescribed benzodiazepines in combination with antipsychotics (HR = 1.48; 95% CI, 1.15-1.91; P = .002). Benzodiazepine-prescribed patients were at greater risk of death by suicide and accidental poisoning as well as from natural causes.
Conclusions: Benzodiazepine use is associated with increased mortality risk in patients with schizophrenia after adjusting for a wide range of potential confounders. Given unproven efficacy, physicians should exercise caution in prescribing benzodiazepines to schizophrenic patients.
J Clin Psychiatry 2016;77(5):661-667
dx.doi.org/10.4088/JCP.15m10271
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Behavioral Health, The Ohio State University Wexner Medical Center, Columbus
bCenter for Biostatistics, The Ohio State University, Columbus
cOhio Department of Mental Health and Addiction Services, Columbus
*Corresponding author: Cynthia A. Fontanella, PhD, 1670 Upham Dr, Columbus, OH 43210 ([email protected]).
Benzodiazepines are widely used in patients with schizophrenia in conjunction with antipsychotic medications for the treatment of anxiety, sleep disorders, and side effects associated with antipsychotics and for sedation in instances of agitated psychosis.1,2 A number of studies estimate rates of benzodiazepine usage ranging from 14% to 79% among patients with schizophrenia.3-6 Despite the high prevalence of benzodiazepine use, there is little evidence that these drugs are efficacious and safe either alone or in combination with antipsychotics in this population.7 There is also concern about the potential for abuse and dependence, adverse effects8,9 including increased risk for dementia10-12 and psychomotor impairments (eg, fatigue, ataxia, falls, road traffic accidents),13-15 cancer,16 pneumonia, and other infections.17 Most importantly, there is limited but troubling evidence suggesting that use of benzodiazepines is associated with increased mortality in patients with schizophrenia.18,19
Two recent studies using linked nationwide databases of hospital treatment, medication prescriptions, and mortality registers found that benzodiazepine use was associated with increased mortality in schizophrenia. In a Finnish study18 of 2,588 patients with schizophrenia hospitalized for the first time between January 1, 2000, and December 31, 2007, benzodiazepine use was associated with a substantial increase in mortality (HR = 1.91); this increase was attributable to both suicidal (HR = 3.83) and nonsuicidal deaths (HR = 1.60). Neither the use of antidepressants nor treatment with 2 or more antipsychotics relative to monotherapy was associated with increased mortality in the same sample. A Danish study19 of antipsychotic-treated schizophrenic patients found the use of benzodiazepine derivatives with long elimination half-lives (more than 24 hours) to be associated with increased risk of natural death (HR = 1.78), whereas the concomitant use of multiple antipsychotics was not.
To the best of our knowledge, no studies have examined the effects of benzodiazepines on mortality in a US population of individuals with schizophrenia. The primary aim of this study was to examine the association between benzodiazepines either alone or in combination with antipsychotics and excess mortality among a statewide Medicaid population of patients with schizophrenia. Based on the available literature, we hypothesized that current use of benzodiazepines alone or in combination with antipsychotics would be associated with higher mortality risk.
METHODS
Overview of Study Design
A retrospective longitudinal study was used to examine the association between benzodiazepines and mortality in patients with schizophrenia. Adults with schizophrenia were followed from July 1, 2006, to December 31, 2013, unless they died or were no longer enrolled in Medicaid. Study subjects were followed for up to 6.5 years to ascertain risk for mortality. All procedures were approved by the Ohio State University Institutional Review Board.

- Although benzodiazepines are widely used as adjunctive medications in the treatment of schizophrenia, the long-term effects of benzodiazepine use on mortality are unknown.
- Given unproven efficacy and safety issues, physicians should exercise caution in prescribing benzodiazepines to patients with schizophrenia.
Sample Selection
Study subjects were included based on the following criteria: (1) age of 18 to 58 years; (2) 2 or more claims for a primary diagnosis of schizophrenia (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-CM-9] code 295) from July 1, 2007, to June 30, 2009; (3) continuous enrollment in Medicaid for an 18-month period (6 months prior to the index claim for schizophrenia and 12 months after the index claim); and (4) no use of benzodiazepines during the 6-month period prior to the index claim for schizophrenia. Because the focus was on outpatient treatment, we excluded all persons who were placed in a long-term institutional facility (eg, nursing home) for 3 months or more. The final sample consisted of 18,953 patients.
Data Sources
Data for this study were abstracted from 2 data sources: Ohio Medicaid claims and encounter and death certificate files. Medicaid claims data were obtained from the Ohio Department of Jobs and Families, while the death records were obtained from the Ohio Department of Health. Medicaid claims data include information on eligibility status and paid claims for prescription drugs and inpatient and outpatient services. The eligibility files included information on monthly enrollment status, eligibility category (eg, covered family and children, disabled), and demographic characteristics of enrollees (eg, age, sex, race/ethnicity, county of residence). The pharmacy files provided information on prescriptions filled by outpatient pharmacies including prescription and dispense dates, generic name and code, national drug code, dosage, day supply, and quantity. Psychotropic medications were identified from pharmacy files using the dispense date and the generic name codes. The institutional and professional files provided information on service claims for inpatient hospitalizations, physician visits (office- or hospital-based), and other outpatient services and included the dates of service, Current Procedural Terminology/Healthcare Common Procedure Coding System procedure codes, and up to 7 ICD-CM-9 diagnoses.
Information on mortality was abstracted from death certificates. Data on all causes of deaths were based on ICD-10 codes that were reported on death certificates. Medicaid claims data were linked with the death certificate file using a 4-step matching algorithm that has been used in prior research20-22: (1) Social Security number (SSN), last name, first name, sex; (2) SSN, last name, first name, date of birth (month), sex; (3) SSN, first name, date of birth (month), sex; (4) SSN, first name, last name, date of birth (month and year), sex.
Measures
Outcome measures. The primary outcome measure was time to death or the end of the study in cases of censored data. The secondary outcome measures were deaths due to suicide and accidental poisoning and deaths due to natural causes. Data on all causes of death were based on ICD-10 codes that were recorded in death certificates.
Key explanatory variable. The key explanatory variable was a 3-level variable coded as 0 = no benzodiazepines, 1 = benzodiazepine alone, and 2 = benzodiazepine and antipsychotic polypharmacy. For the purpose of this study, polypharmacy was defined as any overlapping prescription fills for an antipsychotic medication and benzodiazepine for 60 days or more.
Covariates. Fixed and time-varying covariates were used to measure relevant demographic, clinical, and treatment characteristics. Demographic variables included the patient’s age (18-30, 31-40, or 41-58 years), sex, race/ethnicity (white, black, or other), marital status (single, married, separated/divorced, or widowed), area of residence (metro or non-metro), and months of enrollment. Based on claims in the 6 months prior to the index diagnosis date, subjects were classified as having treatment for substance abuse disorder (ICD-9-CM 291, 292, and 303-305), anxiety disorder (ICD-9-CM 300.00, 300.2, 300.3, and 308.3), depressive disorder (ICD-9-CM 296.2, 296.3, 300.4, and 311), bipolar disorder (ICD-9-CM 296.0, 296.1, 296.4-296.9, and 301.13), and other mental health disorders (ICD-9-CM 290-319 except as noted previously). The Charlson Comorbidity Index23 was used to capture medical comorbidities. Comedications were treated as time-varying covariates and included the use of antidepressants, mood stabilizers (includes both anticonvulsants and lithium), antiparkinson drugs, and hypnotics.
Statistical Analysis
Cox proportional hazard regression with time-varying drug use was used to establish the association between benzodiazepines alone or in combination with antipsychotics and all-cause mortality. By using the start and stop dates of the benzodiazepines alone or benzodiazepines in combination with antipsychotics and no benzodiazepines by default, we were able to set up a database in which we knew the status (either on or off) of these drugs at all possible time points. In addition, this database was generated simultaneously with all of the start and stop dates of antidepressants, anticonvulsants, mood stabilizers, antiparkinson drugs, and hypnotic drugs. Thus, we had the status (either on or off) of these additional drugs. The stop date was based on the length of the prescription relative to the fill date (coded the start date for these analyses), plus an additional 30 days were added to the stop date to allow for drug washout. Using the Cox model, we ran an unadjusted analysis using only the 3 benzodiazepine states (none, alone, or in combination with antipsychotics) as the risk factor. An adjusted analysis was run using benzodiazepine states, antidepressants, anticonvulsants, lithium, antiparkinson drugs, and hypnotic time-varying drugs and the fixed variables mentioned previously in the Covariates section.
Several subpopulations of the database were created for specific combinations of antipsychotic and benzodiazepines. In each of these subpopulations, the subjects all had the same combination of antipsychotic use, antidepressant use, and mood stabilizers. We generated mortality incidence rates per 1,000 person-years of follow-up for these subpopulations along with the 95% confidence intervals for those with and without benzodiazepine use. All analyses were run using Stata 13.1 (StataCorp LP, College Station, TX).24
RESULTS
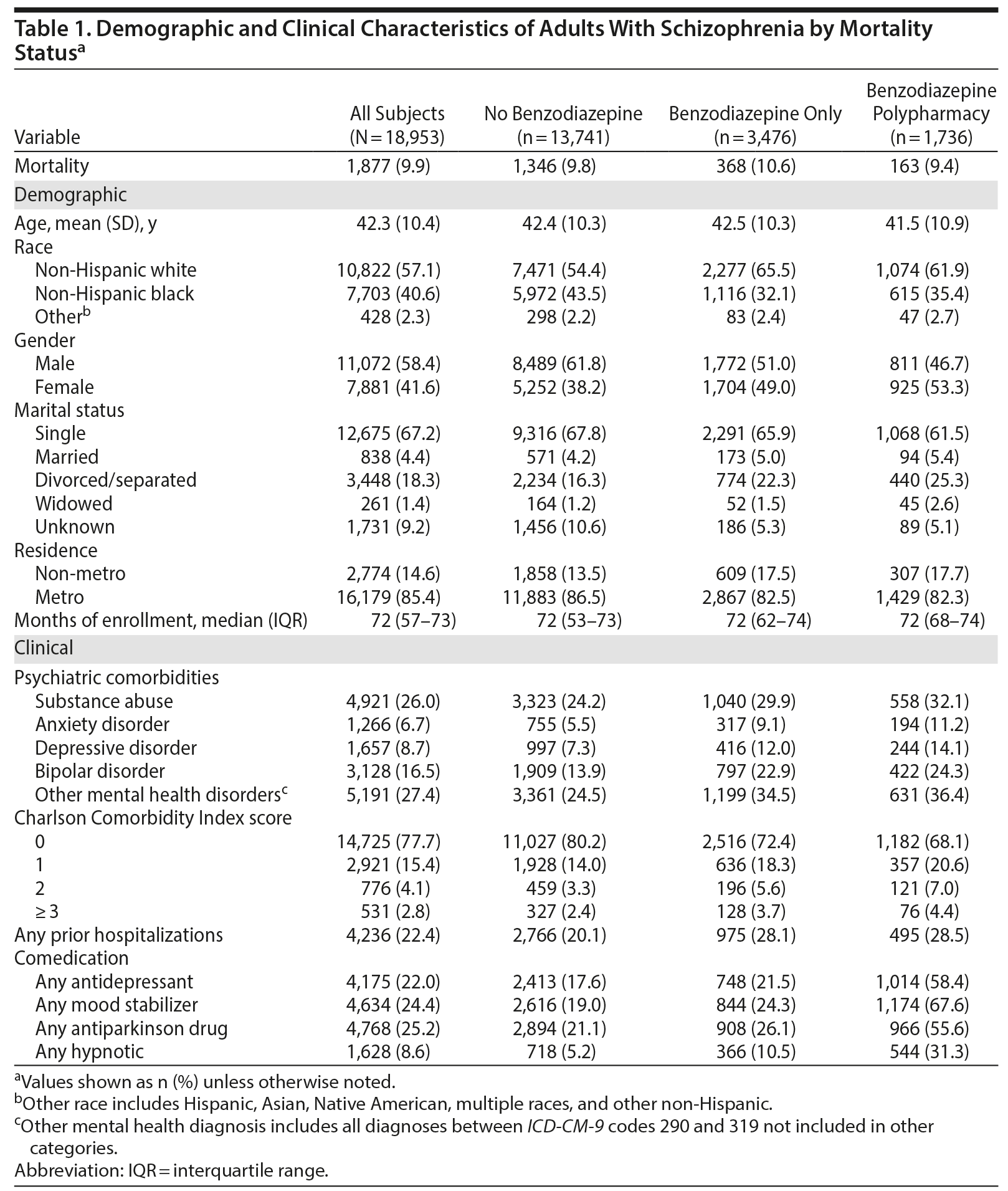
Table 1 shows the demographic and clinical characteristics for the overall study sample and by benzodiazepine use. The mean age of the patients was 42.3 (SD = 10.4) years; 57.1% were non-Hispanic white, 40.6% were non-Hispanic black, and 2.3% were of other racial and ethnic backgrounds; 58.4% were male; and 67.2% were single. The median number of months of enrollment was 72 (IQR = 57-73). Of the 18,953 patients diagnosed with schizophrenia, 13,741 (72.5%) were not prescribed a benzodiazepine, 3,476 (18.3%) were prescribed benzodiazepines alone, and 1,736 (9.2%) were prescribed benzodiazepines in combination with antipsychotics. Nearly 1 in 10 of the patient sample died during the study period. Patients who were prescribed benzodiazepines alone or in combination with antipsychotics were more likely to be non-Hispanic white, female, separated, and divorced. The groups prescribed benzodiazepines, particularly in combination with antipsychotics, also had higher rates of psychiatric and medical comorbidities than nonusers of benzodiazepines and received more prescriptions for other psychotropic drugs (Table 1). During the 6-year follow-up period, 5,212 patients (27.5%) were prescribed benzodiazepines, the most common being lorazepam (55.7%, n = 2,904), clonazepam (42.1%, n = 2,196), and alprazolam (15.5%, n = 810).
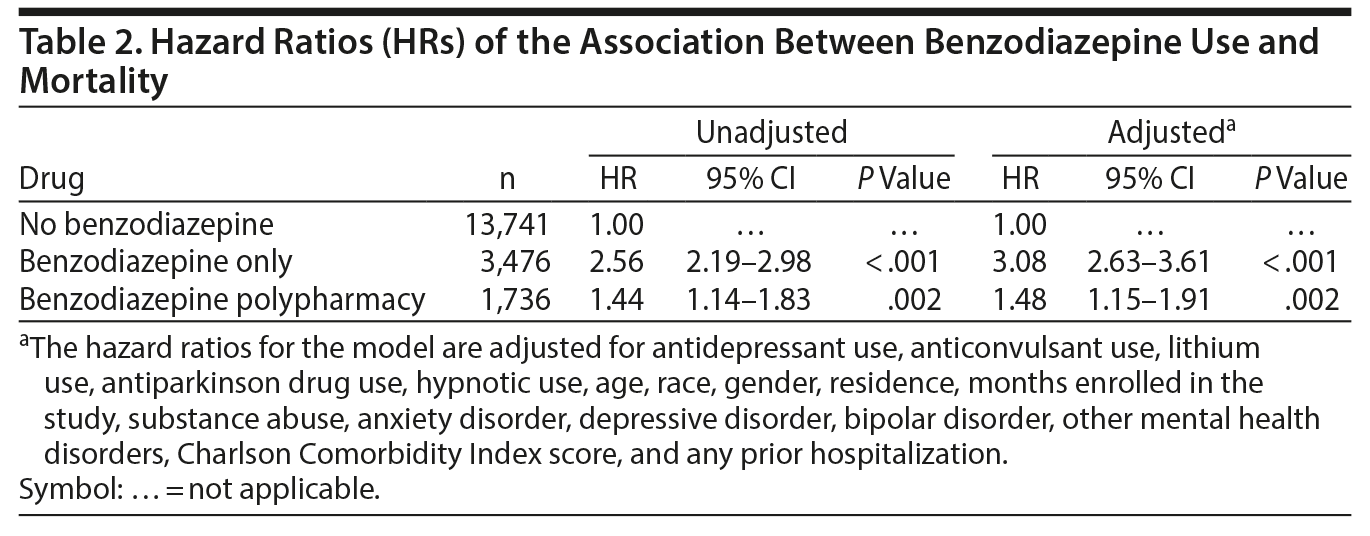
Table 2 presents the unadjusted and adjusted hazard ratios (HRs) from the Cox proportional hazard analysis of the association between benzodiazepines and all-cause mortality. Controlling for a broad array of demographic and clinical variables, the hazard of mortality was 208% higher for patients who were prescribed benzodiazepines without an antipsychotic (HR = 3.08; 95% CI, 2.63-3.61; P < .001) and 48% higher for patients prescribed benzodiazepines in combination with antipsychotics (HR = 1.48; 95% CI, 1.15-1.91; P = .002). The HR for suicide and accidental poisoning was 180% higher for patients prescribed benzodiazepines only (HR = 2.80; 95% CI, 1.45-5.42; P = .002) and 296% higher for patients prescribed benzodiazepines in combination with antipsychotics (HR = 3.96, 95% CI, 2.06-7.63, P < .001). For deaths by natural causes, the HR was 215% higher for patients prescribed benzodiazepines only (HR = 3.15; 95% CI, 2.68-3.71; P < .001) and 33% higher for patients prescribed benzodiazepines in combination with antipsychotics (HR = 1.33; 95% CI, 1.01-1.75; P = .04).
Given previous literature regarding associations between benzodiazepines and specific causes of death,11,16,17 we conducted an exploratory analysis and found that, among the benzodiazepine-treated groups, the crude proportion of patients dying from infectious diseases, nervous disorders, and diseases of the respiratory and digestive systems was higher compared to the non-benzodiazepine group. Accidental poisoning was also notably higher in the benzodiazepine groups compared to the non-benzodiazepine group.
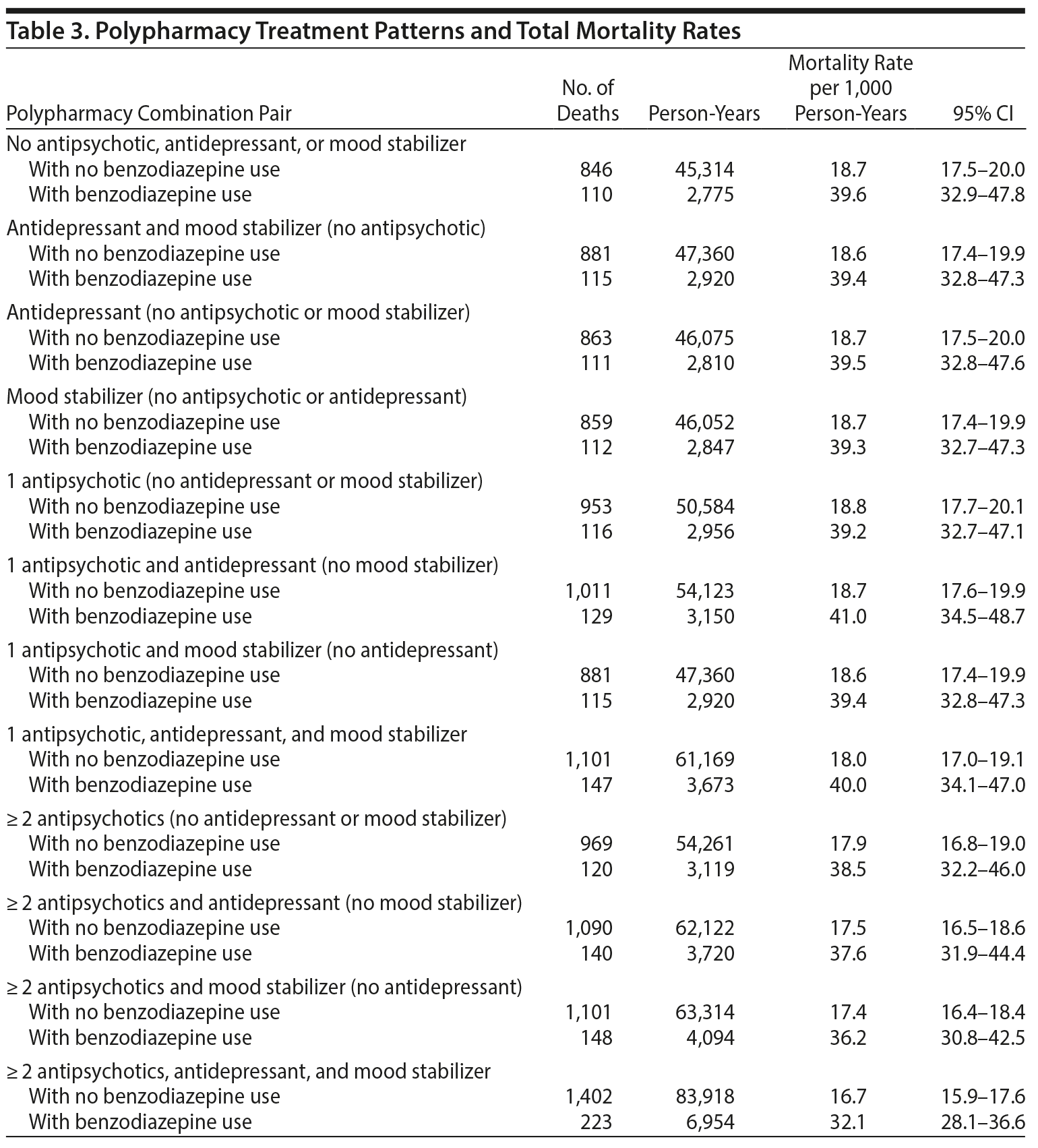
Table 3 shows polypharmacy treatment patterns by benzodiazepine use and total mortality rates. In all examined combinations of antipsychotics, antidepressants, and mood stabilizers, mortality rates were twice as high for benzodiazepine-treated subjects compared to those who did not receive a benzodiazepine.
DISCUSSION
Benzodiazepines are commonly prescribed to patients diagnosed with schizophrenia, with just over one-fourth of the 18,953 patients in this population-based study receiving treatment that included a benzodiazepine. Given how commonly benzodiazepines are prescribed to schizophrenic patients, our finding that benzodiazepine use, both alone and in combination with antipsychotics, is associated with an increased risk of all-cause mortality is particularly troubling. These findings confirm the results of 2 European studies18,19 that have investigated the use of benzodiazepines and mortality in schizophrenia. Although the research designs differ, our results are strikingly similar in terms of the direction and magnitude of effects. In the case of schizophrenic patients prescribed benzodiazepines without an antipsychotic, the hazard of death in the current study was over 3 times higher compared to those not treated with benzodiazepines, even after adjusting for a wide range of potential confounding variables, including physical and psychiatric comorbidities and other psychotropic medications. The risk conferred by benzodiazepine use was significant regardless of whether death was accidental, self-inflicted, or due to “natural causes.” For example, the risks of death classified as due to suicide and accidental poisoning were higher for benzodiazepine users than for nonusers. Similarly, a higher proportion of patients taking benzodiazepines died of infectious diseases, nervous disorders, and diseases of the respiratory and digestive systems in the current study in comparison to those who were benzodiazepine free.
A simple explanation for the observed association between benzodiazepine use and all-cause mortality is not forthcoming, as benzodiazepine-treated patients appear at greater risk to die by suicide and self-poisoning as well as “natural causes.” Prior research suggests that benzodiazepines are associated with increased risk for infectious diseases,17,25 and our results provide some support for these findings. Some have hypothesized that benzodiazepines attenuate the immune response, potentially increasing the risk for infectious diseases and cancer.26,27 Although the exact mechanisms are unknown, animal studies suggest that benzodiazepines increase susceptibility to infection, including pneumonia, perhaps via activation of GABAA receptors on immune cells.28-32 In the case of suicide and accidental poisoning, benzodiazepines may increase the likelihood of impulsive responding and dysphoric mood, potentially increasing the risk for self- injury. For example, in a study of antidepressant-treated adolescents with resistant depression, the use of adjunctive benzodiazepines was found to be associated with a heightened risk for self-harm and suicidal adverse events.33 Use of benzodiazepines may cause disinhibition and increase vulnerability to violent and high-risk behaviors such as suicide.27 Suicide has been reported as a common outcome in chronic benzodiazepine dependence,34,35 and discontinuation of long-term benzodiazepine use may exacerbate anxiety and distress, potentially contributing to suicidal behavior.
With regard to clinical implications, this study in combination with previous work suggests that the routine, ongoing prescription of benzodiazepines in schizophrenia should be discouraged in the absence of additional, prospective study, or at the very least approached with caution. Whether brief, targeted use of benzodiazepines in high-acuity clinical situations should be reevaluated is unclear given this study’s reliance on claims data. Our findings nevertheless suggest that the ongoing prescription of benzodiazepines to patients with schizophrenia should be undertaken only after serious reflection on the balance between potential risks and benefits and appropriate informed consent. Patients, family members, and caregivers should be educated with regard to potential mortality risks associated with benzodiazepine use.
Strengths and Limitations
The strengths of this study include: (1) a large population-based sample of patients with schizophrenia; (2) longitudinal analysis of benzodiazepine use at all time points during the course of the study; (3) control of multiple confounders including demographics, psychiatric and medical comorbidities, and use of other psychotropic medications; and (4) drug data based on prescription data rather than self-report, which is subject to recall bias. However, several limitations need to be considered. First, because the data are from a single state Medicaid population, study findings may not be generalizable to other state programs or non-Medicaid populations, although there is no reason to believe that these results would not be typical. Second, because of the observational nature of the data, it is not possible to infer causality. Third, although we controlled for a wide range of potential confounders, it is impossible to exclude confounding arising from unmeasured factors or measurement error. For example, we were unable to control for lifestyle factors such as smoking and obesity, which may be associated with mortality. Fourth, we must acknowledge that the rationale for benzodiazepine treatment in this observational sample is unclear. One potential concern is that a benzodiazepine may have been initiated in the course of a serious medical illness as a confounder, though our impression on review of the data is that the preponderance of use appeared to be for purely psychiatric reasons. Fifth, diagnoses of schizophrenia were based on claims-based data and were not subject to expert validation through standardized diagnostic procedures. Finally, although pharmacy claims are considered reliable and avoid problems of recall bias associated with self-reports, they do not measure actual consumption of medication.
CONCLUSIONS
Benzodiazepines are commonly prescribed as adjunctive medications in the treatment of schizophrenia. In this population-based study, we found that benzodiazepine use alone or in combination with antipsychotic medications was strongly associated with increased risk for mortality in patients with schizophrenia after adjusting for a wide range of potential confounders. Given the lack of efficacy and apparent safety issues with benzodiazepine use, physicians should exercise caution when considering the routine prescription of benzodiazepines in this vulnerable patient population.
Submitted: July 27, 2015; accepted October 6, 2015.
Drug names: alprazolam (Xanax, Niravam, and others), clonazepam (Klonopin and others), lorazepam (Ativan and others).
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial disclosure: Drs Fontanella, Campo, Lehrer, Klein, and Hurst, Mr Phillips, Ms Hiance-Steelesmith, Ms Sweeney, and Mr Tam have no personal affiliations or financial relationships with any commercial interest to disclose relative to this article.
Funding/support: This project was supported by a grant from the Ohio Department of Mental Health and Addiction Services (OhioMHAS).
Role of the sponsor: The OhioMHAS was responsible for monitoring the operations and conduct of the study and for reviewing and approving the manuscript.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Haw C, Stubbs J. Benzodiazepines—a necessary evil? a survey of prescribing at a specialist UK psychiatric hospital. J Psychopharmacol. 2007;21(6):645-649. PubMed doi:10.1177/0269881106072386
2. Wolkowitz OM, Pickar D. Benzodiazepines in the treatment of schizophrenia: a review and reappraisal. Am J Psychiatry. 1991;148(6):714-726. PubMed doi:10.1176/ajp.148.6.714
3. Chakos MH, Glick ID, Miller AL, et al. Baseline use of concomitant psychotropic medications to treat schizophrenia in the CATIE trial. Psychiatr Serv. 2006;57(8):1094-1101. PubMed doi:10.1176/ps.2006.57.8.1094
4. Novick D, Bousono M, Suarez D, et al; SOHO Advisory Board. Use of concomitant medication with antipsychotic treatment in outpatients with schizophrenia: results from the European Schizophrenia Outpatients Health Outcomes (SOHO) study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(6):972-982. PubMed doi:10.1016/j.pnpbp.2005.06.003
5. Clark RE, Xie H, Brunette MF. Benzodiazepine prescription practices and substance abuse in persons with severe mental illness. J Clin Psychiatry. 2004;65(2):151-155. PubMed doi:10.4088/JCP.v65n0202
6. Wu CS, Lin YJ, Liu SK. Benzodiazepine use among patients with schizophrenia in Taiwan: a nationwide population-based survey. Psychiatr Serv. 2011;62(8):908-914. PubMed doi:10.1176/ps.62.8.pss6208_0908
7. Dold M, Li C, Tardy M, et al. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2012;11(11):CD006391. PubMed
8. Brunette MF, Noordsy DL, Xie H, et al. Benzodiazepine use and abuse among patients with severe mental illness and co-occurring substance use disorders. Psychiatr Serv. 2003;54(10):1395-1401. PubMed doi:10.1176/appi.ps.54.10.1395
9. Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35(suppl):S93-S100. PubMed doi:10.1016/S0920-9964(98)00161-3
10. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. PubMed doi:10.1136/bmj.e6231
11. Gallacher J, Elwood P, Pickering J, et al. Benzodiazepine use and risk of dementia: evidence from the Caerphilly Prospective Study (CaPS). J Epidemiol Community Health. 2012;66(10):869-873. PubMed doi:10.1136/jech-2011-200314
12. Wu CS, Ting TT, Wang SC, et al. Effect of benzodiazepine discontinuation on dementia risk. Am J Geriatr Psychiatry. 2011;19(2):151-159. PubMed doi:10.1097/JGP.0b013e3181e049ca
13. Paterniti S, Dufouil C, Alpérovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22(3):285-293. PubMed doi:10.1097/00004714-200206000-00009
14. Barker MJ, Greenwood KM, Jackson M, et al. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18(1):37-48. PubMed doi:10.2165/00023210-200418010-00004
15. Neutel CI. Risk of traffic accident injury after a prescription for a benzodiazepine. Ann Epidemiol. 1995;5(3):239-244. PubMed doi:10.1016/1047-2797(94)00112-7
16. Kao CH, Sun LM, Su KP, et al. Benzodiazepine use possibly increases cancer risk: a population-based retrospective cohort study in Taiwan. J Clin Psychiatry. 2012;73(4):e555-e560. PubMed doi:10.4088/JCP.11m07333
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170. PubMed doi:10.1136/thoraxjnl-2012-202374
18. Tiihonen J, Suokas JT, Suvisaari JM, et al. Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry. 2012;69(5):476-483. PubMed doi:10.1001/archgenpsychiatry.2011.1532
19. Baandrup L, Gasse C, Jensen VD, et al. Antipsychotic polypharmacy and risk of death from natural causes in patients with schizophrenia: a population-based nested case-control study. J Clin Psychiatry. 2010;71(2):103-108. PubMed doi:10.4088/JCP.08m04818yel
20. Koroukian SM. Linking the Ohio Cancer Incidence System with Medicare, Medicaid and clinical data from home health care and long term care assessment instruments: paving the way for new research endeavors in geriatric oncology. J Registry Manag. 2008;35(4):156-165. PubMed
21. Koroukian SM, Beaird H, Duldner JE, et al. Analysis of injury- and violence-related fatalities in the Ohio Medicaid population: identifying opportunities for prevention. J Trauma. 2007;62(4):989-995. PubMed doi:10.1097/01.ta.0000210359.98816.45
22. Koroukian SM, Cooper GS, Rimm AA. Ability of Medicaid claims data to identify incident cases of breast cancer in the Ohio Medicaid population. Health Serv Res. 2003;38(3):947-960. PubMed doi:10.1111/1475-6773.00155
23. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8 PubMed
24. StataCorp. Stata Statistical Software: Release 12, 13. College Station, TX: StataCorp LP; 2013.
25. Lechin F, van der Dijs B, Vitelli-Flores G, et al. Peripheral blood immunological parameters in long-term benzodiazepine users. Clin Neuropharmacol. 1994;17(1):63-72. PubMed doi:10.1097/00002826-199402000-00007
26. Kripke DF. Possibility that certain hypnotics might cause cancer in skin. J Sleep Res. 2008;17(3):245-250. PubMed doi:10.1111/j.1365-2869.2008.00685.x
27. Mallon L, Broman JE, Hetta J. Is usage of hypnotics associated with mortality? Sleep Med. 2009;10(3):279-286. PubMed doi:10.1016/j.sleep.2008.12.004
28. Sanders RD, Hussell T, Maze M. Sedation & immunomodulation. Crit Care Clin. 2009;25(3):551-570, ix. PubMed doi:10.1016/j.ccc.2009.05.001
29. Laschi A, Descotes J, Tachon P, et al. Adverse influence of diazepam upon resistance to Klebsiella pneumoniae infection in mice. Toxicol Lett. 1983;16(3-4):281-284. PubMed doi:10.1016/0378-4274(83)90188-1
30. Galdiero F, Bentivoglio C, Nuzzo I, et al. Effects of benzodiazepines on immunodeficiency and resistance in mice. Life Sci. 1995;57(26):2413-2423. PubMed doi:10.1016/0024-3205(95)02199-0
31. Huemer HP, Lassnig C, Nowotny N, et al. Diazepam leads to enhanced severity of orthopoxvirus infection and immune suppression. Vaccine. 2010;28(38):6152-6158. PubMed doi:10.1016/j.vaccine.2010.07.032
32. Sanders RD, Myles P, Hubbard R, et al. Benzodiazepines increase mortality from pneumonia: role of GABAergic Signaling, A765. American Society of Anesthesiologists Annual Meeting. October 15-19, 2011; Chicago, IL.
33. Brent DA, Emslie GJ, Clarke GN, et al. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am J Psychiatry. 2009;166(4):418-426. PubMed doi:10.1176/appi.ajp.2008.08070976
34. Allgulander C, Borg S, Vikander B. A 4-6-year follow-up of 50 patients with primary dependence on sedative and hypnotic drugs. Am J Psychiatry. 1984;141(12):1580-1582. PubMed doi:10.1176/ajp.141.12.1580
35. Wines JD Jr, Saitz R, Horton NJ, et al. Suicidal behavior, drug use and depressive symptoms after detoxification: a 2-year prospective study. Drug Alcohol Depend. 2004;76(suppl):S21-S29. PubMed doi:10.1016/j.drugalcdep.2004.08.004
This PDF is free for all visitors!