ABSTRACT
Objective: To describe lemborexant for the treatment of insomnia (DSM-5) in adults using number needed to treat (NNT), number needed to harm (NNH), and likelihood to be helped or harmed (LHH).
Methods: Lemborexant data were obtained from two Phase 3 trials conducted 2016–2018. Efficacy was assessed using different categorical definitions for response, and tolerability was assessed by evaluating rates of adverse events (AEs). Direct comparisons were made with zolpidem extended release (ER), and indirect comparisons were made with other hypnotic agents, including suvorexant, doxepin, ramelteon, zolpidem immediate release, eszopiclone, zaleplon, and selected benzodiazepines, using data from published reports and regulatory documents.
Results: Lemborexant had a clinically relevant magnitude of therapeutic effect, as evidenced by NNT values versus placebo as robust as 3 (95% CI, 2–3). In general, NNH values for lemborexant versus placebo were ≥ 10, suggesting that lemborexant is relatively tolerable. Somnolence was the most common AE, with NNH estimates of 28 (95% CI, 18–61) and 15 (95% CI, 11–22) for lemborexant 5 mg and 10 mg, respectively. Rates of discontinuation of lemborexant because of an AE were low, and for lemborexant 5 mg the rate was lower than that for placebo. LHH contrasting the statistically significant endpoint efficacy measures versus discontinuation because of an AE ranged from 13 to 54. NNT values for lemborexant were generally more robust than for zolpidem ER for the polysomnography and sleep diary outcomes. In indirect comparisons, NNT data for the other hypnotics demonstrated effect sizes that were generally similar to those for lemborexant.
Conclusions: In Phase 3 trials, the benefit-risk ratio for lemborexant is favorable as measured by NNT, NNH, and LHH.
Trial Registration: ClinicalTrials.gov identifiers: NCT02783729, NCT02952820
J Clin Psychiatry 2021;82(4):20m13795
To cite: Citrome L, Juday T, Frech F, et al. Lemborexant for the treatment of insomnia: direct and indirect comparisons with other hypnotics using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Clin Psychiatry. 2021;82(4):20m13795.
To share: https://doi.org/10.4088/JCP.20m13795
© Copyright 2021 Physicians Postgraduate Press, Inc.
aNew York Medical College, Valhalla, New York
bEisai Inc., Woodcliff Lake, New Jersey
*Corresponding author: Leslie Citrome, MD, MPH, 11 Medical Park Drive, Ste 102, Pomona, NY 10970 ([email protected]).
Problems with sleep are commonly encountered in routine clinical practice in both primary and specialty care, and current diagnostic guidance encourages the identification of insomnia disorder whether it occurs as an independent condition or is comorbid with another psychiatric or medical condition.1 Left untreated, insomnia can be associated with marked impairment in function and quality of life as well as psychiatric and physical morbidity.2 Several considerations are involved in the management of insomnia; these considerations include whether insomnia symptoms persist despite good sleep hygiene and/or treatment of any underlying conditions, as well as a patient’s suitability for targeted interventions such as cognitive-behavioral therapy or pharmacotherapy.3
It can be challenging to select among the different hypnotics available, especially for new agents that may be unfamiliar to clinicians and patients. When evaluating potential treatments using data from registrational trials, testing for statistical significance for drug versus placebo is insufficient. Consideration must also be made of the size of the treatment effect. Effect size can describe the potential importance of an intervention’s efficacy and tolerability profile. Clinically intuitive measures of effect size include number needed to treat (NNT) to describe benefit (therapeutic response) and number needed to harm (NNH) to describe untoward events such as an adverse event (AE) or discontinuation due to an AE4,5 (see also Supplementary Box 1). The ratio of NNH to NNT can further describe the benefit-risk ratio and is called “likelihood to be helped or harmed” (LHH).5 This approach can be especially valuable when assessing new treatments and when head-to-head comparisons with other agents are generally not available. A recent example of using NNT, NNH, and LHH is the evaluation of a novel treatment for treatment-resistant major depressive disorder.6
Lemborexant, a dual orexin receptor antagonist (DORA), has been approved by the US Food and Drug Administration (FDA) for the treatment of adult patients with insomnia (as characterized by difficulties with sleep onset and/or sleep maintenance)7 and is also available in Japan and Canada. The mechanism of action of DORAs, which attenuate excessive wakefulness/arousal signaling, differs from that of hypnotic agents such as γ-aminobutyric acid (GABA)-A receptor agonists (for example, the benzodiazepine temazepam and the non-benzodiazepine zolpidem) and others (for example, the melatonin receptor agonist ramelteon) that augment sleep signaling.8
This study reviews the evidence base for lemborexant for the treatment of insomnia in adults using the metrics of NNT, NNH, and LHH to help place this intervention into clinical perspective. In addition to comparisons with placebo, which in turn permit indirect comparisons with other hypnotics for which studies with placebo controls are available, direct comparisons are made with zolpidem extended release (ER), which served as an active treatment arm in one of the two Phase 3 trials that were conducted with lemborexant.
METHODS
Overview
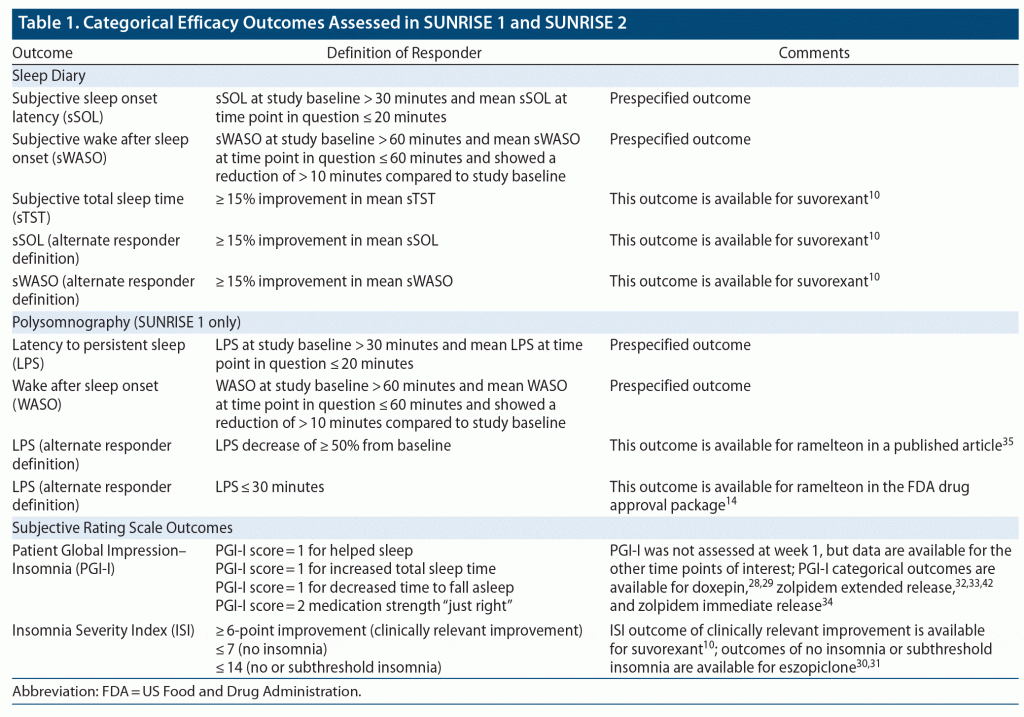
Data were taken from the two Phase 3 randomized placebo-controlled trials of lemborexant for the treatment of insomnia (DSM-5) in adults: SUNRISE 1 (NCT02783729, E2006-G000-304) and SUNRISE 2 (NCT02952820, E2006-G000-303), conducted 2016–2018. The study protocols were reviewed and approved by the relevant Institutional Review Board or Independent Ethics Committee at each study site, and informed consent was obtained from all participants. Outcome measures examined were different categorical definitions for response using several rating thresholds, and several categorical tolerability outcomes including AEs of interest, similar to what has been reported with suvorexant.9,10 Direct comparisons were made with an active control/comparator (zolpidem ER). Indirect comparisons were made with other hypnotics for which similar data are available; to that end, supplementing the data collected in the lemborexant clinical trial program are data as reported in the Drug Approval Packages made available by the FDA (for a description of a drug approval package, see Citrome11). Specifically, the FDA drug approval packages for suvorexant, doxepin, ramelteon, eszopiclone, zaleplon, zolpidem immediate release (IR), and zolpidem ER12–18 were screened for the existence of responder analyses to help inform the selection of additional efficacy outcomes to be extracted from the lemborexant clinical trial database. Relevant drug approval packages are not available for the commonly used benzodiazepine hypnotics triazolam, temazepam, and flurazepam. AE rates for the hypnotics are as extracted from their respective product labels when such data are provided, including those for triazolam, temazepam, and flurazepam,9,19–27 and refined when additional information was available from the relevant drug approval package. Some of the many parallel-group, placebo-controlled, registrational studies that were used to support approval of lemborexant and the other hypnotics as reported in the published literature and in briefing documents28–42 provided additional data. Limited categorical data (see also Table 1) are available on subjective outcomes (including, depending on the agent, at least one of the following measures: subjective total sleep time [sTST], subjective sleep onset latency [sSOL], subjective wake after sleep onset [sWASO], scores on the Insomnia Severity Index [ISI], scores on the Patient Global Impression–Insomnia [PGI-I], or scores on Clinical Global Impression items that are consistent with the PGI-I) for suvorexant,10 doxepin,28,29 eszopiclone,30,31 zolpidem ER,32,33,42 and zolpidem IR34 and on objective outcomes (latency to persistent sleep [LPS]) for ramelteon.14,35 Less information is available for the agents approved decades ago but still in use, namely triazolam (approved in 1982) and temazepam (approved in 1981), and no specific AE rates are available for flurazepam (approved in 1970) (approval years from https://www.accessdata.fda.gov/, accessed April 19, 2020).
Description of Studies SUNRISE 1 and SUNRISE 2
SUNRISE 1 was a 1-month, global, randomized, double-blind, parallel-group, placebo-controlled, active-comparator study conducted at 67 sites in North America and Europe.37 Participants were aged 55 years or older and had insomnia disorder characterized by reported sleep maintenance difficulties and confirmed by sleep history, sleep diary, and polysomnography (PSG). Participants could also have had sleep onset difficulties, but this was not required. Participants received placebo, zolpidem ER 6.25 mg, lemborexant 5 mg, or lemborexant 10 mg for 1 month at bedtime. All patients received instructions consistent with principles of good sleep hygiene. Paired polysomnograms were collected at baseline during a single blind placebo run-in period, the first 2 nights or treatment, and the last 2 nights of treatment. Among 1,006 participants randomized (placebo, n = 208; zolpidem ER 6.25 mg, n = 263; lemborexant 5 mg, n = 266; and lemborexant 10 mg, n = 269), 869 (86.4%) were women, 256 (24.4%) were Black or African American, and the median age was 63 years (range, 55–88 years). Both lemborexant 5 mg and lemborexant 10 mg demonstrated statistically significant greater changes on the primary outcome measure of change from baseline in objective sleep onset as assessed by LPS as measured by PSG at the end of 1 month compared with placebo. The key secondary endpoints of change from baseline in sleep efficiency and wake after sleep onset (WASO) also demonstrated superiority of lemborexant over placebo.
SUNRISE 2 was a 12-month, global, randomized, double-blind (first 6 months), parallel-group, placebo-controlled study conducted at 119 sites in North America, Europe, Asia, and Oceania.38 Participants were aged 18 years or older and had insomnia disorder, with complaints of sleep onset difficulties, sleep maintenance difficulties, or both. Participants received placebo, lemborexant 5 mg, or lemborexant 10 mg for 6 months at bedtime (Period 1), followed by lemborexant 5 mg and lemborexant 10 mg for an additional 6 months (Period 2); subjects randomized to placebo for the first 6 months in Period 1 were re-randomized to receive either lemborexant 5 mg or lemborexant 10 mg in Period 2. All patients received instructions consistent with principles of good sleep hygiene. An Electronic Sleep Diary was completed. Among 971 participants randomized (placebo, n = 325; lemborexant 5 mg, n = 323; and lemborexant 10 mg, n = 323), 643 (66.2%) were women, 76 (8.0%) were Black or African American, and the median age was 55 years (range, 18–88 years). Decreases from baseline in sSOL (the primary endpoint) were significantly greater with lemborexant 5 mg and lemborexant 10 mg versus placebo at month 6. The key secondary endpoints of change from baseline in subjective sleep efficiency and sWASO also demonstrated superiority of lemborexant over placebo.
Efficacy Outcomes
Examined were categorical efficacy outcomes of clinical interest, occurring during the double-blind period, as listed in Table 1; in addition to prespecified protocol-determined definitions of response, additional responder categories were assessed based on available data for the other hypnotics. The denominator was the number of randomized subjects who received at least one dose of study drug and had a post-baseline assessment on the efficacy outcome of interest. Data were extracted by study arm. Time points examined for both studies for non-PSG measures included week 1 and month 1. For SUNRISE 2, additional time points were month 3 and month 6. For SUNRISE 1, the time points examined for the PSG outcomes were day 1, day 2, day 29, and day 30.
Tolerability Outcomes
Examined were discontinuation from the clinical trial because of an AE and treatment emergent AEs occurring at any time during the double-blind period. The denominator was the number of all randomized subjects who had received at least one dose of study drug. Data were extracted by study arm for each study. Threshold for reporting AEs was a rate of ≥ 1% for any individual active arm of SUNRISE 1 or SUNRISE 2. When pooling the AE data for SUNRISE 1 and SUNRISE 2, only events occurring in the first month of SUNRISE 2 were included; threshold for reporting was a rate of ≥ 1% for any dose of lemborexant, with reporting of the following AEs regardless of rate: sleep paralysis, dizziness, and fall.
Data Analysis
NNT and NNH, with their respective 95% CIs, were calculated for lemborexant 5/10 mg versus placebo, individually for each study and pooled as appropriate. If there was an active control, analogous analyses were done comparing the active control versus placebo and lemborexant was directly compared with the active control. LHH was calculated to illustrate potential trade-offs for efficacy and tolerability outcomes, specifically response versus the most encountered AE and for discontinuation because of an AE. In all instances, if the 95% CI included “infinity,” the result was considered not statistically significant at the P < .05 threshold. The terms statistically significant and not statistically significant are used descriptively and not inferentially. The notation NS is used rather than showing the non-continuous 95% CIs generated when statistical significance was not achieved. If the AE rates were the same or lower for drug versus placebo, the notation no difference was made. Formulae used are listed in Supplementary Box 2.
RESULTS
Results are provided as follows and in Table 2 and Table 3, Supplementary Tables 1–21, Figure 1 and Figure 2, and Supplementary Figure 1. Discussed first are the efficacy and tolerability outcomes from SUNRISE 1 and SUNRISE 2, followed by indirect comparisons with other agents using data from other clinical trials.
Direct Comparisons of Efficacy
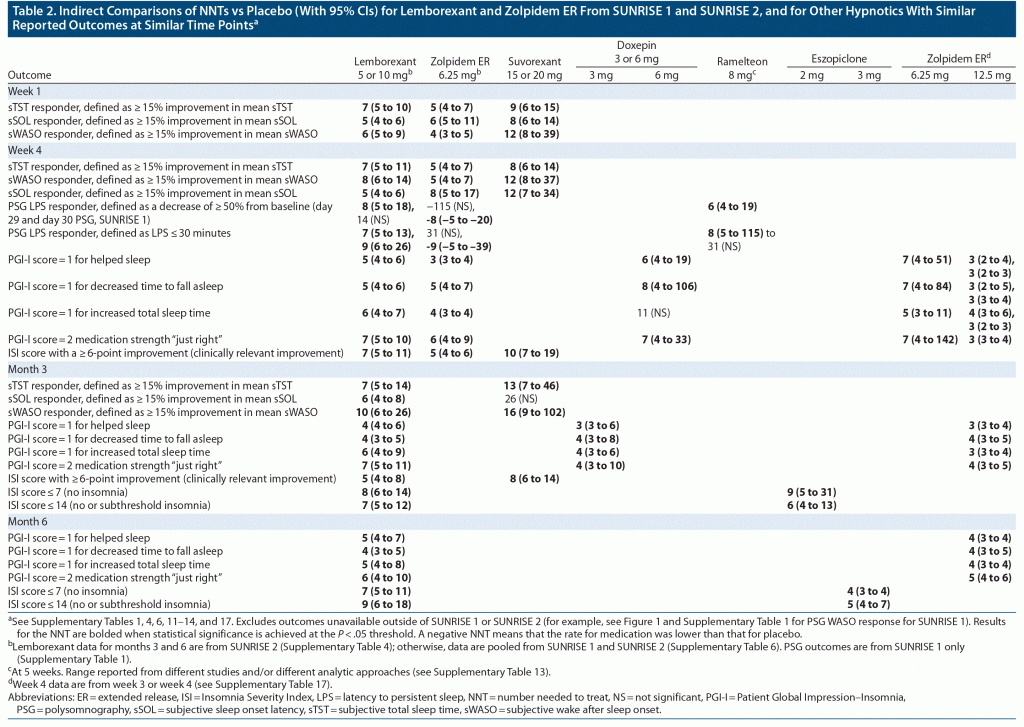
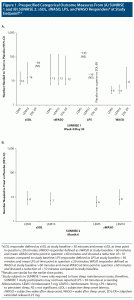
In SUNRISE 1, effect sizes for the subjective efficacy outcomes for lemborexant 5/10 mg versus placebo were similar between week 1 and week 4 (prespecified sSOL and sWASO response at week 4 is illustrated in Figure 1), indicating that there is little or no lag time between start of therapy and onset of efficacy (see Supplementary Table 1). In general, sTST/sWASO/sSOL outcomes based on 15% improvement thresholds had more robust NNT values than the prespecified sSOL/sWASO outcomes based on absolute time thresholds. Most NNT values versus placebo were < 10, and some were as low as 4, suggesting that lemborexant 5/10 mg had a clinically relevant magnitude of therapeutic effect. Results for zolpidem ER 6.25 mg versus placebo in this study showed a similar pattern, but with generally weaker effect sizes except for the PGI-I and ISI outcomes. When directly comparing lemborexant 5/10 mg with zolpidem ER 6.25 mg (Supplementary Table 2), NNT values < 10 were observed at week 4 for sSOL response defined by ≥ 15% improvement for lemborexant 10 mg and pooled lemborexant 5 mg/lemborexant 10 mg, demonstrating a small advantage for lemborexant on this outcome.
The PSG prespecified categorical outcomes of LPS and WASO response at month 1 from the SUNRISE 1 study are shown in Figure 1. PSG outcomes at days 1, 2, 29, and 30 are listed in Supplementary Tables 1 and 2, the latter including direct comparisons of lemborexant 5/10 mg versus zolpidem ER 6.25 mg. LPS response, defined in the study protocol as LPS at study baseline > 30 minutes and mean LPS at time point in question ≤ 20 minutes, demonstrated statistically significantly superiority of lemborexant 10 mg to placebo only at day 29 (NNT = 13; 95% CI, 7–625). Of note, subjects did not need to report sleep onset difficulties for inclusion in SUNRISE 1. WASO response, defined in the study protocol as WASO at study baseline > 60 minutes and mean WASO at time point in question ≤ 60 minutes and showing a reduction of > 10 minutes compared to study baseline, consistently demonstrated statistically significant superiority of lemborexant 5/10 mg to placebo, with robust effect sizes as low as a NNT of 3. When subjects with missing information due to early withdrawal or other reasons were considered as nonresponders, effect sizes for WASO response remained robust for both lemborexant 5/10 mg and zolpidem ER 6.25 mg (Supplementary Table 3). For lemborexant 5/10 mg and zolpidem ER 6.25 mg, the effect sizes for WASO response were stronger at day 1/2 versus day 29/30. Zolpidem ER 6.25 mg in SUNRISE 1 performed poorly on the LPS measures (Supplementary Table 1), with “negative” NNT values versus placebo that were statistically significant at day 30 (ie, placebo superior to zolpidem ER release 6.25 mg on this outcome). Although not a prespecified outcome in the original statistical analysis plan as such, when directly comparing lemborexant 5/10 mg with zolpidem ER 6.25 mg, NNT values were < 10 in favor of lemborexant 5/10 mg for all listed PSG outcomes at day 30 (LPS and WASO response) and for many of the outcomes at the earlier time points of day 1, 2, and 29 (Supplementary Table 2).
In SUNRISE 2 (Figure 1, Supplementary Table 4), the pattern of results for lemborexant 5/10 mg versus placebo was similar to that for SUNRISE 1 but with more robust effect sizes (ie, smaller NNT values) for the prespecified outcome measures of sSOL and sWASO. When subjects with missing information due to early withdrawal or other reasons were considered as nonresponders in the analysis, effect sizes for sWASO response did not become consistently statistically significant until toward the end of the study, and more so for lemborexant 5 mg than for lemborexant 10 mg (Supplementary Table 5). The most robust effect sizes were noted at months 4 and 6.
Pooled subjective efficacy results for lemborexant 5/10 mg from both SUNRISE 1 and SUNRISE 2 for the first 4 weeks are shown in Supplementary Table 6. The pattern of results remains the same, with NNT values versus placebo < 10 for the majority of the outcomes for lemborexant 5/10 mg.
Indirect Comparisons of Efficacy
Table 2 describes indirect comparisons of the NNTs versus placebo for lemborexant 5/10 mg and zolpidem ER 6.25 mg from SUNRISE 1 and SUNRISE 2 and for other hypnotics with similar reported outcomes at similar time points (Supplementary Tables 1, 4, 6, 11–14, and 17). In general, effect sizes versus placebo for lemborexant 5/10 mg were larger (NNT values smaller) than those for the other available DORA, suvorexant, at week 1, week 4, and month 3 (the time points for which data are available for both agents) for sTST/sWASO/sSOL outcomes based on based on 15% improvement thresholds and for ISI response as defined by a ≥ 6-point improvement (clinically relevant improvement).10,12,36 Regarding hypnotics with fundamentally different mechanisms of action, doxepin 3 mg at month 3 and 6 mg at week 4 demonstrated effect sizes versus placebo similar to that for lemborexant 5/10 mg on PGI-I outcomes.28,29 Data for eszopiclone were limited to ISI outcomes at month 6 (nonelderly adults) and week 12 (elderly adults), and NNT values for the 2-mg dose were similar to those for lemborexant 5/10 mg; an apparent dose-response is observed for eszopiclone, with more robust NNT values observed at the higher dose (and more robust than seen with lemborexant 5/10 mg).30,31 Zolpidem IR ≤ 10 mg outcomes (Supplementary Table 16)34 mirrored the effect sizes observed in the SUNRISE 1 study for zolpidem ER 6.25 mg, as did outcomes for the registrational studies for zolpidem ER that examined zolpidem ER 12.5 mg (Supplementary Table 17).32,33 However, although taken from different studies, week 3 data on the PGI-I outcomes for zolpidem ER 6.25 mg from the zolpidem ER registrational studies were somewhat weaker than the corresponding data for the 12.5 mg dose.32,33,42
Only one other hypnotic—ramelteon—had available PSG categorical outcome results allowing for indirect comparison, which evidenced effect sizes similar to those for lemborexant 5/10 mg with NNT versus placebo < 10 on LPS response (defined as LPS decrease of ≥ 50% from baseline or LPS ≤ 30 minutes) at 1 month (Table 2, Supplementary Table 13).14,35
Direct Comparisons of Tolerability
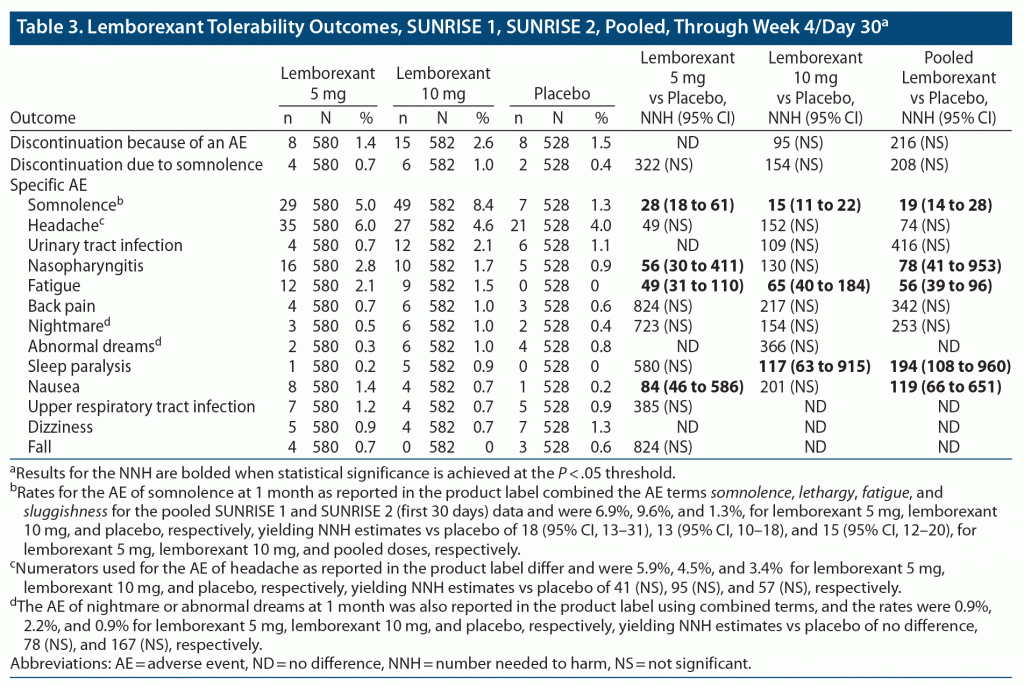
Table 3 provides the pooled lemborexant tolerability outcomes from SUNRISE 1 and SUNRISE 2 (through week 4/day 30). Supplementary Tables 7–9 provide the data from the individual studies, including direct comparisons of lemborexant 5/10 mg versus zolpidem ER 6.25 mg from SUNRISE 1. From pooled data through week 4/day 30, the rates of discontinuation because of an AE were similar for lemborexant 5 mg and placebo (1.4% vs 1.5%), but about double for lemborexant 10 mg (2.6%), with resultant NNH estimates versus placebo of no difference, 95 (NS), and 216 (NS) for lemborexant 5 mg, 10 mg, and pooled doses, respectively. The most common reason for discontinuation of lemborexant was somnolence, with rates of 0.7% for lemborexant 5 mg, 1.0% for lemborexant 10 mg, and 0.4% for placebo, with resultant NNH estimates versus placebo of 322 (NS), 154 (NS), and 208 (NS) for lemborexant 5 mg, lemborexant 10 mg, and pooled doses, respectively. Somnolence was the most common AE, with statistically significant NNH estimates versus placebo of 28 (95% CI, 18–61), 15 (95% CI, 11–22), and 19 (95% CI, 14–28) for lemborexant 5 mg, lemborexant 10 mg, and pooled doses, respectively. Supplementary Figure 1 illustrates the risk of somnolence across SUNRISE 1 and SUNRISE 2 treatment arms. Other AEs had lower incidence rates, smaller differences from placebo, and thus very small effect sizes (ie, high NNH values).
For SUNRISE 1, discontinuation rates because of an AE were low overall for both doses of lemborexant, 0.8% and 1.1% for lemborexant 5 mg and lemborexant 10 mg, respectively, and were like that observed for placebo (1.0%). The discontinuation rate because of an AE was higher for zolpidem ER 6.25 mg (2.7%); however, the NNH versus placebo of 59 for that agent was not statistically significant. In the SUNRISE 1 study, no discontinuation rates because of any specific AE met the threshold of 1% in any study arm. Discontinuation rates because of somnolence were low (0.4%, 0%, 0.4%, and 0.5%, for lemborexant 5 mg, lemborexant 10 mg, zolpidem ER 6.25 mg, and placebo, respectively) and did not demonstrate a dose-response. Regarding the specific AE of somnolence, although the NNH versus placebo for somnolence for lemborexant 5 mg was not statistically significant, it was statistically significant for lemborexant 10 mg (NNH = 20; 95% CI, 12–64) and for the two doses pooled (NNH = 27; 95% CI, 16–100); thus, somnolence appears dose-related. Although rates of somnolence were lower for zolpidem ER 6.25 mg than for placebo, zolpidem ER 6.25 mg evidenced a statistically significant NNH versus placebo for fatigue (NNH = 66; 95% CI, 34–2,393). Further details about other AEs can be found in Supplementary Tables 7 and 8. In direct comparisons of lemborexant 5/10 mg with zolpidem ER 6.25 mg (Supplementary Table 8), NNH for somnolence for lemborexant 5 mg versus zolpidem ER 6.25 mg was 39 (NS) but for lemborexant 10 mg versus zolpidem ER 6.25 mg was 18 (95% CI, 12–47). Differences regarding other AEs were smaller in magnitude.
The time interval for reporting AEs in SUNRISE 2 was 6 months, allowing for more events of different types to be enumerated than for the 1-month duration of SUNRISE 1. Rates of discontinuation because of an AE were similar for lemborexant 5 mg and placebo (4.1% vs 3.8%), but about double for lemborexant 10 mg (8.3%), yielding a statistically significant NNH for lemborexant 10 mg versus placebo of 23 (95% CI, 13–122). Rates of discontinuation because of somnolence were 1.0% for lemborexant 5 mg, 2.9% for lemborexant 10 mg, and 0.6% for placebo, with a NNH versus placebo for discontinuation because of somnolence of 305 (NS), 45 (95% CI, 24–499), and 78 (NS) for lemborexant 5 mg, lemborexant 10 mg, and pooled doses, respectively. Rates of discontinuation because of nightmare were 0.3% for lemborexant 5 mg, 1.3% for lemborexant 10 mg, and 0% for placebo, with a NNH versus placebo for discontinuation because of nightmare of 314 (NS), 79 (95% CI, 40–2,990), and 126 (95% CI, 68–990) for lemborexant 5 mg, lemborexant 10 mg, and pooled doses, respectively. Preexisting history of nightmares was not known. Somnolence was the most common AE and, consistent with SUNRISE 1, was dose-related. NNH for somnolence for lemborexant 5/10 mg versus placebo was statistically significant, and for lemborexant 10 mg (but not lemborexant 5 mg) the NNH was < 10; NNH for both doses pooled was 11 (95% CI, 9–16). At the 3-month time point (of interest because of data available for other hypnotics), somnolence rates were 26/323 (8.0%), 38/323 (11.8%), and 4/325 (1.2%) for lemborexant 5 mg, lemborexant 10 mg, and placebo, respectively, resulting in NNH values versus placebo of 15 (95% CI, 10–28), 10 (95% CI, 7–15), and 12 (95% CI, 9–17) for lemborexant 5 mg, lemborexant 10 mg, and pooled doses, respectively. Fatigue was the other AE that achieved statistical significance for NNH versus placebo for lemborexant 5/10 mg, with estimates of 29 (95% CI, 18–77), 32 (95% CI, 19–94), and 30 (95% CI, 21–57) for lemborexant 5 mg, lemborexant 10 mg, and pooled doses, respectively. Other AEs evidenced less important effect sizes and were more commonly encountered with lemborexant 10 mg than with lemborexant 5 mg. Overall, lemborexant 5 mg appears to have been better tolerated than lemborexant 10 mg.
Indirect Comparisons of Tolerability
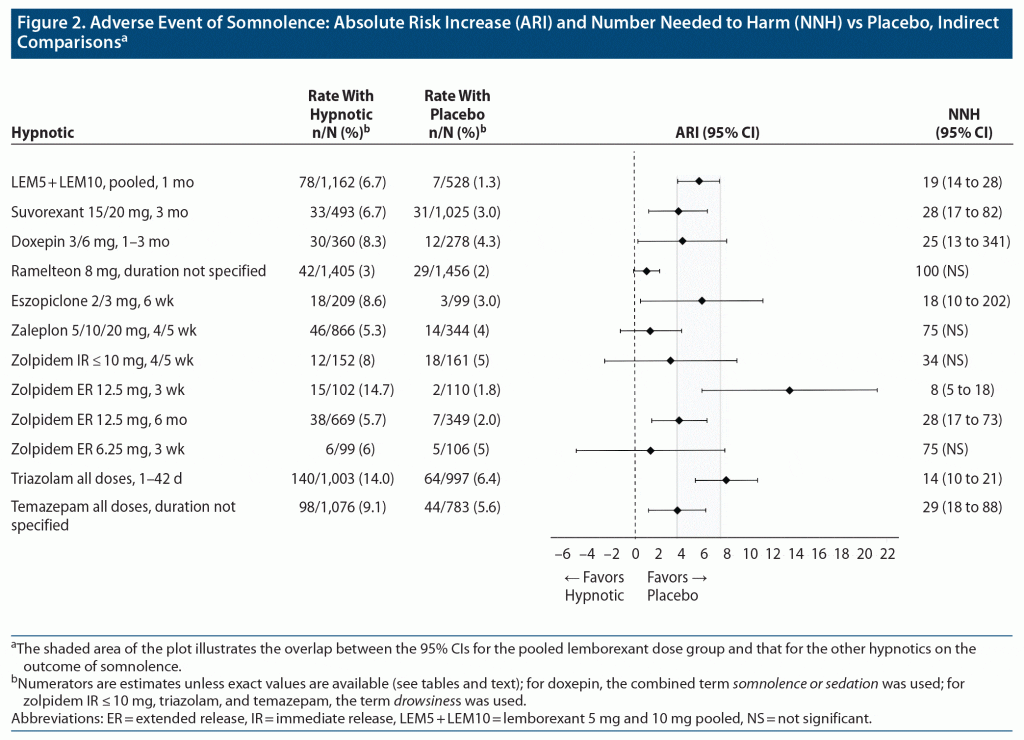
Figure 2 shows a forest plot of the absolute risk increase versus placebo for the AE of somnolence for the pooled doses of lemborexant from SUNRISE 1 and SUNRISE 2 (30 days) and the AE of somnolence for hypnotics from other studies. For doxepin, the combined AE terms somnolence and sedation were reported; for zolpidem IR ≤ 10 mg, triazolam, and temazepam, the AE term drowsiness was reported. Except for ramelteon, there was overlap of the 95% CIs with lemborexant and all of the other included hypnotics.
NNH estimates for somnolence for eszopiclone for non-elderly adults were like those for lemborexant (Supplementary Tables 10 and 14A). Somnolence with zolpidem IR appeared dose dependent and could also be clinically relevant (Supplementary Table 16). In one study of zolpidem ER 12.5 mg, the NNH versus placebo for somnolence at 3 weeks was 8 (95% CI, 5–18); however, in another study at the lower dose of 6.25 mg, the NNH versus placebo at 3 weeks was 75 (NS) (Supplementary Table 17). In a longer study of zolpidem ER 12.5 mg, the NNH versus placebo at 6 months was 28 (95% CI, 17–73) (Supplementary Table 17). NNH estimates for suvorexant and doxepin versus placebo for somnolence were statistically significant and were also similar to the NNH for lemborexant 5 mg for the single AE term for somnolence but were generally more favorable than that for lemborexant for the combined terms as reported in product labeling (see notes in Table 3). Both ramelteon and zaleplon did not appear to carry significant risk for somnolence, with NNH values versus placebo of 100 (NS) and 75 (NS), respectively (Supplementary Tables 13 and 15).
Overall, in general when examining the rates of AEs for other hypnotics (as per Supplementary Tables 11–19), NNH values < 10 were seldomly encountered. However, they could be found for unpleasant taste with eszopiclone (Supplementary Tables 14A and 14B) and “nervous system disorders” and somnolence with zolpidem ER 12.5 mg (Supplementary Table 17). Supplementary Table 10 provides a “heat map” for indirect comparisons of NNHs versus placebo (with 95% CIs) for lemborexant 5/10 mg (Table 3) and zolpidem ER 6.25 mg (Supplementary Table 7) from SUNRISE 1 and SUNRISE 2 (see text for month 3 data) and for other hypnotics (Supplementary Tables 11–19) and when statistical significance was achieved. Risk for an AE is considered higher for NNH < 10, intermediate for NNH between 10 and 19, and low for NNH ≥ 20. These levels of risk are represented in Supplementary Table 10 by red, yellow, and green highlighting, respectively. Note that dosing may mitigate some of the AE risk for somnolence and related events.
Likelihood to be Helped or Harmed
Pooling the data from SUNRISE 1 and SUNRISE 2, the rates of discontinuation because of an AE were low, and for lemborexant 5 mg the rate was lower than that for placebo. Pooling both doses provided a NNH estimate of 216 (NS) versus placebo on this outcome. After dividing this figure by any of the NNT estimates for the statistically significant endpoint efficacy measures, the resultant LHH ranged from 13 to 43 for the subjective outcomes and from 24 to 54 for the PSG outcomes. Thus, in the clinical trials, lemborexant was much more likely to result in a therapeutic response than a discontinuation because of an AE. The effect sizes for endpoint therapeutic benefit were most pronounced for the day 30 PSG outcome of WASO response in the SUNRISE 1 study (Supplementary Table 1) and the month 6 subjective outcome of PGI-I score = 1 for decreased time to fall asleep for the SUNRISE 2 study (Supplementary Table 4), with both having NNT estimates of 4. Taking the NNH for the most common AE associated with lemborexant 5/10 mg, somnolence, with a NNH of 19 (Table 3) and dividing by the NNT of 4 gives a LHH of 4.8; thus, lemborexant was about 5 times likelier to result in a PSG outcome of WASO response, or patient reported decreased time to fall asleep, than an AE of somnolence. When assuming that subjects with missing information due to early withdrawal or other reasons were nonresponders, the NNT for WASO response for lemborexant 5/10 mg at day 1/2 was 3 and at day 29/30 was 5 (Supplementary Table 3), resulting in LHH values (therapeutic response vs AE somnolence) of 6.3 and 3.8 for day 1/2 and day 29/30, respectively.
Supplementary Table 20 provides the LHH for hypnotics for which statistically significant values for a NNT versus placebo for any efficacy measure and a statistically significant NNH versus placebo for somnolence were available. The most robust (smallest) NNT values for efficacy available for each medication were used to calculate LHH. All LHH values were > 1; thus, for each medication (lemborexant, suvorexant, doxepin, eszopiclone, and zolpidem ER), it is more likely to encounter therapeutic response than somnolence. A limitation is that the actual efficacy outcome measure and the length of observation differed among the listed hypnotics. When comparing the two DORA hypnotics currently available, lemborexant 5/10 mg and suvorexant 15/20 mg, 3-month data are available for sTST, sSOL, or sWASO response defined by ≥ 15% improvement and ISI response defined as a mean improvement of ≥ 6 points. Pairing the NNT versus placebo for these outcomes versus NNH of somnolence at 3 months, LHH values are comparable and range from 1.2 to 2.4 for lemborexant and 1.1 to 3.5 for suvorexant (Supplementary Table 21).
DISCUSSION
NNT values versus placebo that are < 10, and NNH values versus placebo that are ≥ 10, are desirable.5 Most NNT values for lemborexant 5/10 mg versus placebo were < 10, and some were as low as 3, suggesting that lemborexant has a clinically relevant magnitude of therapeutic effect.4,5 The most robust NNT values were generally for patient-reported outcomes and WASO response. All NNH values versus placebo for lemborexant from the pooled AE data were ≥ 10, evidencing that lemborexant is relatively tolerable. Rates of discontinuation because of an AE were low, and for lemborexant 5 mg these rates were similar to those for placebo (1.4% and 1.5%, respectively). Moreover, the NNH versus placebo for discontinuation because of an AE for pooled doses of lemborexant through day 30 was 216, and the 95% CI includes infinity, and thus was not statistically significant. LHH contrasting the statistically significant endpoint efficacy measures versus discontinuation because of an AE ranged from 13 to 54.
In SUNRISE 1, NNT values for lemborexant 5/10 mg were generally more robust than for zolpidem ER 6.25 mg for PSG and sleep diary outcomes, but generally not for the PGI-I or ISI categorical outcomes. The degree of overlap in effect sizes across all measures was considerable except for some of the PSG outcomes, particularly at day 30 when placebo was superior to zolpidem ER 6.25 mg on LPS categorical outcomes.
In indirect comparisons, lemborexant 5/10 mg demonstrated a numerically larger effect size (lower NNT) versus placebo than suvorexant on sleep measures at week 1, week 4, and month 3 (the time points for which data were available for both agents); however, the 95% CIs generally overlapped, and an appropriately designed head-to-head study would be required to properly compare these two medications. Except for the limited categorical PSG data available for ramelteon (with results similar to that for lemborexant 5/10 mg), NNT data for the other hypnotics for which comparison is possible are restricted to PGI-I and ISI outcomes with effect sizes that are similar to or more robust than that for lemborexant and with 95% CIs that are also generally overlapping.
Although somnolence was the most common AE observed with lemborexant 5/10 mg, with a NNH versus placebo between 15 and 28 for the first 30 days when pooling SUNRISE 1 and SUNRISE 2 data, this did not usually result in discontinuation of treatment within the first 30 days (SUNRISE 1 and SUNRISE 2), or within the first 6 months (SUNRISE 2). Moreover, having an AE of somnolence does not equal having impairment. Nonetheless, given the similar efficacy for lemborexant 5 mg and lemborexant 10 mg, the optimal starting dose for lemborexant appears to be 5 mg, which is consistent with approved prescribing information. Compared to starting at the 10 mg dose, initiating lemborexant at 5 mg carries a lower risk for somnolence as well as a lower risk for discontinuation because of an AE.
It is not surprising that somnolence would be reported as a common adverse effect with many hypnotic medications. Although more or better quality of sleep is the expected benefit of the treatment, next-day somnolence may need to be managed for some patients. This can include an adjustment of the time to retire to bed, or in the case of somnolence being dose-related, then dose reduction could be considered.
Although there may have been instances in which NNH values versus placebo were < 10, for example in SUNRISE 2 for somnolence with lemborexant 10 mg, unpleasant taste with eszopiclone, and “nervous system disorders” and somnolence with zolpidem ER 12.5 mg, a single-digit NNH may be acceptable if the adverse event is mild or moderate, does not lead to discontinuation, is temporary or causes little distress, and does not pose a serious health risk or if a treatment has good (single-digit NNT) efficacy and there is a compelling need for efficacy that mitigates the low NNH tolerability limitation.5 A NNH in the range of 10–100 may be acceptable for adverse events that may lead to discontinuation, but are not associated with serious immediate health risks, or when alternatives do not have a better profile.5 LHH values can help better understand these tradeoffs, and although a LHH >> 1 on its face is desirable, there is sometimes the need to accept a LHH that approximates 1 or is < 1.43–45
Limitations
The data analyzed in this study are limited to dichotomous outcomes from trials in which medications were taken daily (and not “as needed”). The results may not be generalizable to patients outside the confines of a clinical trial; this is always a concern for results of registrational trials because of the strict inclusion/exclusion criteria that these studies require. Definitions of insomnia also may vary from trial to trial and reflect diagnostic criteria that have evolved over time, including the inclusion or exclusion of patients with somatic and/or psychiatric comorbidities. Exposure to lemborexant has been systematically studied up through 12 months in SUNRISE 2, although the double-blind period was 6 months in duration; the optimal length of medication treatment necessary to address insomnia was not examined. Although the two lemborexant studies that were pooled for the 30-day outcomes were similar, there were important differences in design (SUNRISE 1 employed PSG and recruited only older patients, although median age remained high in SUNRISE 2 at 55 years, versus 63 years in the SUNRISE 1; in addition, SUNRISE 1 required the presence of sleep maintenance difficulties [participants could also have had sleep-onset difficulties, but this was not required], and SUNRISE 2 enrolled patients with sleep onset and/or sleep maintenance difficulties). In the lemborexant trials but not necessarily in studies of other agents, all patients received instructions consistent with principles of good sleep hygiene, which may have contributed to the improvement in sleep, especially among patients randomized to placebo. The metrics of NNT and NNH are not appropriate for continuous outcomes, such as WASO, and such outcomes require dichotomization for NNT to be directly calculated. Reasons for clinical trial discontinuation can be complex, so that the NNH for discontinuation due to AEs in a study may not always generalize to overall tolerability in clinical practice. We did not calculate discontinuation rates per month or time to discontinuation; such data would be of interest and should be considered when planning any future head-to-head comparisons of hypnotics and their acceptability to patients with insomnia. Some patients may be more sensitive to somnolence or other AEs than other patients, and thus all prescribing decisions should be individualized. The brief durations of the available controlled studies of lemborexant limit the sensitivity of calculating NNH for delayed adverse outcomes, and the relatively small sample sizes of the studies limit sensitivity of calculating NNH for uncommon adverse outcomes and subpopulation effects. Indirect comparisons of NNT, NNH, and LHH with other hypnotics as calculated in other studies of these agents versus placebo must be approached with caution because of heterogeneity in study design, including age of participants, dosing, and duration, as well as differences in available study outcome measures. The less commonly used benzodiazepine hypnotics estazolam and quazepam were not included in this report.
CONCLUSIONS
The data support the use of lemborexant as a potentially beneficial hypnotic for adults with insomnia, but no definitive conclusions can be drawn regarding whether its efficacy is substantially better or worse than that of other choices. Evidence for efficacy was demonstrable as early as day 1 based on PSG outcomes. Except for ramelteon, the occurrence of somnolence as a side effect is similar to other choices. However, orexin receptor antagonists such as lemborexant and suvorexant serve as an alternative to the older hypnotics, and because of the different mechanism of action, DORAs largely avoid the obstacles of physiologic tolerance, rebound, and withdrawal.7,9 Head-to-head trials among DORAs versus other hypnotics, as well as between lemborexant and suvorexant, are desirable to better understand their similarities and differences in clinically relevant populations.
Submitted: November 18, 2020; accepted March 30, 2021.
Published online: June 1, 2021.
Potential conflicts of interest: In the past 12 months, Dr Citrome has served as a consultant to AbbVie, Acadia, Alkermes, Allergan, Angelini, Astellas, Avanir, Axsome, BioXcel, Cadent Therapeutics, Eisai, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Luye, Lyndra, Medavante-ProPhase, Merck, Neurocrine, Noven, Osmotica, Otsuka, Relmada, Sage, Shire, Sunovion, Takeda, Teva, and University of Arizona and provided one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; has served as a speaker for AbbVie, Acadia, Alkermes, Allergan, Angelini, Eisai, Intra-Cellular Therapies, Janssen, Lundbeck, Merck, Neurocrine, Noven, Otsuka, Sage, Shire, Sunovion, Takeda, Teva, and Continuing Medical Education (CME) activities organized by medical education companies such as Medscape, North American Center for Continuing Medical Education (NACCME), Neuroscience Education Institute (NEI), Vindico, and Uuniversities and professional organizations/societies; owns stocks (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer (purchased > 10 years ago); and has received royalties from Wiley (Editor-in-Chief, International Journal of Clinical Practice, through end of 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (Topic Editor: Psychiatry, Clinical Therapeutics). Drs Juday, Frech, and Atkins are employees of Eisai Inc.
Funding/support: This study was funded by Eisai Inc., Woodcliff Lake, New Jersey.
Role of the sponsor: Although personnel at Eisai Inc., reviewed the manuscript, final approval for the decisions to submit the manuscript was the sole decision of the authors.
Previous presentation: Citrome L, Juday T, Atkins N, Frech F, Malhotra M. Lemborexant for the Treatment of Insomnia: Number Needed to Treat, Number Needed to Harm, and Likelihood to Be Helped or Harmed. Poster Abstract G52. Journal of Managed Care & Specialty Pharmacy. 2020; 26(4-a):S47 • Citrome L, Juday T, Atkins N, Frech F, Malhotra M. Assessing Lemborexant Efficacy and Safety in the Treatment of Insomnia. Poster Abstract. Journal of the National Medical Association. 2020;112(5S):S34 • Citrome L, Juday T, Atkins N, Frech F, Malhotra M. Lemborexant for the Treatment of Insomnia: Number Needed to Treat, Number Needed to Harm, and Likelihood to Be Helped or Harmed. Poster Presentation. American Academy of Nurse Practitioners National Congress [presented virtually September 10, 2020, through December 31, 2020] • Citrome L, Juday T, Atkins N, Frech F, Malhotra M. Lemborexant and suvorexant for treating insomnia: An indirect comparison using number needed to treat, number needed to harm, and likelihood to be helped or harmed. Poster Presentation. Psych Congress 2020 Virtual Experience. September 10–13, 2020.
Supplementary material: Available at Psychiatrist.com.
CLINICAL POINTS
- Insomnia is common, and there are many pharmacologic treatment options available to choose from.
- Using number needed to treat and number needed to harm can help place new hypnotics, such as lemborexant, into clinical perspective.
References (45)

- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
- Krystal AD, Prather AA, Ashbrook LH. The assessment and management of insomnia: an update. World Psychiatry. 2019;18(3):337–352. PubMed CrossRef
- Palagini L, Manni R, Aguglia E, et al. Expert opinions and consensus recommendations for the evaluation and management of insomnia in clinical practice: joint statements of five Italian scientific societies. Front Psychiatry. 2020;11:558. PubMed CrossRef
- Citrome L. Compelling or irrelevant? using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412–419. PubMed CrossRef
- Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407–411. PubMed CrossRef
- Citrome L, DiBernardo A, Singh J. Appraising esketamine nasal spray for the management of treatment-resistant depression in adults: number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Affect Disord. 2020;271:228–238. PubMed CrossRef
- DAYVIGO (lemborexant) tablets, for oral use. Product label. Eisai Inc. Revised March 2021. Accessed April 24, 2021. https://www.dayvigohcp.com/-/media/Files/DAYVIGOHCP/PDF/prescribing-information.pdf
- Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. PubMed CrossRef
- BELSOMRA (suvorexant) tablets, for oral use. Product label. Merck & Co., Inc. Revised July 2018. Accessed January 19, 2020. https://www.merck.com/product/usa/pi_circulars/b/belsomra/belsomra_pi.pdf
- Citrome L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429–1441. PubMed CrossRef
- Citrome L. Iloperidone redux: a dissection of the Drug Approval Package for this newly commercialised second-generation antipsychotic. Int J Clin Pract. 2010;64(6):707–718. PubMed CrossRef
- Suvorexant Drug Approval Package (Application No.: 204569, Approval Date: August 13, 2014). US Food and Drug Administration. Date created: September 12, 2014. Accessed January 19, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/204569Orig1s000TOC.cfm
- Doxepin 3 and 6 mg Oral Tablets Drug Approval Package (Application No.: 022036, Approval Date: March 17, 2010). US Food and Drug Administration.Date created: February 11, 2011. Accessed January 19, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022036s000_silenor_toc.cfm
- Ramelteon Drug Approval Package (Application No.: 021782, Approval Date: July 22, 2005). US Food and Drug Administration. Date created: October 20, 2005. Accessed January 19, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021782s000_RozeremTOC.cfm
- Eszopiclone Drug Approval Package (Application No.: 021476, Approval Date: December 15, 2004). US Food and Drug Administration. Date created: April 22, 2005. Accessed January 19, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021476_Lunesta.cfm
- Zaleplon Drug Approval Package (Application No.: 020859, Approval Date: August 13, 1999). US Food and Drug Administration. Date created: March 30, 2001. Accessed January 19, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20859_Sonata.cfm
- Zolpidem Tartrate Drug Approval Package (Application No.: 019908, Approval Date: December 16, 1992). US Food and Drug Administration. Date created: July 13, 2005. Accessed January 19, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/019908_S000_AmbienTOC.cfm
- Zolpidem Tartrate Extended Release Drug Approval Package (Application No.: 021774, Approval Date: June 1, 2005). US Food and Drug Administration. Date created: July 13, 2005. Accessed January 19, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021774s000_AmbienTOC.cfm
- Silenor (doxepin) tablets for oral administration. Product label. Pernix Therapeutics LLC. Revised March 2010. Accessed January 19, 2020. https://www.silenor.com/Content/pdf/prescribing-information.pdf
- ROZEREM (ramelteon) tablets, for oral use. Product label. Takeda Pharmaceuticals America, Inc. Revised December 2018. Accessed January 19, 2020. https://general.takedapharm.com/rozerempi/
- LUNESTA (eszopiclone) tablets, for oral use. Product label. Sunovion Pharmaceuticals Inc. Revised August 2019. Accessed January 19, 2020. https://www.lunesta.com/PostedApprovedLabelingText.pdf
- SONATA- zaleplon capsule. Product label. Pfizer Inc. Revised December 2019. Accessed January 19, 2020. http://labeling.pfizer.com/ShowLabeling.aspx?id=710
- AMBIEN (zolpidem tartrate) tablets, for oral use. Product label. Sanofi-Aventis US, LLC. Revised August 2019. Accessed January 19, 2020. http://products.sanofi.us/ambien/Ambien.pdf
- AMBIEN CR (zolpidem tartrate) extended-release tablets, for oral use. Product label. Sanofi-Aventis US, LLC. Revised August 2019. Accessed January 19, 2020. http://products.sanofi.us/ambien_cr/ambien_CR.pdf
- HALCION (triazolam) tablets, for oral use. Product label. Pfizer Inc. Revised October 2019. Accessed January 19, 2020. http://labeling.pfizer.com/ShowLabeling.aspx?id=586
- Restoril (temazepam) Capsules USP. Product label. US Food and Drug Administration. Revised December 2018. Accessed January 19, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018163s065lbl.pdf
- FLURAZEPAM HYDROCHLORIDE capsules, for oral use. Product label. DailyMed. Revised December 2018. Accessed January 19, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=2f2db2f5-49d3-4d47-a08a-628df49d2120
- Krystal AD, Durrence HH, Scharf M, et al. Efficacy and safety of doxepin 1 mg and 3 mg in a 12-week sleep laboratory and outpatient trial of elderly subjects with chronic primary insomnia. Sleep. 2010;33(11):1553–1561. PubMed CrossRef
- Lankford A, Rogowski R, Essink B, et al. Efficacy and safety of doxepin 6 mg in a four-week outpatient trial of elderly adults with chronic primary insomnia. Sleep Med. 2012;13(2):133–138. PubMed CrossRef
- Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30(8):959–968. PubMed CrossRef
- Ancoli-Israel S, Krystal AD, McCall WV, et al. A 12-week, randomized, double-blind, placebo-controlled study evaluating the effect of eszopiclone 2 mg on sleep/wake function in older adults with primary and comorbid insomnia. Sleep. 2010;33(2):225–234. PubMed CrossRef
- Krystal AD, Erman M, Zammit GK, et al; ZOLONG Study Group. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31(1):79–90. PubMed CrossRef
- Roth T, Soubrane C, Titeux L, et al; Zoladult Study Group. Efficacy and safety of zolpidem-MR: a double-blind, placebo-controlled study in adults with primary insomnia. Sleep Med. 2006;7(5):397–406. PubMed CrossRef
- Dockhorn RJ, Dockhorn DW. Zolpidem in the treatment of short-term insomnia: a randomized, double-blind, placebo-controlled clinical trial. Clin Neuropharmacol. 1996;19(4):333–340. PubMed CrossRef
- Mini L, Wang-Weigand S, Zhang J. Ramelteon 8 mg/d versus placebo in patients with chronic insomnia: post hoc analysis of a 5-week trial using 50% or greater reduction in latency to persistent sleep as a measure of treatment effect. Clin Ther. 2008;30(7):1316–1323. PubMed CrossRef
- Suvorexant Advisory Committee Meeting Briefing Document. Merck Sharp & Dohme Corporation. May 22, 2013. Accessed February 9, 2020.http://klinikfarmakoloji.com/sites/default/files/2019-07/uykufda.pdf
- Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. PubMed CrossRef
- Kärppä M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. PubMed CrossRef
- Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433–1442. PubMed
- Zammit GK, McNabb LJ, Caron J, et al. Efficacy and safety of eszopiclone across 6-weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20(12):1979–1991. PubMed CrossRef
- Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26(7):793–799. PubMed CrossRef
- Walsh JK, Soubrane C, Roth T. Efficacy and safety of zolpidem extended release in elderly primary insomnia patients. Am J Geriatr Psychiatry. 2008;16(1):44–57. PubMed CrossRef
- Ketter TA, Citrome L. Sensitive and personalized determinations of likelihood of being helped or harmed. J Clin Psychiatry. 2017;78(5):e554. PubMed CrossRef
- Citrome L. Likelihood to be helped or harmed can assist in clinical decision-making. J Clin Psychiatry. 2011;72(2):261–262, author reply 262. PubMed CrossRef
- Citrome L, Kantrowitz J. Antipsychotics for the treatment of schizophrenia: likelihood to be helped or harmed, understanding proximal and distal benefits and risks. Expert Rev Neurother. 2008;8(7):1079–1091. PubMed CrossRef
This PDF is free for all visitors!