Background: Substance abuse adds to diagnostic uncertainty in psychosis and may increase the risk of transition from brief and affective psychoses to schizophrenia. This study examined whether comorbid substance disorder was associated with diagnostic instability and progression from other psychosis diagnoses to schizophrenia and whether effects differed for cannabis and stimulant-related disorders.
Method: We identified 24,306 individuals admitted to hospital with an ICD-10 psychosis diagnosis between 2000 and 2011. We examined agreement between initial diagnosis and final diagnosis over 2-5 years and predictors of diagnostic change toward and away from a final diagnosis of schizophrenia.
Results: Nearly half (46%) of participants with initial brief, atypical, or drug-induced psychoses were later diagnosed with schizophrenia. Persisting illicit drug disorders did not increase the likelihood of progression to schizophrenia (OR = 0.97; 95% CI, 0.89-1.04) but increased the likelihood of revision of index psychosis diagnosis away from schizophrenia (OR = 1.55; 95% CI, 1.40-1.71). Cannabis disorders predicted an increased likelihood of progression to schizophrenia (OR = 1.12; 95% CI, 1.01-1.24), while stimulant disorders predicted a reduced likelihood (OR = 0.81; 95% CI, 0.67-0.97). Stimulant disorders were associated with greater overall diagnostic instability.
Conclusions: Many people with initial diagnoses of brief and affective psychoses are later diagnosed with schizophrenia. Cannabis disorders are associated with diagnostic instability and greater likelihood of progression to schizophrenia. By contrast, comorbid stimulant disorders may be associated with better prognosis in psychosis, and it may be important to avoid premature closure on a diagnosis of schizophrenia when stimulant disorders are present.
J Clin Psychiatry 2014;75(4):349-356
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: November 9, 2013; accepted January 29, 2014.
Online ahead of print: Month 00, 2014 (doi:10.4088/JCP.13m08878).
Corresponding author: Grant E. Sara, MM, InforMH, Macquarie Hospital, PO Box 169, North Ryde NSW 1670 Australia ([email protected]).
This CME activity is expired. For more CME activities, visit CMEInstitute.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders

ABSTRACT
Background: Substance abuse adds to diagnostic uncertainty in psychosis and may increase the risk of transition from brief and affective psychoses to schizophrenia. This study examined whether comorbid substance disorder was associated with diagnostic instability and progression from other psychosis diagnoses to schizophrenia and whether effects differed for cannabis and stimulant-related disorders.
Method: We identified 24,306 individuals admitted to hospital with an ICD-10 psychosis diagnosis between 2000 and 2011. We examined agreement between initial diagnosis and final diagnosis over 2-5 years and predictors of diagnostic change toward and away from a final diagnosis of schizophrenia.
Results: Nearly half (46%) of participants with initial brief, atypical, or drug-induced psychoses were later diagnosed with schizophrenia. Persisting illicit drug disorders did not increase the likelihood of progression to schizophrenia (OR = 0.97; 95% CI, 0.89-1.04) but increased the likelihood of revision of index psychosis diagnosis away from schizophrenia (OR = 1.55; 95% CI, 1.40-1.71). Cannabis disorders predicted an increased likelihood of progression to schizophrenia (OR = 1.12; 95% CI, 1.01-1.24), while stimulant disorders predicted a reduced likelihood (OR = 0.81; 95% CI, 0.67-0.97). Stimulant disorders were associated with greater overall diagnostic instability.
Conclusions: Many people with initial diagnoses of brief and affective psychoses are later diagnosed with schizophrenia. Cannabis disorders are associated with diagnostic instability and greater likelihood of progression to schizophrenia. By contrast, comorbid stimulant disorders may be associated with better prognosis in psychosis, and it may be important to avoid premature closure on a diagnosis of schizophrenia when stimulant disorders are present.
J Clin Psychiatry 2014;75(4):349-356
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: November 9, 2013; accepted January 29, 2014 (doi:10.4088/JCP.13m08878).
Corresponding author: Grant E. Sara, MM, InforMH, Macquarie Hospital, PO Box 169, North Ryde NSW 1670 Australia ([email protected]).
A person with psychosis may receive different diagnoses over time. “Diagnostic shifts”1 may reflect interrater variation, changes in available information, the evolution of illness, or a combination of all of these.1,2 These shifts are of clinical relevance; the diagnosis first made by a person’s treating team may determine his or her subsequent care3,4 and may shape the expectations of the person, his or her family, and treating clinicians.
Comorbid substance use is common in psychosis5-9 and may contribute to diagnostic shifts in several ways. First, substance use creates clinical uncertainty; it is difficult to judge causation of psychosis when substance use persists.10 Second, substance use may influence the course of psychosis. Substances may trigger recurrence of symptoms and relapse of illness11-16 and therefore may increase the likelihood of progression toward enduring psychoses such as schizophrenia. However, findings on this issue are conflicting; comorbid substance use in brief or affective psychoses has been associated with reduced,1,17 increased,4 or unchanged18 likelihood of diagnostic shift to schizophrenia.
Cannabis and stimulants may differ in their effects on diagnostic stability. Cannabis interacts with personal vulnerabilities to increase the risk of developing schizophrenia.19 Therefore, persistent cannabis use is likely to predict a diagnostic trajectory from brief psychoses to schizophrenia. Amphetamine, cocaine, and other stimulants are used in up to 30% of young people with psychosis.20,21 They are powerful dopamine agonists that can induce psychotic symptoms in healthy volunteers22 and whose effects may increase with repeated use due to sensitization.23,24 Dopamine overactivity may play a role in schizophrenia25,26; therefore, persistent stimulant use may be even more likely than persistent cannabis use to cause diagnostic progression from other psychoses to schizophrenia.
However, the only study27 that has examined cannabis and stimulants separately found that 46% of people with cannabis-induced psychosis later received a diagnosis of schizophrenia, compared with only 30% of people with amphetamine-induced psychosis. That study examined people with a specific diagnosis of substance-induced psychosis, and, when multiple substances were related to the psychosis episode, they were classed as “other or unknown” substances. It is important to know whether these findings can be generalized to other clinical situations that often involve a wide range of psychosis diagnoses and where cannabis and stimulant use often coexist.
Our study examined admissions to mental health units in the state of New South Wales (NSW) (population 7.2 million), Australia. This provided a population-based sample with sufficient power to examine diagnostic stability in brief psychoses (including brief, atypical, and drug-induced psychoses) and affective psychoses (bipolar disorder and psychotic depression) and to examine the effects of cannabis and stimulant comorbidity separately and in combination. We focused on substance problems occurring during the follow-up period rather than at the first (index) admission because baseline substance problems have been shown to have a more limited effect on outcome in psychosis than ongoing substance use.11,12,14
METHOD
Sample
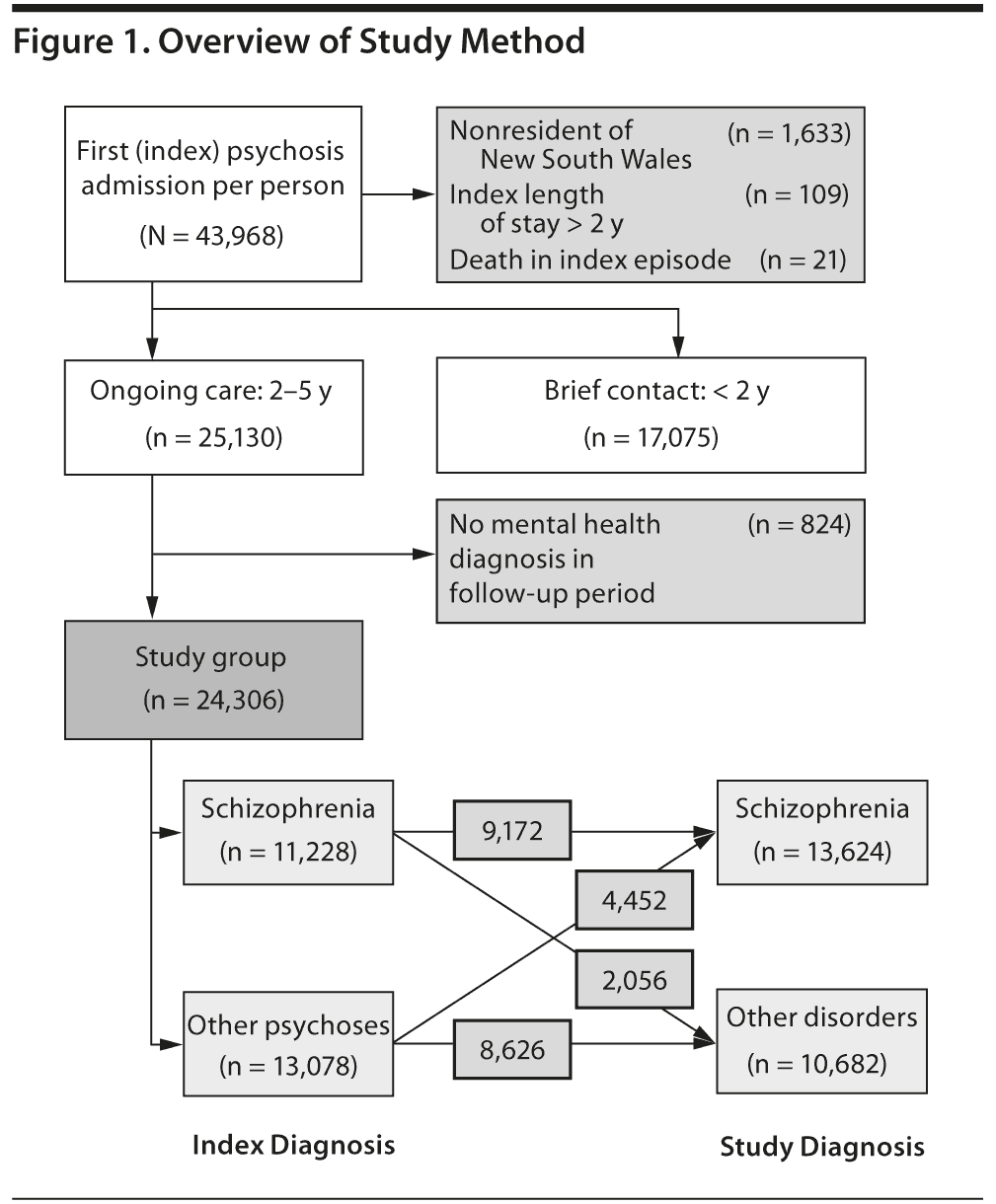
Admissions of NSW residents to state-operated (“public”) hospitals from July 1, 2000, to June 30, 2011, were screened. The first (index) admission with psychosis was identified for each person with the use of a unique person identifier (Figure 1). Participants were aged 18-50 years and had an index admission of more than 1 day’s duration to a designated mental health unit, with a primary or secondary diagnosis of psychosis. People whose index admissions were longer than 2 years or ended in death were excluded.
For each participant, we identified all subsequent admissions for mental health care to NSW public hospitals and all subsequent contacts with specialized community mental health services in the 5 years from the end of the index admission. Diagnostic stability was examined only in persons with at least 2 years of ongoing service contact to avoid overestimating diagnostic agreement where the time between assessments was limited.
The study was approved by the NSW Population and Health Services Research Ethics Committee.
Psychosis Diagnoses
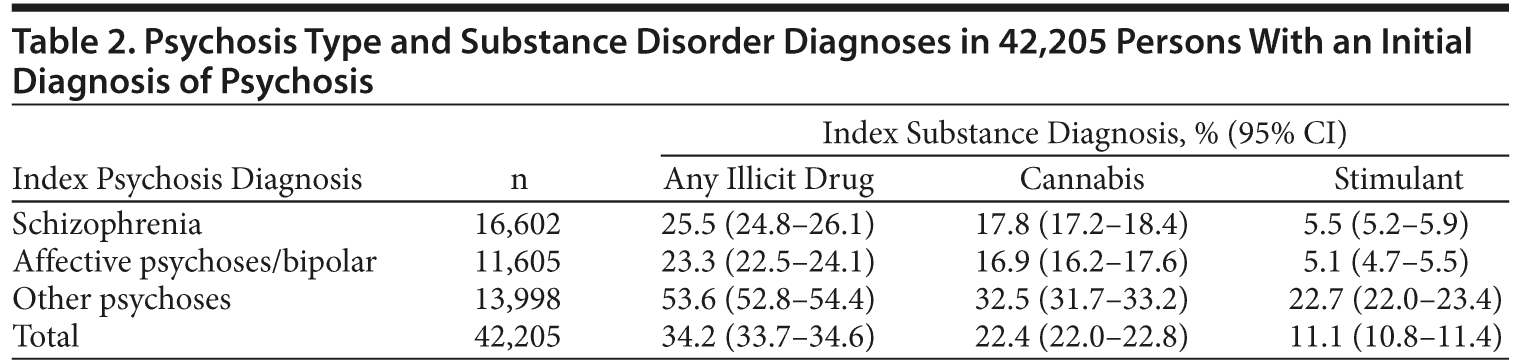
New South Wales health services recorded diagnoses using the International Classification of Diseases, Tenth Revision, Australian Modification (ICD-10–AM).28 Hospital episodes with a primary or additional ICD-10 diagnosis of psychosis were grouped into (1) “schizophrenia,” including schizophrenia (F20) and schizoaffective disorder (F25); (2) “affective psychoses,” including bipolar disorder (F30, F31) and psychotic depression (F32.3, F32.30, F32.31, F33.3); and (3) other psychoses, including acute and transient psychoses (F23), delusional disorders (F22, F24), other or unspecified psychosis (F28, F29), and drug-induced psychoses. Drug-induced psychoses included ICD-10 substance codes (F10-F19) in which psychosis was specified (eg, F10.5, F10.9). DSM-IV schizophreniform psychosis is classified with acute and transient psychoses in ICD-10. Organic psychoses and schizotypal disorder were excluded.

- Nearly half of initial diagnoses of drug-induced or atypical psychosis are later revised to schizophrenia.
- Comorbid substance use increases diagnostic uncertainty and also makes it more likely that initial diagnoses of schizophrenia will be revised to other diagnoses.
- Ongoing stimulant use disorders are associated with greater diagnostic uncertainty and reduced risk of diagnostic change to schizophrenia when compared to cannabis use or no drug use.
Index diagnosis was obtained from the first admission for each person. Final diagnosis was the mental health diagnosis at the end of the 2- to 5-year follow-up period in inpatient and community care episodes. When multiple diagnoses were recorded on the last date, priority was given to the “primary” diagnosis, that is, the diagnosis identified by the treating team as being responsible for the episode of care. Final diagnoses of mental health conditions other than psychosis were grouped as “nonpsychotic conditions.” Persons with no specific mental health diagnosis recorded in the study period were excluded from analysis.
Binary variables were created for schizophrenia or other psychosis diagnoses at index admission and during ongoing community-based care.
Substance Diagnoses
Substance disorders were identified by primary or additional diagnosis codes for abuse, dependence, intoxication, poisoning by specific illicit drugs, or alcohol-related liver disease. Drug-induced psychoses counted as both a psychosis and a substance disorder. Amphetamines and cocaine were grouped as stimulant disorders. Opiate disorders included illicit opiates (eg, heroin) and nonmedical use of prescription opiates. Polydrug disorder was recorded only where specifically diagnosed (ICD code F19). We distinguished between substance disorders occurring only at the index admission and substance disorders occurring in the study period (ie, at least 1 substance disorder diagnosis occurring subsequent to the index admission). Only substance diagnoses in the study period were examined in analyses of diagnostic stability.
A binary variable was constructed indicating the presence of any illicit drug diagnosis (cannabis, stimulant, hallucinogen, opiate, or polydrug) during the study period (excluding the index admission). A composite “illicit drug use group” variable was created, with 5 mutually exclusive categories: (1) no illicit drug diagnoses, (2) cannabis, (3) stimulants, (4) cannabis plus stimulants, and (5) other/polydrug only. Some persons in groups 2-4 had additional substance diagnoses. People in the other/polydrug only category had only specific substance diagnoses (eg, opiate disorders) or a polydrug diagnosis without indication of the substances involved and no cannabis or stimulant diagnoses.
Other Variables
Demographic variables were measured from index admission. Migration status was based on country of birth. Rurality and disadvantage measures were based on Australian Bureau of Statistics reference data for the area of residence, collapsed for rurality (major metropolitan vs regional and rural residence) and disadvantage (most disadvantaged 40% of local areas vs least disadvantaged 60%). Ongoing contact was defined as having at least 1 community mental health contact or admission to a mental health unit in the 2 to 5 years after discharge from the index admission.
Statistical Analysis
Predictors of ongoing contact were examined using binary logistic regression. Univariate odds ratios (ORs) and 95% CIs were calculated separately for demographic, diagnostic, and prior care variables. Multivariate analysis included all variables with significant univariate associations (P ≤ .05). Multicollinearity was assessed and collinear variables excluded.29
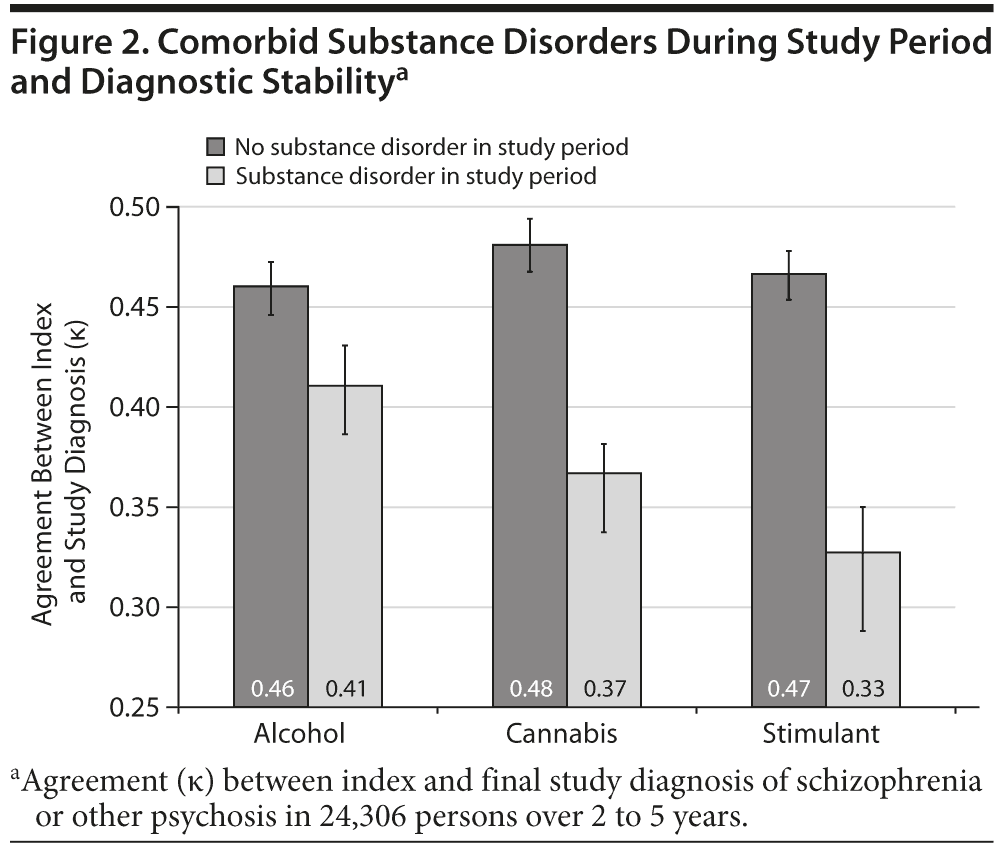
Index and final diagnoses were cross-tabulated, and overall diagnostic agreement was calculated as the percentage of persons with both index and final diagnosis in the same group. Diagnostic agreement was calculated separately for persons with and without comorbid substance diagnoses and compared using Pearson χ2 test. For the binary schizophrenia/no schizophrenia variable, agreement between index and final diagnosis was calculated using Cohen κ, calculated separately for persons with and without cannabis, stimulant, and alcohol disorders.
Predictors of diagnostic change to or from schizophrenia were examined using binary logistic regression analyses, conducted separately for people with (1) schizophrenia and (2) other psychoses at index admission. Within each group, 2 regression models were constructed. The first examined the effect of any illicit drug diagnosis, and the second examined the effects of cannabis and stimulants separately. Regression diagnostics were conducted as described above. Analyses were conducted using Stata v13 (StataCorp, College Station, TX).
RESULTS
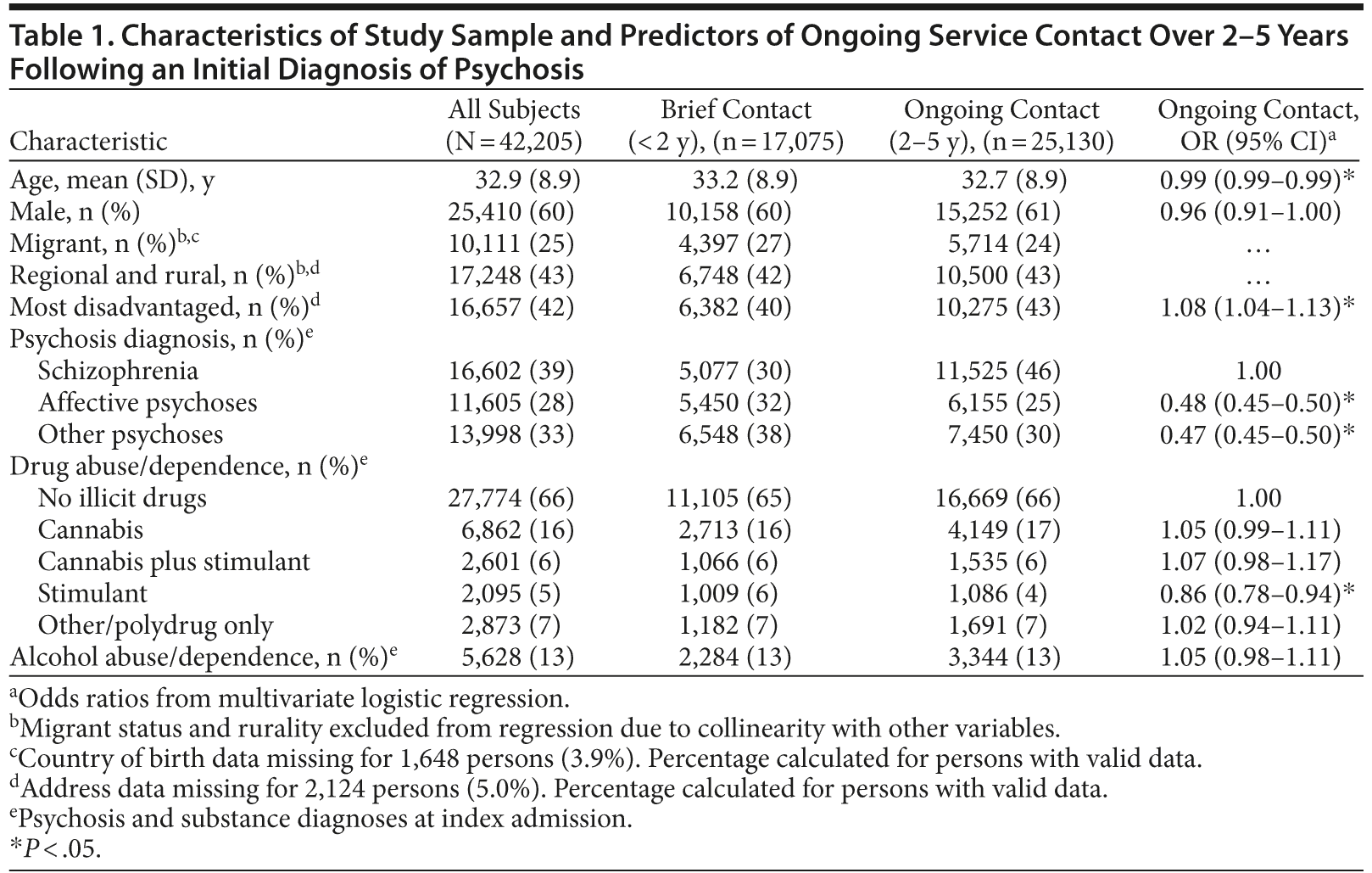
After exclusions, an index psychosis admission was identified for 42,205 persons aged 18-50 years (Figure 1). Sixty percent were male (Table 1), and more than half were aged between 21 and 35. The most common diagnoses at index admission were schizophrenia (39%) and other (brief, atypical, drug-induced, and unspecified) psychoses (33%). One-third had comorbid illicit drug diagnoses at index admission, most commonly a cannabis disorder (22%) and/or stimulant disorder (11%). More than half of those with stimulant disorders also had cannabis disorder. The rate and pattern of cannabis and stimulant disorders were similar for schizophrenia and affective psychoses (Table 2). Comorbid substance disorders were most common in brief, atypical, and drug-induced psychoses.
Thirty-nine percent of subjects had 2 or fewer years of mental health service contact and were excluded from analysis of diagnostic stability. Ongoing contact was more likely in people who were younger, lived in disadvantaged areas, and had index diagnoses of schizophrenia. Overall substance diagnoses at index admission did not predict ongoing contact; however, contact was less likely (OR = 0.86; 95% CI, 0.78-0.94) in people whose only substance comorbidity at index admission was a stimulant disorder.
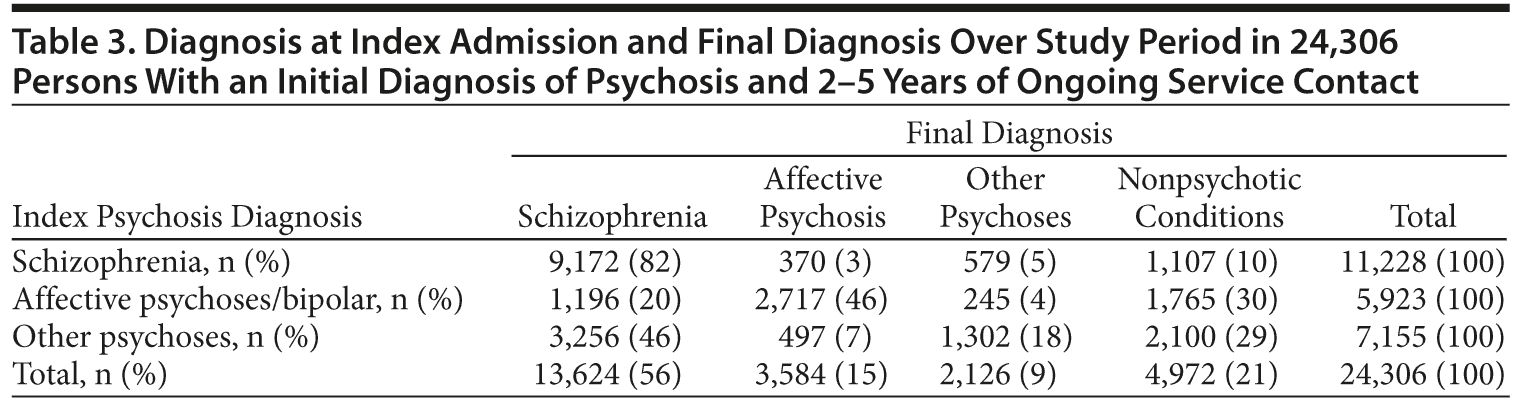
Diagnoses at index admission were compared with diagnosis over the following 2 to 5 years (Table 3). Only 18% of those with an index diagnosis of brief, atypical, or drug-induced psychosis retained a diagnosis within that group. Others were diagnosed with schizophrenia (46%), nonpsychotic conditions (29%), and affective psychoses (7%). Index diagnoses of schizophrenia were stable: 82% retained that diagnosis in the study period.
One-third of people with ongoing service contact had at least 1 illicit substance diagnosis during the study period. Substance disorders predicted lower diagnostic stability. Index and final diagnoses agreed in 60% of persons without substance disorders but only in 47% of people with substance disorders (χ2 = 423, P < .001). Diagnostic agreement was lower for stimulant disorders (40% agreement between index and final diagnosis) than for cannabis disorders (47% agreement, χ2 = 57, P < .001). Stability between index and final diagnosis was also examined by measuring κ for agreement between index and final diagnosis after grouping these into binary schizophrenia/no schizophrenia variables. Diagnostic stability was lower for those with comorbid stimulant disorders and cannabis disorders in the study period compared with those with alcohol disorders in the study period (Figure 2).
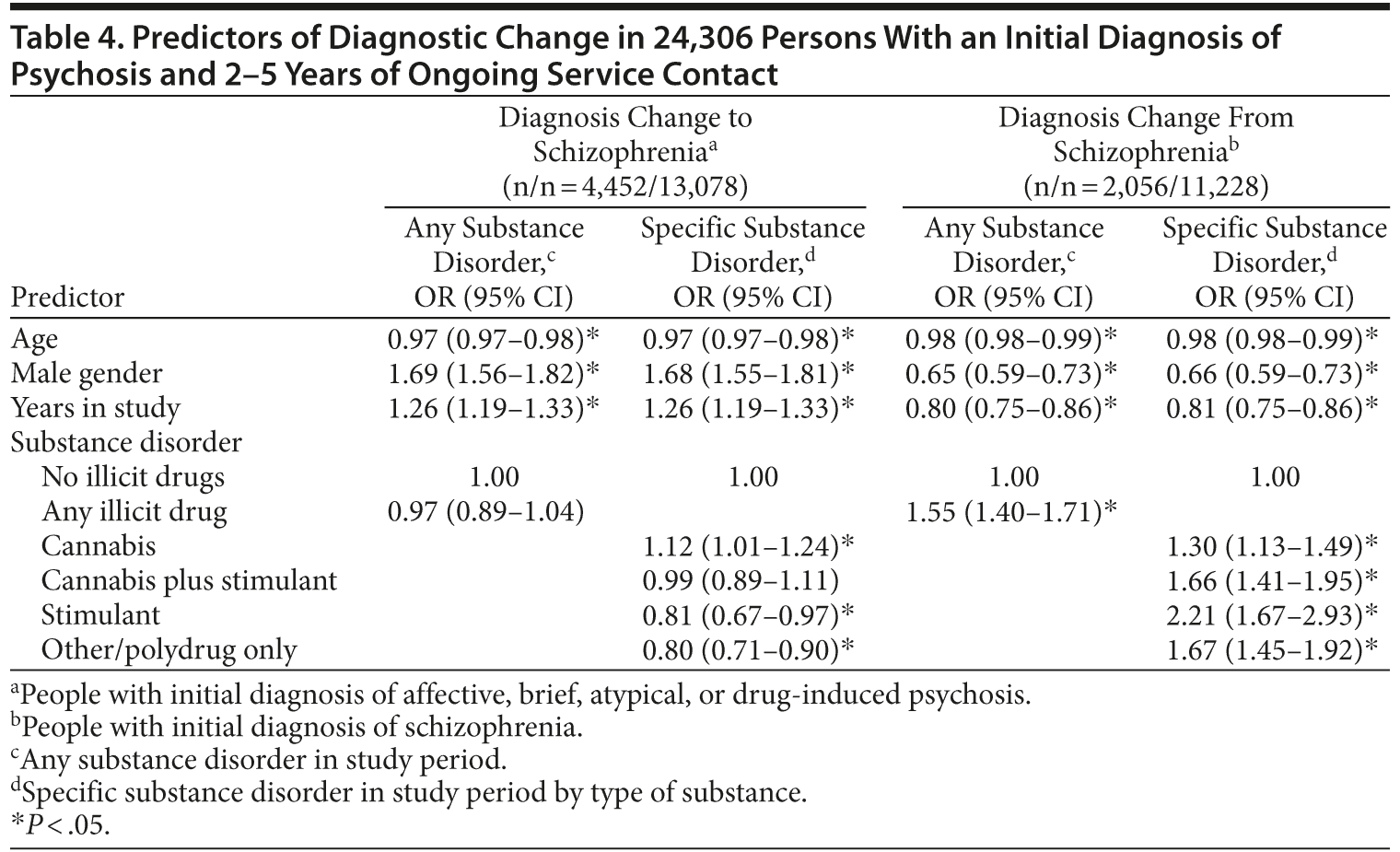
Predictors of diagnostic change were examined separately for people with diagnoses of schizophrenia (n = 11,228) or other psychoses (n = 13,078) at index admission (Table 4). In people with initial diagnoses of affective, brief, or atypical psychoses, male gender and longer duration of observation were associated with increased odds of diagnostic change to schizophrenia. Conversely, in people with initial diagnoses of schizophrenia, female gender was associated with diagnostic revision away from schizophrenia toward other diagnoses. Younger age was associated with diagnostic instability in both directions.
In people with initial diagnoses of affective, brief, or atypical psychoses, illicit substance disorders during the study period were not associated with diagnostic change to schizophrenia after controlling for age, sex, and duration of observation. However, when examined separately, cannabis and stimulant disorders had significant but opposing effects: diagnostic change to schizophrenia was more likely with comorbid cannabis disorders (OR = 1.12; 95% CI, 1.01-1.24) and less likely with stimulant disorders (OR = 0.81; 95% CI, 0.67-0.97) or other drug disorders (OR = 0.80; 95% CI, 0.71-0.90) without cannabis disorders. Of people with an index diagnosis of affective or other psychoses and no substance diagnosis, 34.0% were later diagnosed with schizophrenia compared with 36.6% of those with affective/other psychoses and ongoing cannabis disorders (risk difference, 2.6%; number needed to harm, 39).
In people with initial diagnoses of schizophrenia, all comorbid substance disorders were associated with greater likelihood of diagnostic change toward other diagnoses. This effect was greatest for those with only stimulant disorders, least for those with only cannabis disorders, and intermediate for those with both cannabis and stimulant disorders.
DISCUSSION
We examined diagnostic stability in 24,306 individuals 2 to 5 years after an admission for psychosis. More than 80% of initial diagnoses of brief, atypical, or drug-induced psychoses were later revised, nearly half (46%) to schizophrenia. Index diagnoses of schizophrenia were more stable, but 18% were revised to other conditions over 5 years. The rate of diagnostic change in our sample was consistent with other studies that have found between 29% and 50% of people with brief or drug-induced psychoses later receive a diagnosis of schizophrenia.3,18,30,31 Initial diagnoses of schizophrenia have been revised to other conditions in 8% of people at 2 years17 and up to 21% of people at 5 years.32
Our first aim was to examine whether comorbid substance disorders were associated with diagnostic instability. Nearly half of people with ongoing service contact had at least 1 comorbid illicit substance disorder. Considered together, ongoing substance disorders did not increase the likelihood of diagnostic progression from affective or other psychoses to schizophrenia. These findings are consistent with reports17,33 that substance use after a first psychosis admission predicts unchanged or reduced risk of a later schizophrenia diagnosis. However, we found that substance disorders were associated with diagnostic instability. In people with comorbid substance disorders, initial and final psychosis diagnoses agreed less than half of the time, because there was greater likelihood of revision of diagnosis away from index diagnoses of schizophrenia in people with substance disorders.
Our second aim was to examine whether cannabis and stimulant disorders differed in their associations with diagnostic change. We found that cannabis disorders had a modest association with diagnostic progression to schizophrenia; 1 additional person received this diagnosis for every 39 persons with an ongoing cannabis disorder diagnosis. In contrast, stimulant disorders were associated with diagnoses of briefer psychoses and with diagnostic change away from schizophrenia. These findings add to those of Niemi-Pynttפri and colleagues,27 who examined substance-induced psychoses. Together, these studies underline the importance of examining cannabis and stimulants separately rather than grouping them together.
In people with comorbid diagnoses of both cannabis and stimulant disorders, an additive risk and an increase in the likelihood of developing a more chronic psychosis may be expected. However, we found that people with both diagnoses had an intermediate risk of diagnostic transition to schizophrenia when compared to those with stimulant or cannabis diagnoses alone. There are a number of possible explanations for this finding, which warrant further research. If stimulants have greater potential than cannabis to trigger psychotic states, then they may precipitate psychosis in individuals with a lower personal vulnerability to the development of schizophrenia. Persons with a first admission psychosis who also use stimulants may also have other factors associated with more positive outcomes, such as later age of drug use or psychosis onset and a higher socioeconomic status.20 It is also possible that stimulants and cannabis are associated with different responses to treatment. Stimulants act on the same dopamine pathways through which antipsychotic medications are thought to act,23 and so antipsychotic medications may be more effective in stimulant-related psychoses. Psychoses associated with cannabis use may be less responsive to treatment because they involve abnormalities in other chemical pathways.
Limitations
The hospital data used do not have unique person identifiers prior to the start of the study period. Therefore, we were unable to identify whether individuals had admissions prior to their index admission, and, if so, how many admissions they had. Older participants in our study are more likely to have had prior admissions, while, for younger participants, their index admission in the study period is more likely to have been their first ever hospital admission. Therefore, age and stage of illness are likely to be confounded in our study.
We included only persons with at least 2 years of service contact. People with stimulant disorders and affective, brief, drug-induced, and atypical psychoses were more likely to have brief contact and therefore be excluded from the study. People with no ongoing service contact are less likely to have had severe or enduring psychoses such as schizophrenia. Therefore, we may have underestimated any association between stimulant disorders and positive outcomes.
To obtain a population-wide sample, we have used clinical diagnoses from administrative datasets. Routine diagnoses are less reliable than research diagnoses, and substance comorbidities may be particularly underrecorded.34 In this study, the types of diagnoses, rates of substance comorbidity, and patterns of diagnostic change were similar to those reported in clinical studies. However, caution is needed in interpreting this study’s conclusions, and further evidence from clinical studies using more rigorous diagnoses is required.
We considered substance disorder diagnoses as a measure of ongoing substance problems. This measure is imprecise and cannot distinguish between different levels of duration or severity of substance disorder. Apparent differences between cannabis and stimulant groups in our study may have been due to the effects of different comorbidity with other drugs such as hallucinogens or opiates. However, the rate of comorbid diagnoses with these substances was low.
We derived a single study diagnosis from the most recent diagnosis in the study period. This is 1 of several ways in which multiple diagnoses may be combined.35 Our choice of method was based on testing of competing algorithms against NSW administrative data and research diagnoses (G.E.S., unpublished data, 2014).
CONCLUSIONS
Substance comorbidity is common in people with psychosis and may contribute to diagnostic change by causing diagnostic uncertainty and by influencing the course of illness. Cannabis and stimulants differed in their impact on diagnostic change. Stimulant disorders were associated with diagnostic instability, a lower likelihood of change to schizophrenia, and greater likelihood of diagnostic revision away from schizophrenia. While many people with initial diagnoses of brief and affective psychoses may progress to a diagnosis of schizophrenia, it is important to avoid premature closure on a diagnosis of schizophrenia, particularly when stimulant disorders are present.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this activity.
Author affiliations: InforMH, Mental Health and Drug and Alcohol Office, New South Wales (NSW) Ministry of Health, North Ryde (Dr Sara); Discipline of Psychiatry, Sydney Medical School, University of Sydney (Drs Sara and Malhi); Department of Academic Psychiatry, CADE Clinic, Royal North Shore Hospital (Dr Malhi), Sydney; and School of Population Health, Queensland Centre for Mental Health Research (Drs Sara, Burgess, and Whiteford), and Centre for Clinical Research (Dr Hall), University of Queensland, Brisbane, Australia.
Financial disclosure: Dr Malhi has served on advisory boards for and received research funds and honoraria from AstraZeneca, Eli Lilly, Janssen-Cilag, Lundbeck, Organon, Pfizer, Ranbaxy, Servier, and Wyeth. Dr Hall is supported by a National Medical Research Council Australia Fellowship. Drs Sara, Burgess, and Whiteford have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: No direct financial support was received for the conduct of this study. Data extraction and analysis were conducted by Dr Sara in his role as an employee of the NSW Ministry of Health.
Role of the sponsor: The Ministry of Health played no role in the design, conduct, or publication of the study.
Additional information: Data for NSW public hospital admissions are contained in the NSW Admitted Patient Data Collection (APDC), owned by the NSW Ministry of Health. The APDC data dictionary and information on access can be obtained from the Centre for Electronic Health Record Linkage (http://www.cherel.org.au/data-dictionaries). Reference data for the Australian population was obtained from the Australian Bureau of Statistics Socio-Economic Indices for Areas (http://www.abs.gov.au/ausstats/[email protected]/mf/2033.0.55.001) and the Accessibility/Remoteness Index of Australia (http://www.abs.gov.au/websitedbs/d3310114.nsf/home/remoteness+structure).
REFERENCES
1. Bromet EJ, Kotov R, Fochtmann LJ, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168(11):1186-1194. PubMed doi:10.1176/appi.ajp.2011.11010048
2. Haahr U, Friis S, Larsen TK, et al. First-episode psychosis: diagnostic stability over one and two years. Psychopathology. 2008;41(5):322-329. PubMed doi:10.1159/000146070
3. Crebbin K, Mitford E, Paxton R, et al. First-episode drug-induced psychosis: a medium term follow up study reveals a high-risk group. Soc Psychiatry Psychiatr Epidemiol. 2009;44(9):710-715. PubMed doi:10.1007/s00127-008-0490-2
4. Whitty P, Clarke M, McTigue O, et al. Diagnostic stability four years after a first episode of psychosis. Psychiatr Serv. 2005;56(9):1084-1088. PubMed doi:10.1176/appi.ps.56.9.1084
5. Addington J, Addington D. Patterns, predictors and impact of substance use in early psychosis: a longitudinal study. Acta Psychiatr Scand. 2007;115(4):304-309. PubMed doi:10.1111/j.1600-0447.2006.00900.x
6. Wade D, Harrigan S, Edwards J, et al. Patterns and predictors of substance use disorders and daily tobacco use in first-episode psychosis. Aust N Z J Psychiatry. 2005;39(10):892-898. PubMed
7. Kavanagh DJ, Waghorn G, Jenner L, et al. Demographic and clinical correlates of comorbid substance use disorders in psychosis: multivariate analyses from an epidemiological sample. Schizophr Res. 2004;66(2-3):115-124. PubMed doi:10.1016/S0920-9964(03)00130-0
8. Moore E, Mancuso SG, Slade T, et al. The impact of alcohol and illicit drugs on people with psychosis: the second Australian National Survey of Psychosis. Aust N Z J Psychiatry. 2012;46(9):864-878. PubMed doi:10.1177/0004867412443900
9. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518. PubMed doi:10.1001/jama.1990.03450190043026
10. Mathias S, Lubman DI, Hides L. Substance-induced psychosis: a diagnostic conundrum. J Clin Psychiatry. 2008;69(3):358-367. PubMed doi:10.4088/JCP.v69n0304
11. González-Pinto A, Alberich S, Barbeito S, et al. Cannabis and first-episode psychosis: different long-term outcomes depending on continued or discontinued use. Schizophr Bull. 2011;37(3):631-639. PubMed doi:10.1093/schbul/sbp126
12. Wade D, Harrigan S, Edwards J, et al. Substance misuse in first-episode psychosis: 15-month prospective follow-up study. Br J Psychiatry. 2006;189(3):229-234. PubMed doi:10.1192/bjp.bp.105.017236
13. Wade D, Harrigan S, McGorry PD, et al. Impact of severity of substance use disorder on symptomatic and functional outcome in young individuals with first-episode psychosis. J Clin Psychiatry. 2007;68(5):767-774. PubMed doi:10.4088/JCP.v68n0517
14. Sorbara F, Liraud F, Assens F, et al. Substance use and the course of early psychosis: a 2-year follow-up of first-admitted subjects. Eur Psychiatry. 2003;18(3):133-136. PubMed doi:10.1016/S0924-9338(03)00027-0
15. Bertelsen M, Jeppesen P, Petersen L, et al. Course of illness in a sample of 265 patients with first-episode psychosis—five-year follow-up of the Danish OPUS trial. Schizophr Res. 2009;107(2-3):173-178. PubMed doi:10.1016/j.schres.2008.09.018
16. Malla A, Norman R, Bechard-Evans L, et al. Factors influencing relapse during a 2-year follow-up of first-episode psychosis in a specialized early intervention service. Psychol Med. 2008;38(11):1585-1593. PubMed doi:10.1017/S0033291707002656
17. Schwartz JE, Fennig S, Tanenberg-Karant M, et al. Congruence of diagnoses 2 years after a first-admission diagnosis of psychosis. Arch Gen Psychiatry. 2000;57(6):593-600. PubMed doi:10.1001/archpsyc.57.6.593
18. Naz B, Bromet EJ, Mojtabai R. Distinguishing between first-admission schizophreniform disorder and schizophrenia. Schizophr Res. 2003;62(1-2):51-58. PubMed doi:10.1016/S0920-9964(02)00332-8
19. Hall W, Degenhardt L. Cannabis and the increased incidence and persistence of psychosis. BMJ. 2011;342:d719. PubMed doi:10.1136/bmj.d719
20. Sara G, Burgess P, Malhi GS, et al. Differences in associations between cannabis and stimulant disorders in first admission psychosis. Schizophr Res. 2013;147(2-3):216-222. PubMed doi:10.1016/j.schres.2013.04.017
21. Hides L, Dawe S, Kavanagh DJ, et al. Psychotic symptom and cannabis relapse in recent-onset psychosis: prospective study. Br J Psychiatry. 2006;189(2):137-143. PubMed doi:10.1192/bjp.bp.105.014308
22. Angrist B, Corwin J, Bartlik B, et al. Early pharmacokinetics and clinical effects of oral D-amphetamine in normal subjects. Biol Psychiatry. 1987;22(11):1357-1368. PubMed doi:10.1016/0006-3223(87)90070-9
23. Hermens DF, Lubman DI, Ward PB, et al. Amphetamine psychosis: a model for studying the onset and course of psychosis. Med J Aust. 2009;190(suppl):S22-S25. PubMed
24. Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. Br J Psychiatry. 2004;185(3):196-204. PubMed doi:10.1192/bjp.185.3.196
25. Keshavan MS, Tandon R, Boutros NN, et al. Schizophrenia, “just the facts”: what we know in 2008 part 3: neurobiology. Schizophr Res. 2008;106(2-3):89-107. PubMed doi:10.1016/j.schres.2008.07.020
26. Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358-371. PubMed doi:10.1177/026988119901300405
27. Niemi-Pynttפri JA, Sund R, Putkonen H, et al. Substance-induced psychoses converting into schizophrenia: a register-based study of 18,478 Finnish inpatient cases. J Clin Psychiatry. 2013;74(1):e94-e99. PubMed doi:10.4088/JCP.12m07822
28. National Centre for Classification in Health. The International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification. 7th ed. Sydney, Australia: National Centre for Classification in Health, Faculty of Health Sciences, The University of Sydney; 2010.
29. Belsley D. A guide to using the collinearity diagnostics. Comput Sci Econ Managet. 1991;4:33-50.
30. Arendt M, Rosenberg R, Foldager L, et al. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Br J Psychiatry. 2005;187(6):510-515. PubMed doi:10.1192/bjp.187.6.510
31. Castagnini A, Bertelsen A, Berrios GE. Incidence and diagnostic stability of ICD-10 acute and transient psychotic disorders. Compr Psychiatry. 2008;49(3):255-261. PubMed doi:10.1016/j.comppsych.2007.10.004
32. Baca-Garcia E, Perez-Rodriguez MM, Basurte-Villamor I, et al. Diagnostic stability of psychiatric disorders in clinical practice. Br J Psychiatry. 2007;190(3):210-216. PubMed doi:10.1192/bjp.bp.106.024026
33. Bromet EJ, Naz B, Fochtmann LJ, et al. Long-term diagnostic stability and outcome in recent first-episode cohort studies of schizophrenia. Schizophr Bull. 2005;31(3):639-649. PubMed doi:10.1093/schbul/sbi030
34. Large MM, Smith GS, Sara G, et al. Meta-analysis of self-reported substance use compared with laboratory substance assay in general adult mental health settings. Int J Methods Psychiatr Res. 2012;21(2):134-148. PubMed doi:10.1002/mpr.1350
35. Morgan VA, Jablensky AV. From inventory to benchmark: quality of psychiatric case registers in research. Br J Psychiatry. 2010;197(1):8-10. PubMed doi:10.1192/bjp.bp.109.076588