ABSTRACT
Objective: In one of the largest and most comprehensive studies investigating the link between objective parameters of sleep and biological rhythms with peripartum mood and anxiety to date, we prospectively investigated the trajectory of subjective and objective sleep and biological rhythms, levels of melatonin, and light exposure from late pregnancy to postpartum and their relationship with depressive and anxiety symptoms across the peripartum period.
Methods: One hundred women were assessed during the third trimester of pregnancy, of whom 73 returned for follow-ups at 1–3 weeks and 6–12 weeks postpartum. Participants were recruited from an outpatient clinic and from the community from November 2015 to May 2018. Subjective and objective measures of sleep and biological rhythms were obtained, including 2 weeks of actigraphy at each visit. Questionnaires validated in the peripartum period were used to assess mood and anxiety.
Results: Discrete patterns of longitudinal changes in sleep and biological rhythm variables were observed, such as fewer awakenings (F = 23.46, P < .001) and increased mean nighttime activity (F = 55.41, P < .001) during postpartum compared to late pregnancy. Specific longitudinal changes in biological rhythm parameters, most notably circadian quotient, activity during rest at night, and probability of transitioning from rest to activity at night, were most strongly linked to higher depressive and anxiety symptoms across the peripartum period.
Conclusions: Biological rhythm variables beyond sleep were most closely associated with severity of depressive and anxiety symptoms across the peripartum period. Findings from this study emphasize the importance of biological rhythms and activity beyond sleep to peripartum mood and anxiety.
J Clin Psychiatry 2022;83(2):21m13991
To cite: Slyepchenko A, Minuzzi L, Reilly JP, et al. Longitudinal changes in sleep, biological rhythms, and light exposure from late pregnancy to postpartum and their impact on peripartum mood and anxiety. J Clin Psychiatry. 2022;83(2):21m13991.
To share: https://doi.org/10.4088/JCP.21m13991
© Copyright 2022 Physicians Postgraduate Press, Inc.
aWomen’s Health Concerns Clinic, St Joseph’s Healthcare Hamilton, Hamilton, Ontario, Canada
bNeuroscience Graduate Program, McMaster University, Hamilton, Ontario, Canada
cMood Disorders Treatment and Research Centre, Department of Psychiatry and Behavioural Neurosciences, McMaster University, Hamilton, Ontario, Canada
dDepartment of Electrical and Computer Engineering, McMaster University, Hamilton, Ontario, Canada
*Corresponding author: Benicio N. Frey, MD, MSc, PhD, 100 West 5th St, Ste C124, Hamilton, ON, L8N 3K7, Canada ([email protected]).
The peripartum period is a vulnerable time for women’s mental health—it is estimated that 15%–18% of women experience anxiety and 7%–13% of women experience depression during this period.1,2 Anxiety and depression have a high rate of comorbidity during the peripartum period, with a prevalence of 9.5% during pregnancy and 9.3% postpartum.3
Peripartum depression and anxiety have a negative impact on mothers, including worsened quality of life, relationship problems, social functioning,4 body image and self-confidence, and obstetric outcomes, among others.5,6 Importantly, peripartum depression and anxiety might affect children’s language development, bonding between mother and child, emotional and behavioral issues, and even gray matter volume and fractional anisotropy at age 10 years.5,7–9 Though a number of psychosocial and risk factors have been described for peripartum depression and anxiety,10–13 there are no reliable biological predictors of these peripartum psychiatric disorders.
Studies indicate worsening of sleep quality during pregnancy and throughout postpartum, particularly during the third trimester of pregnancy and the first month postpartum.14,15 However, understanding of the longitudinal changes in biological rhythms beyond sleep across this period remains limited.16 Systematic evidence suggests that subjective sleep disturbance during pregnancy is linked to postpartum depression,17–19 with similar, though less consistent findings for objective markers of sleep disturbance.17,19–22 Emerging evidence suggests that sleep during the third trimester may also be linked to postpartum anxiety, though findings have not been consistent.20,23,24
Few studies have investigated the impact of biological rhythms on mood or anxiety during the peripartum period. Interestingly, findings suggest that worsened subjective biological rhythm disturbances beyond sleep quality, but not subjective sleep quality itself, may be linked to worsened mood from the third trimester of pregnancy to 6–12 weeks postpartum.25,26 Additionally, a couple of small studies27,28 have linked changes in melatonin secretion to postpartum depressive symptoms.
In this investigation, we aimed to characterize the longitudinal changes in subjective and objective sleep and biological rhythms, levels of melatonin, and light exposure from the third trimester of pregnancy to 1–3 weeks and 6–12 weeks postpartum. We also aimed to identify which changes in sleep, biological rhythm, and light exposure variables were associated with postpartum depression and anxiety. Our study is the largest to date to investigate objective changes in biological rhythms across this period and to provide a thorough characterization of biological rhythms and, to our knowledge, is the first to investigate the impact of biological rhythms on peripartum anxiety.
METHODS
Participants
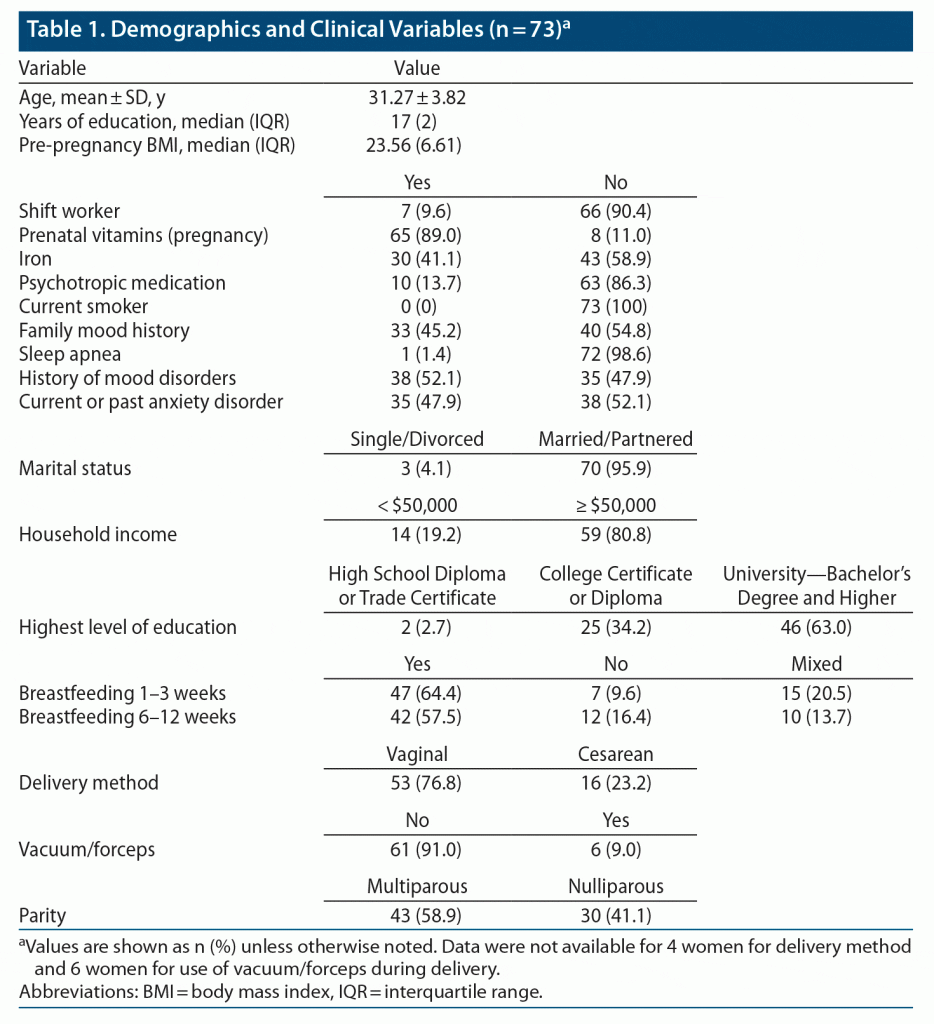
During the third trimester of pregnancy, 100 women were recruited from the community, including advertising in obstetric and midwifery practices, family physicians’ offices, and ultrasound clinics, and from an outpatient psychiatric clinic (Women’s Health Concerns Clinic at St Joseph’s Healthcare, Hamilton, Ontario, Canada) from November 2015 to May 2018. This clinic specializes in psychiatric disorders during the peripartum, premenstrual, and perimenopausal periods and receives referrals from family physicians in the community and from obstetric and midwifery clinics as well as self-referrals.29 The following inclusion and exclusion criteria were applied: age ≥ 16 years; no history of head trauma with loss of consciousness ≥ 5 minutes; no current major depressive or (hypo)manic episode; and ≥ 27 weeks of pregnancy at enrollment. Current mood state and diagnosis were established using the Mini-International Neuropsychiatric Interview (MINI) Version 6.00,30 following DSM-IV-TR criteria. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Study procedures were approved by the Hamilton Integrated Research Ethics Board (Project #0602), and all participants provided signed written informed consent before study entry.
Clinical Assessments
Participants were interviewed using the MINI to assess psychiatric diagnosis. The Postpartum Depression Predictors Inventory–Revised (PDPI-R)31 assessed psychosocial risk factors for postpartum depression. Depressive symptoms were assessed using the self-report Edinburgh Postnatal Depression Scale (EPDS)32 and the clinician-rated Montgomery-Asberg Depression Rating Scale (MADRS).33 Manic symptoms were evaluated using the Young Mania Rating Scale (YMRS),34 a clinical interview. Anxiety symptoms were assessed using the Generalized Anxiety Disorder 7-item scale (GAD-7).35
The Epworth Sleepiness Scale (ESS)36 measured daytime sleepiness, and the Pittsburgh Sleep Quality Index (PSQI)37 assessed subjective sleep quality. The Biological Rhythms Interview of Assessment in Neuropsychiatry (BRIAN)38 evaluated subjective disruptions in biological rhythms, and chronotype (not part of main score).
Study Procedures
Participants attended 3 study visits: during the third trimester of pregnancy, at 1–3 weeks postpartum (commonly-described period of postpartum blues onset39), and 6–12 weeks postpartum (period of higher incidence of postpartum depression2).
During the third trimester of pregnancy, following informed consent procedures, participants were interviewed using clinical tools (MINI, MADRS, YMRS). Next, participants completed a series of questionnaires (PDPI-R, EPDS, PSQI, GAD-7, ESS, BRIAN) assessing a broad range of clinical characteristics. At 1–3 and 6–12 weeks postpartum, participants were interviewed using the MADRS and YMRS and completed the EPDS, PSQI, GAD-7, ESS, and BRIAN questionnaires.
At each of the 3 timepoints, participants were fitted with an actigraph, to be worn on the non-dominant wrist for 2 weeks (Actiwatch 2; Philips Respironics Inc; Biolynx; Montreal, Quebec, Canada). Participants also filled out a sleep log to record actigraph removal periods, bedtime, wake-up time, and naps. Participants collected a first morning urine sample on the last day of actigraph wear and completed salivary melatonin sampling during all 3 visits for 1 night, from 6:00 pm until midnight or until they went to sleep that evening.
Urinary 6-Sulfatoxymelatonin
The major urinary metabolite of melatonin, 6-sulfatoxymelatonin (6-SM), was extracted from the first morning urine samples. Levels of 6–SM were analyzed using enzyme-linked immunosorbent assay for 6-SM (Buhlmann Diagnostics Corporation; Amherst, New Hampshire, USA; assay sensitivity = 0.14 ng/mL, intraassay coefficient of variation = 7.1%; interassay coefficient of variation = 11.9%). Concentration of 6-SM was calculated as a ratio of 6-SM (ng) to creatinine (mg) to control for urine volume.40–42
Dim Light Onset Melatonin
Saliva samples were analyzed using a salivary melatonin enzyme-linked immunoassay kit (Salimetrics; State College, Pennsylvania, USA). Assay sensitivity was 1.42 pg/mL, with an interassay coefficient of variation for low endogenous melatonin of 23.6% and intra-assay coefficient of variation for the mean of low endogenous melatonin levels from 5.9% to 6.5%. From available samples, dim light melatonin onset (DLMO) was calculated using the absolute threshold method, for which DLMO was determined to occur at the interpolated time that first sample exceeded and maintained a level of melatonin above a threshold of 7 pg/mL for at least 2 hours.43 We were able to obtain complete samples for all 3 visits that could be used to calculate DLMO for only 12 participants. Results are presented in Supplementary Table 1, though we considered this to be a failed method.
Actigraphy
Actigraphs were worn continuously for 2 weeks. Pre-processing of the actigraph data involved removal of periods of actigraph non-wear as reported in the sleep log and visual inspection to remove intervals during which actigraphs captured no activity for at least 20 minutes. Days on which ≥ 4 hours of recording were unavailable were removed from analysis. Further details can be obtained from a previous report.44
Sleep Variables
The following sleep variables were assessed: total sleep time (TST, in hours); sleep onset latency (in minutes), which encompasses transition from wakefulness to sleep; sleep efficiency (SE), ie, percentage of time in bed spent asleep; and wake after sleep onset (WASO), ie, number of wakeful minutes following sleep onset. The mean midpoint between sleep onset and offset was manually calculated to produce mean mid-sleep time.
Light Exposure Variables
Illuminance data from the epoch-by-epoch CSV files were used to calculate time above light threshold (TAT) and mean timing of TAT (MLiT) across 24-hour periods,45 using 10-, 100-, 500-, and 1,000-lux light thresholds.
Cosinor Analysis
Period-adjusted cosinor analysis was performed using the cosinor R package (v.1.1). The following parameters were extracted: midline estimating statistic of the rhythm (MESOR), ie, mean activity adjusted to the rhythm; amplitude; acrophase, ie, timing of peak activity; and circadian quotient (CQ), a measure of rhythm strength, estimated by dividing amplitude by MESOR. Mean and standard deviation of nighttime activity detected during sleep periods were calculated, yielding nighttime activity mean and SD values.
Nonparametric Circadian Activity Rhythm Analysis
We calculated the following variables: interdaily stability (IS; range: 0–1, with 1 indicating perfect stability), which describes invariability of the data between days, indicating the degree of coupling of the rhythm to environmental zeitgebers; intradaily variability (IV; range: typically 0–2, with 2 indicating higher fragmentation of the rhythm within a day.46) Nonparametric circadian activity rhythm analysis, using the nparACT (v.0.8) package,47 yielded the following: L5, 5 consecutive hours with the lowest amplitude; L5 start time; M10, 10 consecutive hours with highest amplitude); M10 start time; and relative amplitude (RA), the ratio of L5 subtracted from M10 to L5 added to M10.46
Transition Probabilities
The Python hmmlearn package (v.0.2.0; https://github.com/hmmlearn/hmmlearn) was used in Python v.2.7.6 (Python Software Foundation; https://www.python.org/) to obtain the following variables: probability of transitioning from rest to active states (pRA), probability of transitioning from active to rest states (pAR), and mean activity counts for each state during nighttime and daytime (μR day/night, μA day/night). Details regarding the analysis are available in Ortiz et al48 and in prior publications from our group.44,49
Statistical Analysis
Longitudinal changes in clinical variables, sleep, and biological rhythms. Longitudinal changes in clinical, sleep, and biological rhythm variables were examined using repeated-measures analyses of variance (ANOVAs) and Friedman tests. The Greenhouse-Geisser sphericity correction was applied to factors violating the sphericity assumption in the repeated-measures ANOVAs. A significance level of .05 was used, and the Benjamini-Hochberg procedure for false discovery rate (FDR) was applied to control for multiple comparisons. Post hoc tests were applied to significantly different variables according to the repeated-measures analyses. Analyses were performed using R v.4.0.2.50
Effect of sleep and biological rhythm variables on depression and anxiety symptoms. Generalized estimating equations (GEEs) were used to model depression (EPDS) and anxiety (GAD-7) symptoms, using sleep/biological rhythms variables as predictors. In instances of missing data from actigraph malfunction, 5-nearest-neighbor imputation was performed for predictor variables for visits with a filled-out EPDS. Visits for which EPDS scores were not available were not imputed.
GEE models were fit with EPDS and GAD-7 scores as primary outcomes and sleep and biological rhythm variables as predictors. In each model, visit (maternal status), an interaction term with the sleep/biological rhythm variable and visit; years of education; body mass index; history of mood disorders; and history of anxiety disorders according to the MINI were included. The aim of this analysis was to find sleep and biological rhythm variables that were most associated with severity of depressive or severity of anxiety symptoms over time. Further details of our methodological approach are provided in Supplementary Appendix 1.
RESULTS
Demographic and Clinical Variables
Demographic and clinical variables are detailed in Table 1. A total of 73 women returned for all 3 visits, completing clinical questionnaires. A participant flowchart is provided in Supplementary Figure 1. Of these women, 57 completed actigraphy and 6-SM sampling across all 3 visits. Of the participants, 52.1% had a history of major depressive or bipolar disorder, though all were euthymic at enrollment, and 47.9% had a current or past anxiety disorder. As expected, these conditions were highly comorbid in our sample: 35.6% of participants had both a history of mood disorders and a current or past anxiety disorder, 16.4% had a history of mood disorders only, and 12.3% had a past or current anxiety disorder only.
Longitudinal Trajectory of Sleep and Biological Rhythms
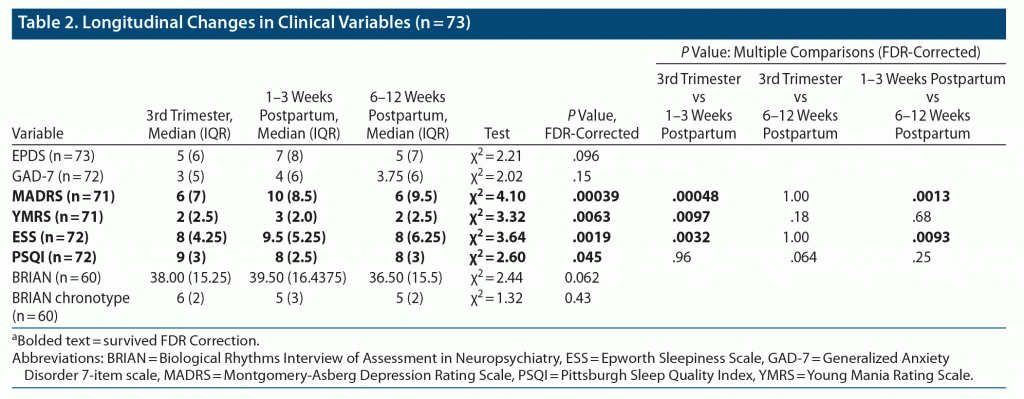
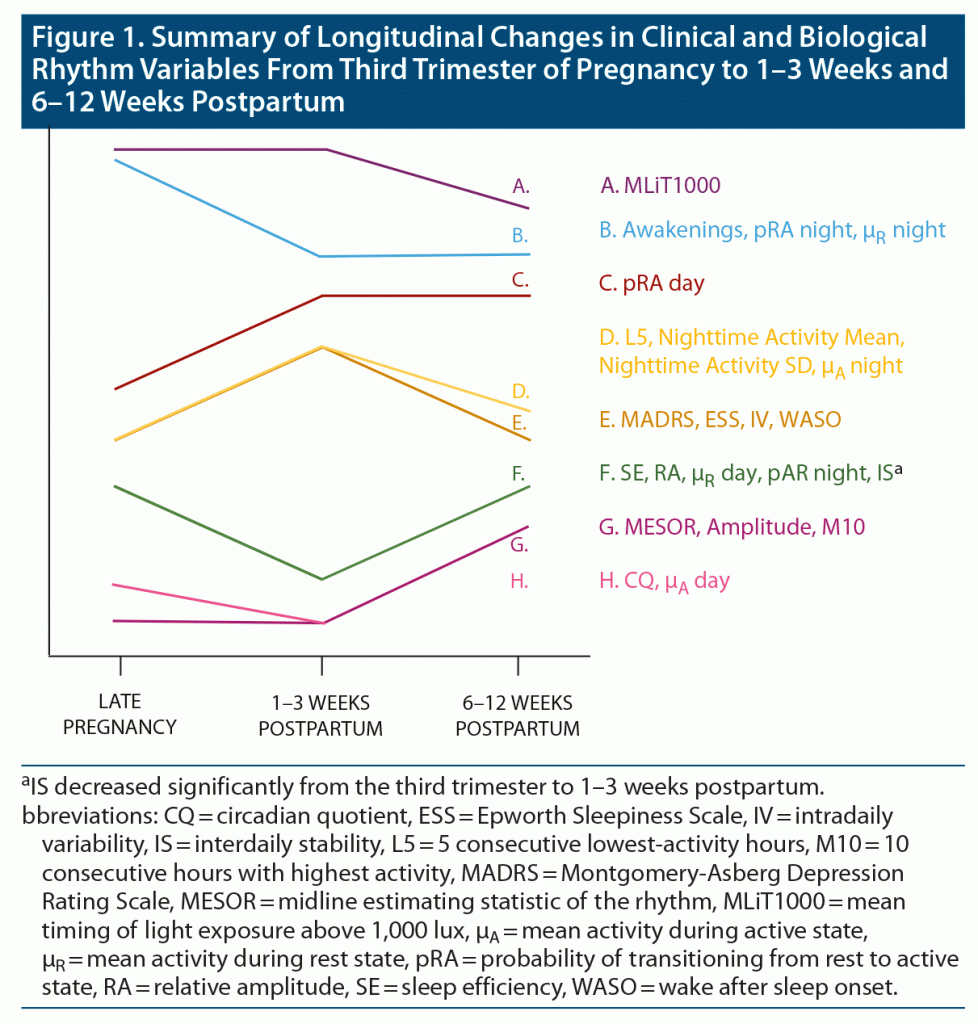
Figure 1, Table 2, and Supplementary Table 2 detail longitudinal changes in sleep and biological rhythm variables and clinical variables across the peripartum period. Significant changes were seen in sleep variables, variables obtained from cosinor analysis, nonparametric circadian activity rhythm analysis, transition probability analysis, and light exposure. Figure 1 displays only the variables with a statistically significant pattern of change across the peripartum period. No significant changes were observed in urinary 6-SM between timepoints (P > .05).
Specific patterns of longitudinal changes in sleep and biological rhythm variables were seen, as summarized in Figure 1. A number of variables increased (IV, F = 19.00; WASO, F = 28.73; ESS, χ2 = 3.64) or decreased (SE, χ2 = 5.43; RA, χ2 = 7.23; μR day, χ2 = 5.58; pAR night, F = 18.62) at 1–3 weeks postpartum, and returned to pregnancy levels at 6–12 weeks postpartum. SE and WASO reflect the amount of time spent awake during the night, indicating that at 1–3 weeks postpartum, women spent more time awake during the night compared to late pregnancy and 6–12 weeks postpartum. pAR night, a measure of nighttime fragmentation of activity, was decreased during this time as well, perhaps indicating sustained bouts of wakefulness during the night. Daytime sleepiness also increased at 1–3 weeks postpartum, compared to the other 2 timepoints, potentially indicating that sleep during this period was not sufficiently restful. Several of these variables described a disturbance in biological rhythms during this time: increased IV indicates a more fragmented rhythm throughout the 24-hour day, while decreased RA seen during this time indicates that there is a decrease in the difference between activity during active periods and rest periods.
Some variables increased at 1–3 weeks postpartum compared to pregnancy and reduced somewhat at 6–12 weeks postpartum, not returning to pregnancy levels (nighttime activity mean, F = 55.41; nighttime activity SD, F = 40.33; μA night, F = 37.76; L5, F = 7.84). These variables reflect increased activity during the night, especially during active periods, and increased variance in activity during the night. Several variables increased (pRA day, χ2 = 7.12) or decreased (number of awakenings F = 23.46; pRA night, χ2 = 4.87; μR night, χ2 = 2.99) at 1–3 weeks postpartum compared to pregnancy and maintained this state through 6–12 weeks postpartum. Increased pRA day indicates higher fragmentation in the rest-activity pattern during the day, whereas a decreased pRA night means a decreased transition probability from rest to activity and is likely related to interruptions to sustained periods of rest and, likely, the number of awakenings. Several variables remained constant from pregnancy to 1–3 weeks postpartum and increased (MESOR, F = 15.13; amplitude, χ2 = 4.59; M10, F = 17.94) or decreased (MLiT1000, χ2 = 2.76) at 6–12 weeks postpartum. This indicates a shift to an earlier timing of light exposure above 1,000 lux from pregnancy and early postpartum to 6–12 weeks postpartum. Additionally, higher MESOR during 6–12 weeks postpartum indicates a higher rhythm-adjusted mean of activity during this time. An increase in activity during the most active period of the day was also reflected by an increase in M10. Some variables changed at 6–12 weeks postpartum compared to 1–3 weeks postpartum, but the change from pregnancy was not significant (increased CQ, χ2 = 4.31; μA day, χ2 = 3.28). Higher CQ at 6–12 weeks postpartum indicates increased robustness of the daily rhythms, representing the ratio of amplitude to MESOR. Meanwhile, μA day describes the mean activity during active periods during the day. Taken together, these changes indicate higher daytime activity during 6–12 weeks postpartum compared to late pregnancy and 1–3 weeks postpartum.
Finally, IS was higher during pregnancy compared to 1–3 weeks postpartum but not compared to 6–12 weeks postpartum (χ2 = 3.00). This shows a decrease in the synchronicity of the rhythm to the external environment during 1–3 weeks postpartum, which suggests that this period in the early postpartum is associated with the greatest degree of disruption in the stability of daily activity rhythms.
GEE Models: Association of Longitudinal Changes in Sleep and Biological Rhythms With Longitudinal Changes in Mood and Anxiety
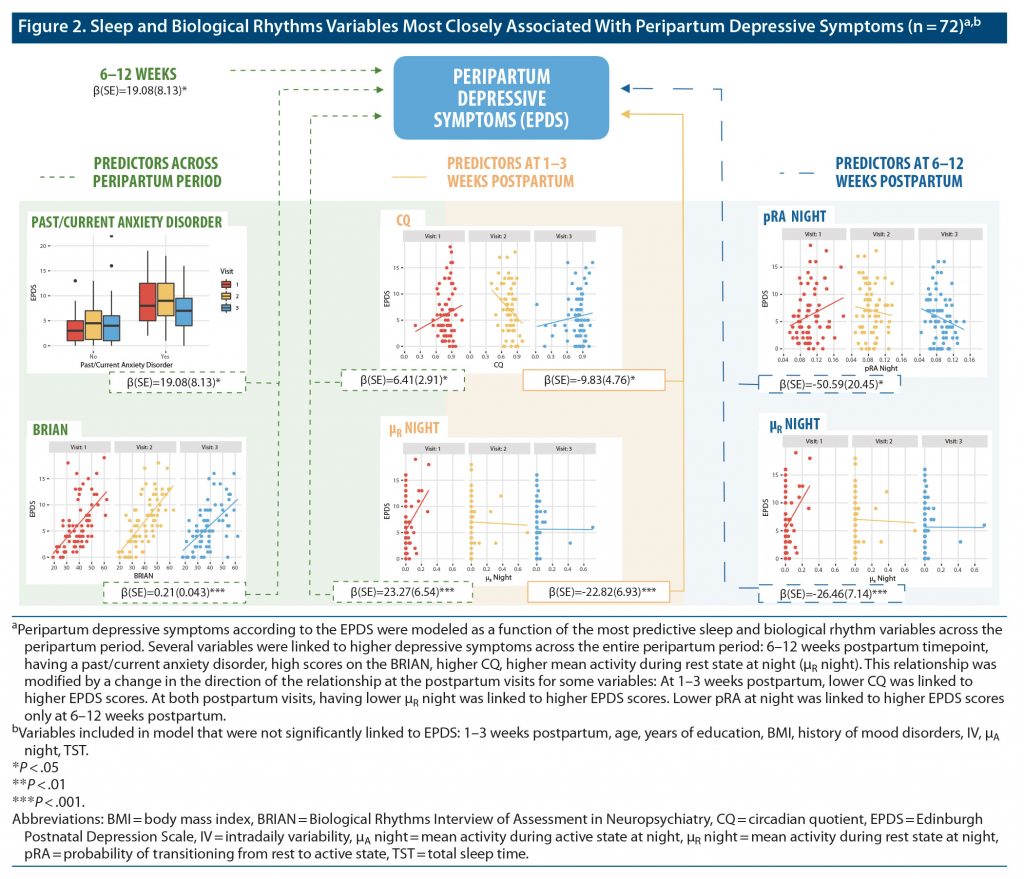
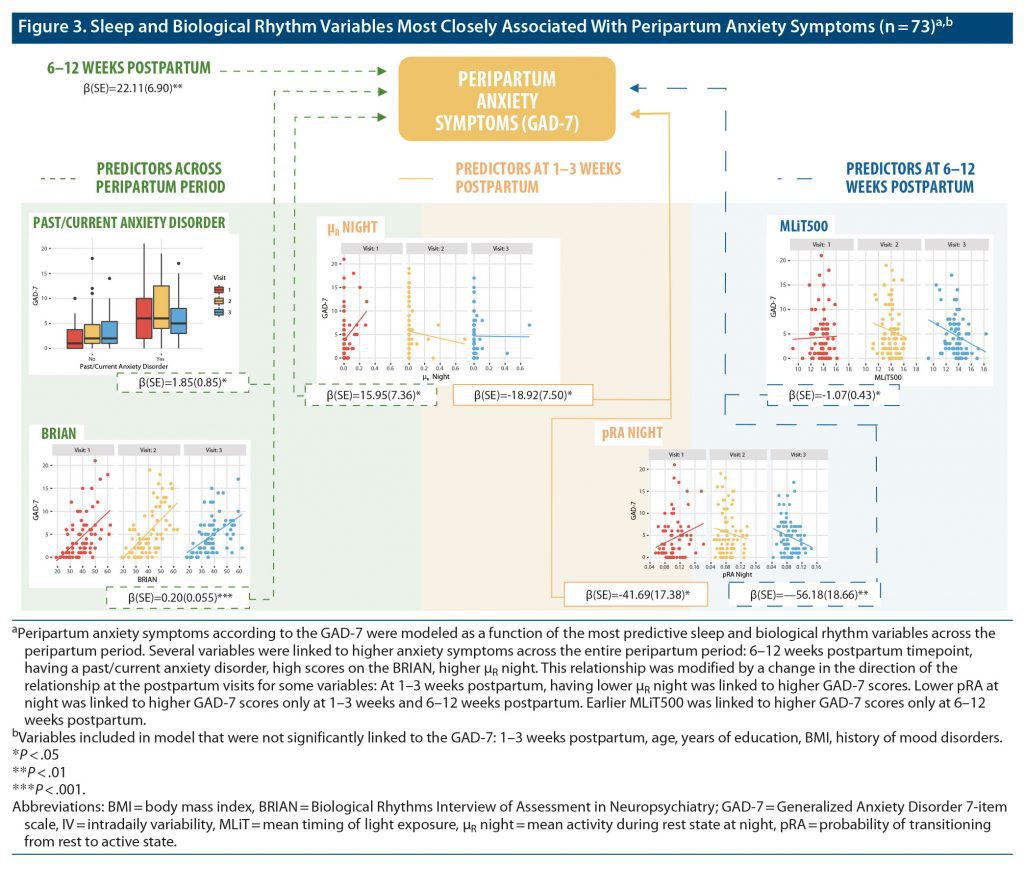
EPDS and GAD-7 scores were modeled as a function of sleep and biological rhythm variables from the third trimester of pregnancy to 1–3 weeks and 6–12 weeks postpartum (Figure 2 and Figure 3; Supplementary Tables 3 and 4). EPDS scores were predicted by past or current anxiety disorder (β = 2.44, P = .00073), BRIAN scores (β = 0.21, P = .000001), CQ (β = 6.41, P = .027), μR night (β = 23.27, P = .00037), interaction of Visit 2 with μR night (β = −22.82, P = .0010), interaction of Visit 3 with μR night (β = −26.46, P = .00021), interaction of Visit 2 with CQ (β = −9.83, P = .039), and interaction of pRA night with Visit 3 (β = −50.59, P = .013). Data from 1 participant were removed following outlier analysis. These longitudinal analyses indicate that specific variables reflecting nighttime activity and robustness of daily activity rhythms are the most robust predictors of depressive symptoms between late pregnancy and the early postpartum period.
Significant predictors of GAD-7 scores during the peripartum period included past or current anxiety disorder (β = 1.85, P = .030), BRIAN scores (β = 0.20, P = .00028), μR night (β = 15.95, P = .030), the interaction between Visit 2 and μR night (β = −18.92, P = .012), the interaction between Visit 2 and pRA night (β = −41.69, P = .016), the interaction between Visit 3 and pRA night (β = −56.18, P = .0026), and the interaction of Visit 3 with MLiT500 (β = −1.07, P = .012). Overall, these results indicate that specific variables reflecting nighttime activity and earlier timing of light exposure were the strongest predictors of severity of anxiety symptoms between late pregnancy and the early postpartum period.
DISCUSSION
Association of Sleep and Biological Rhythms With Depressive and Anxiety Symptoms During the Peripartum Period
The primary aim of our study was to determine which sleep and biological rhythm variables were most strongly associated with the longitudinal trajectory of depressive and anxiety symptoms from the third trimester of pregnancy to 1–3 weeks and 6–12 weeks postpartum. Our findings indicate that biological rhythm variables beyond sleep were the most closely associated with the severity of depressive and anxiety symptoms across the peripartum period. We found a set of objective parameters, as passively acquired through actigraphy, that were associated with the trajectory of depressive symptoms from the third trimester of pregnancy to the third postpartum month. Specifically, higher circadian quotient (CQ), which is a measure of circadian rhythm strength, and higher μR night—a measure of mean activity during rest states at night—were strongly linked to higher depressive symptoms. Prior findings from an independent study in our laboratory26 indicated that changes in subjective biological rhythms and sleep efficiency (SE) were associated with depressive symptoms, though changes in interdaily stability (IS), mood history, and risk factors for postpartum depression were not.
Interestingly, we found that there were objective variables that were significant predictors of depressive symptoms when interacting with maternal status (ie, late pregnancy or 1–3 weeks or 6–12 weeks postpartum). This may indicate that different biological rhythm parameters may be important to depressive symptoms at different points in the peripartum period. For instance, we found that higher pRA night was linked to decreased depressive symptoms at 6–12 weeks postpartum, a period that coincides with the higher risk of development of postpartum depression. This variable describes the probability of transitioning from rest to activity during the night, with higher values indicating higher fragmentation of nighttime rest, suggesting that women with more fragmented nighttime sleep at 6–12 weeks postpartum had lower depressive symptoms. Similarly, higher μR night was associated with lower depressive symptoms at 1–3 weeks and 6–12 weeks postpartum, indicating that higher activity during rest at night at 1–3 weeks and 6–12 weeks postpartum was linked to lower depressive symptoms. While these results might sound counterintuitive at first, they may indicate that mothers with lower depressive symptoms were more actively responsive to the babies’ needs overnight, since these same variables were not predictive of depressive symptoms in late pregnancy. Low stability and high fragmentation of activity rhythms as measured by interdaily stability and intradaily variability have been linked to findings such as mood instability and impulsivity,51 whereas measures of variability in activity have, overall, been linked to bipolar disorder.52 Additionally, although individuals with more robust daily rhythms (eg, those with higher IS and CQ) exhibit more mood stability, they are less likely to shift with changing environmental demands. For instance, shift workers with high IS display worsened cognitive performance as they start their night shifts.53 It should be noted that nighttime pRA is a more specific measure of fragmentation than intradaily variability (IV), as it marks the quantity of transitions out of sustained, stable rest bouts at night, whereas IV marks overall fragmentation throughout the 24 hours. Nighttime pRA is more closely linked specifically to awakenings during the night.
Next, we found a unique set of objective parameters that were linked to the trajectory of anxiety across the peripartum period. The parameters that were most strongly linked to increased anxiety symptoms across the peripartum period included increased μR night. At 1–3 weeks postpartum, however, lower μR night was linked to higher anxiety symptoms. Additionally, earlier mean timing of light exposure above 500 lux—that is, light exposure occurring during an earlier time in the day—was significantly associated with anxiety symptoms at 6–12 weeks postpartum; meanwhile, higher pRA night was predictive of lower anxiety symptoms at 1–3 weeks and 6–12 weeks postpartum. Previous findings have suggested subjective sleep quality, but not objective nighttime sleep, to be linked to peripartum anxiety.20,23,54 Timing of light exposure during the day has not been previously investigated in the context of postpartum anxiety, to our knowledge. While it is unclear from the results of this study whether timing of light exposure reflects a particular behavioral pattern, it is possible that this variable is linked to the timing of outdoor activity, therefore indirectly reflecting circadian phase. It should be noted that during the model selection process, acrophase and the start time of the lowest 5 hours of activity during the day at 1–3 weeks postpartum—2 markers of rhythm phase—were significant predictors of severity of anxiety (GAD-7 scores) in univariate models, though they did not reach the threshold for model selection. Additionally, a previous study55 found that daily light exposure patterns in combination with activity were able to predict circadian phase for dim light melatonin onset as part of the Kronauer limit-cycle model. Notably, when sleep variables were analyzed along with daily activity rhythms and light exposure, we did not find actigraphy-derived sleep parameters to be among the most predictive factors for depressive or anxiety symptoms during the peripartum period.
Consistent with findings that objective biological rhythm disturbances were linked to both peripartum depressive and peripartum anxiety symptoms, we found that increased subjective biological rhythm disruptions according to the BRIAN were associated with depressive and anxiety symptoms throughout the peripartum period. Self-reported biological rhythm disruption may therefore be an important and consistent marker for both depressive and anxiety symptoms throughout the peripartum period. While depressive symptoms during the peripartum period have previously been linked to self-reported biological rhythm disruptions across the peripartum period,25,26 to our knowledge, this study is the first to report self-reported biological rhythm disruption to be associated with anxiety during the peripartum period. Importantly, there was some overlap between variables that were most closely associated with depressive and anxiety symptoms, including subjective biological rhythms disturbances, μR night at 1–3 weeks postpartum, pRA night at 6–12 weeks postpartum, and having a current or past anxiety disorder. This finding is not entirely surprising, given the great degree of comorbidity between anxiety and depressive symptoms in the peripartum period.3 However, we did find a number of distinct sleep and biological rhythm variables that were the most important predictors of depressive (eg, CQ) and anxiety (eg, MLiT500) symptoms. Overall, findings that changes in biological rhythms beyond sleep were most influential on peripartum depressive and anxiety symptoms indicate that stabilization of biological rhythms and activity patterns beyond sleep is novel and highlights the important role of stabilizing biological rhythms to maintain healthy mood and minimize anxiety during the peripartum period.
Longitudinal Trajectories of Sleep and Biological Rhythms During the Peripartum Period
Another aim of our study was to investigate the longitudinal trajectory of biological rhythms and sleep across the peripartum period, which have been little investigated to date.26,56 We observed several possible trajectories of change in clinical variables, sleep, and biological rhythms across the peripartum period. A number of changes were transient, when variables changed during the early postpartum timepoint and returned to pregnancy levels by 6–12 weeks postpartum (eg, MADRS scores, ESS scores, IV, SE, WASO, RA, μR day, pAR night). These alterations in clinical, sleep, and biological rhythm variables may be indicative of maternal adjustment to new roles and patterns of sleep and activity. Transient changes in SE and WASO in the early postpartum are consistent with prior literature.57,58 We did not find TST to change over the peripartum period, indicating that there was no significant change in total sleep duration across the peripartum period, which is consistent with some,57 but not other studies.58 Some patterns of change that occurred in the postpartum period appeared to be related mostly to increased activity during the night, likely corresponding to meeting the infants’ needs (eg, nighttime feedings). Some of these changes occurred at 1–3 weeks postpartum, and values did not return to pregnancy levels (eg, nighttime activity mean and SD, μA night, L5). Meanwhile, other variables changed at 1–3 weeks postpartum compared to pregnancy and maintained the changes through 6–12 weeks postpartum (eg, pRA day, number of awakenings, pRA night). Further investigations should evaluate whether differences in trajectories of change in sleep and biological rhythm symptoms result from specific behavioral patterns across the peripartum period, and examine breastfeeding and infant behaviors during the night, to see whether these are related to changes in number of awakenings across the perinatal period.
The peripartum period is marked by dramatic changes in hormones, first by a robust rise of estrogen and progesterone throughout pregnancy, which subsequently drop following delivery. Progesterone and its metabolites, such as allopregnanolone, have sedative effects through positive allosteric modulation of γ-aminobutyric acid (GABA)A receptors59—in fact, sedation and somnolence are some of the major treatment-emergent effects from intravenous brexanolone, a synthetic form of allopregnanolone used for treatment of postpartum depression.60 It is conceivable that the robust changes in reproductive hormones during the peripartum period influence the changes in sleep and activity observed during this period, with potential sedating periods during late pregnancy, when progesterone levels are high, and disappearance of these sedating effects when progesterone levels suddenly drop after child delivery.
We found no changes in levels of overnight secretion of melatonin’s primary metabolite, 6-SM, from pregnancy to the postpartum period, and 6-SM levels were not found to be associated with peripartum depressive or anxiety symptoms. Additionally, we did not find changes in dim light melatonin onset from pregnancy to the postpartum period in a subsample of our participants. Sharkey and colleagues28 previously reported phase shifts in dim light melatonin onset from the third trimester of pregnancy to 6 weeks postpartum in a sample of women, emphasizing the potential role of internal misalignment of the timing of biological rhythms in postpartum depression. Other studies have also linked peripartum melatonin secretion to be associated with depressive,27 obsessive-compulsive, and manic symptoms across the peripartum period.61 A single sample of 6-SM from urine therefore may not provide enough granularity to allow observation of subtle dynamic changes in melatonin secretion over the peripartum period. Future studies should consider monitoring 6-SM secretion through multiple samples of urine throughout 24 hours, as this would allow measurement of 6-SM onset and offset and provide a more accurate measurement of 6-SM secretion. We also did not see evidence of a shift in phase of activity across the peripartum period, as marked by a lack of significant differences in variables such as mean mid-sleep time (ie, sleep midpoint), acrophase, and the start times of L5 and M10—the lowest 5 and highest 10 hours of consecutive activity from late pregnancy to either of the postpartum time points. From our results, it appears that changes in sleep patterns over the course of the peripartum period do not seem to be attributed to changes in circadian timing, given that the transient changes in SE and WASO at 1–3 weeks postpartum were not accompanied by changes in circadian phase. However, we did see a statistically significant shift from 1–3 weeks postpartum to 6–12 weeks postpartum toward an earlier mean timing of light exposure above 1,000 lux, which may be a marker of timing of outdoor activity. An alternative explanation for the shift toward an earlier mean timing of light exposure above 1,000 lux from 1–3 weeks to 6–12 weeks postpartum could be changes in infant behavior, such as earlier morning awakenings, as the infant’s sleep patterns begin to stabilize. Another implication of our study is the relevance of conducting transition probability analysis.44,48,49 This is a relatively novel technique, and we found that some of the most important predictors of depressive and anxiety symptoms were derived from this analysis. Moreover, the majority of the variables derived from this analysis showed robust changes across the peripartum period.
A limitation to this study is that actigraphy is thought to be less reliable than polysomnography in differentiating quiet wakefulness from sleep. We included a longer period of actigraphy monitoring (2 weeks), which is thought to improve sleep assessment.62 Importantly, we focused on understanding variables such as biological rhythms beyond sleep, which we would not be able to obtain through polysomnography, collection with which is limited to nighttime. Given the strong association of biological rhythm variables with peripartum mood and anxiety symptoms, future studies should investigate interventions targeting biological rhythms as treatment or prevention strategies for peripartum depression and anxiety. We found that timing of light exposure was an important predictor of anxiety symptoms at 6–12 weeks postpartum. To our knowledge, bright light therapy has not been previously investigated as an intervention for peripartum anxiety, though several small studies indicated preliminary evidence for posttreatment improvement following bright light therapy as an intervention for peripartum depression.63–67 Bright light therapy has been primarily used as an intervention for seasonal and non-seasonal depression,68,69 although some limited evidence also suggests that bright light therapy reduces symptoms of anxiety beyond symptoms of depression.70,71 Additionally, a study found preliminary evidence for posttreatment improvement for blue-light–blocking glasses as a treatment for peripartum depression,72 though it should be noted that several studies using blue-light–blocking glasses to treat insomnia or sleep disturbances in mood disorders were not effective in changing actigraphy-derived sleep variables, or depressive symptoms.73,74
CONCLUSIONS
In the largest and most comprehensive study conducted to date investigating the link between objective parameters of sleep and biological rhythms and peripartum mood and anxiety, we found that a specific set of biological rhythm markers beyond sleep were associated with the trajectory of depressive and anxiety symptoms from the third trimester of pregnancy to the third postpartum month. Findings from this study emphasize the importance of stabilization of biological rhythms and activity patterns beyond sleep to maintain healthy mood and minimize anxiety during the peripartum period.
Submitted: March 15, 2021; accepted August 18, 2021.
Published online: January 18, 2022.
Potential conflicts of interest: Dr Slyepchenko has received grant support from Pfizer, outside of this work. Drs Frey, Minuzzi, and Reilly have no conflicts to disclose.
Funding/support: This study was supported in part by an award from The Research Institute of St. Joe’s Hamilton and the Teresa Cascioli Charitable Foundation Research Award in Women’s Health.
Role of the sponsor: The funders did not have any role in the design, implementation, or publication of the study.
Acknowledgments: We would like to thank Jodi Gilchrist, MSc (St Joseph’s Healthcare Hamilton, Hamilton, Ontario), and Marg Coote, MSc (St Joseph’s Healthcare Hamilton, Hamilton, Ontario), for completing the laboratory analysis of the urinary melatonin samples. We are thankful to the Community Midwives of Hamilton for their help with recruitment of study participants. Additionally, we would like to thank Gabriella Mattina, PhD (Unity Health TO, Toronto, Ontario); Melissa Furtado, MSc (McMaster University, Hamilton, Ontario); and Sawayra Owais, MSc (McMaster University, Hamilton, Ontario), for their teamwork in study recruitment. We thank Aljeena Qureshi (McMaster University, Hamilton, Ontario) for her help with quality control and data entry. None of the acknowledged individuals has any potential conflicts of interest relevant to the subject of this article.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- While sleep disturbance has been widely associated with increased risk for postpartum depression, much less is known about how changes in the internal clock beyond sleep are associated with worse depression and anxiety in the postpartum period.
- Although dynamic changes in sleep patterns were observed, changes in activity and biological rhythms beyond sleep were most closely associated with depression and anxiety symptoms across the peripartum period.
- These results can inform future interventions focused on stabilization of daily routine, sleep, light exposure, and activity patterns in the prevention of postpartum depression and anxiety worsening.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Women’s Mental Health section. Please contact Marlene P. Freeman, MD, at [email protected].
References (74)

- Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315–323. PubMed CrossRef
- Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. PubMed CrossRef
- Falah-Hassani K, Shiri R, Dennis C-L. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol Med. 2017;47(12):2041–2053. PubMed CrossRef
- Weissman MM. Postpartum depression and its long-term impact on children: many new questions. JAMA Psychiatry. 2018;75(3):227–228. PubMed CrossRef
- Goodman JH, Watson GR, Stubbs B. Anxiety disorders in postpartum women: a systematic review and meta-analysis. J Affect Disord. 2016;203:292–331. PubMed CrossRef
- Andersson L, Sundström-Poromaa I, Wulff M, et al. Implications of antenatal depression and anxiety for obstetric outcome. Obstet Gynecol. 2004;104(3):467–476. PubMed CrossRef
- Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–1819. PubMed CrossRef
- Bánhidy F, Ács N, Puhó E, et al. Association between maternal panic disorders and pregnancy complications and delivery outcomes. Eur J Obstet Gynecol Reprod Biol. 2006;124(1):47–52. PubMed CrossRef
- Zou R, Tiemeier H, van der Ende J, et al. Exposure to maternal depressive symptoms in fetal life or childhood and offspring brain development: a population-based imaging study. Am J Psychiatry. 2019;176(9):702–710. PubMed CrossRef
- Furtado M, Chow CHT, Owais S, et al. Risk factors of new onset anxiety and anxiety exacerbation in the perinatal period: a systematic review and meta-analysis. J Affect Disord. 2018;238:626–635. PubMed CrossRef
- Hutchens BF, Kearney J. Risk factors for postpartum depression: an umbrella review. J Midwifery Womens Health. 2020;65(1):96–108. PubMed CrossRef
- Biaggi A, Conroy S, Pawlby S, et al. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62–77. PubMed CrossRef
- Lancaster CA, Gold KJ, Flynn HA, et al. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202(1):5–14. PubMed CrossRef
- Bei B, Coo Calcagni S, Milgrom J, et al. Day-to-day alteration of 24-hour sleep pattern immediately before and after giving birth. Sleep Biol Rhythms. 2012;10(3):212–221. CrossRef
- Sedov ID, Cameron EE, Madigan S, et al. Sleep quality during pregnancy: a meta-analysis. Sleep Med Rev. 2018;38:168–176. PubMed CrossRef
- Gallaher KGH, Slyepchenko A, Frey BN, et al. The role of circadian rhythms in postpartum sleep and mood. Sleep Med Clin. 2018;13(3):359–374. PubMed CrossRef
- Coo Calcagni S, Bei B, Milgrom J, et al. The relationship between sleep and mood in first-time and experienced mothers. Behav Sleep Med. 2012;10(3):167–179. PubMed CrossRef
- Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65–77. PubMed CrossRef
- Park EM, Meltzer-Brody S, Stickgold R. Poor sleep maintenance and subjective sleep quality are associated with postpartum maternal depression symptom severity. Arch Women Ment Health. 2013;16(6):539–547. PubMed CrossRef
- Bei B, Milgrom J, Ericksen J, et al. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33(4):531–538. PubMed CrossRef
- McEvoy KM, Rayapati D, Washington Cole KO, et al. Poor postpartum sleep quality predicts subsequent postpartum depressive symptoms in a high-risk sample. J Clin Sleep Med. 2019;15(9):1303–1310. PubMed CrossRef
- Wolfson AR, Crowley SJ, Anwer U, et al. Changes in sleep patterns and depressive symptoms in first-time mothers: last trimester to 1-year postpartum. Behav Sleep Med. 2003;1(1):54–67. PubMed CrossRef
- Osnes RS, Roaldset JO, Follestad T, et al. Insomnia late in pregnancy is associated with perinatal anxiety: a longitudinal cohort study. J Affect Disord. 2019;248:155–165. PubMed CrossRef
- Tham EK, Tan J, Chong YS, et al. Associations between poor subjective prenatal sleep quality and postnatal depression and anxiety symptoms. J Affect Disord. 2016;202:91–94. PubMed CrossRef
- Krawczak EM, Minuzzi L, Hidalgo MP, et al. Do changes in subjective sleep and biological rhythms predict worsening in postpartum depressive symptoms? a prospective study across the perinatal period. Arch Women Ment Health. 2016;19(4):591–598. PubMed CrossRef
- Krawczak EM, Minuzzi L, Simpson W, et al. Sleep, daily activity rhythms and postpartum mood: a longitudinal study across the perinatal period. Chronobiol Int. 2016;33(7):791–801. PubMed CrossRef
- Parry BL, Meliska CJ, Sorenson DL, et al. Plasma melatonin circadian rhythm disturbances during pregnancy and postpartum in depressed women and women with personal or family histories of depression. Am J Psychiatry. 2008;165(12):1551–1558. PubMed CrossRef
- Sharkey KM, Pearlstein TB, Carskadon MA. Circadian phase shifts and mood across the perinatal period in women with a history of major depressive disorder: a preliminary communication. J Affect Disord. 2013;150(3):1103–1108. PubMed CrossRef
- Caropreso L, Saliba S, Hasegawa L, et al. Quality assurance assessment of a specialized perinatal mental health clinic. BMC Pregnancy Childbirth. 2020;20(1):485. PubMed CrossRef
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33, quiz 34–57. PubMed
- Beck CT. Revision of the postpartum depression predictors inventory. J Obstet Gynecol Neonatal Nurs. 2002;31(4):394–402. PubMed CrossRef
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782–786. PubMed CrossRef
- Montgomery S, Åsberg M. A New Depression Scale Designed to be Sensitive to Change: Acad. Department of Psychiatry, Guy’s Hospital; 1977.
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–435. PubMed CrossRef
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. PubMed CrossRef
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. PubMed CrossRef
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. PubMed CrossRef
- Giglio LMF, Magalhães PV, Andreazza AC, et al. Development and use of a biological rhythm interview. J Affect Disord. 2009;118(1-3):161–165. PubMed CrossRef
- Henshaw C. Mood disturbance in the early puerperium: a review. Arch Women Ment Health. 2003;6(suppl 2):S33–S42. PubMed CrossRef
- Chang YS, Lin MH, Lee JH, et al. Melatonin supplementation for children with atopic dermatitis and sleep disturbance: a randomized clinical trial. JAMA Pediatr. 2016;170(1):35–42. PubMed CrossRef
- Nowak R, McMillen IC, Redman J, et al. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: two non-invasive techniques for monitoring human circadian rhythmicity. Clin Endocrinol (Oxf). 1987;27(4):445–452. PubMed CrossRef
- Sturgeon SR, Doherty A, Reeves KW, et al. Urinary levels of melatonin and risk of postmenopausal breast cancer: women’s health initiative observational cohort. Cancer Epidemiol Biomarkers Prev. 2014;23(4):629–637. PubMed CrossRef
- Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4(1):66–69. PubMed CrossRef
- Slyepchenko A, Allega OR, Leng X, et al. Association of functioning and quality of life with objective and subjective measures of sleep and biological rhythms in major depressive and bipolar disorder. Aust N Z J Psychiatry. 2019;53(7):683–696. PubMed CrossRef
- Reid KJ, Santostasi G, Baron KG, et al. Timing and intensity of light correlate with body weight in adults. PLoS One. 2014;9(4):e92251. PubMed CrossRef
- van Someren EJ, Hagebeuk EE, Lijzenga C, et al. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol Psychiatry. 1996;40(4):259–270. PubMed CrossRef
- Blume C, Santhi N, Schabus M. ‘nparACT’ package for R: A free software tool for the non-parametric analysis of actigraphy data. 2016;3:430–435.
- Ortiz A, Bradler K, Radu L, et al. Exponential state transition dynamics in the rest-activity architecture of patients with bipolar disorder. Bipolar Disord. 2016;18(2):116–123. PubMed CrossRef
- Allega OR, Leng X, Vaccarino A, et al. Performance of the biological rhythms interview for assessment in neuropsychiatry: an item response theory and actigraphy analysis. J Affect Disord. 2018;225:54–63. PubMed CrossRef
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. https://www.R-project.org/.
- McGowan NM, Goodwin GM, Bilderbeck AC, et al. Actigraphic patterns, impulsivity and mood instability in bipolar disorder, borderline personality disorder and healthy controls. Acta Psychiatr Scand. 2020;141(4):374–384. PubMed CrossRef
- Scott J, Murray G, Henry C, et al. Activation in bipolar disorders: a systematic review. JAMA Psychiatry. 2017;74(2):189–196. PubMed CrossRef
- Rosa DE, Marot LP, de Mello MT, et al. Shift rotation, circadian misalignment and excessive body weight influence psychomotor performance: a prospective and observational study under real life conditions. Sci Rep. 2019;9(1):19333. PubMed CrossRef
- Volkovich E, Tikotzky L, Manber R. Objective and subjective sleep during pregnancy: links with depressive and anxiety symptoms. Arch Women Ment Health. 2016;19(1):173–181. PubMed CrossRef
- Woelders T, Beersma DGM, Gordijn MCM, et al. Daily light exposure patterns reveal phase and period of the human circadian clock. J Biol Rhythms. 2017;32(3):274–286. PubMed CrossRef
- Thomas KA, Burr RL, Spieker S, et al. Mother-infant circadian rhythm: development of individual patterns and dyadic synchrony. Early Hum Dev. 2014;90(12):885–890. PubMed CrossRef
- Matsumoto K, Shinkoda H, Kang M, et al. Longitudinal study of mothers’ sleep-wake behaviors and circadian time patterns from late pregnancy to postpartum: monitoring of wrist actigraphy and sleep logs. Biol Rhythm Res. 2003;34(3):265–278. CrossRef
- Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, et al. Normative longitudinal maternal sleep: the first 4 postpartum months. Am J Obstet Gynecol. 2010;203(5):465.e1–465.e7. PubMed CrossRef
- Lancel M, Faulhaber J, Holsboer F, et al. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol. 1996;271(4 Pt 1):E763–E772. PubMed CrossRef
- Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058–1070. PubMed CrossRef
- Obeysekare JL, Cohen ZL, Coles ME, et al. Delayed sleep timing and circadian rhythms in pregnancy and transdiagnostic symptoms associated with postpartum depression. Transl Psychiatry. 2020;10(1):14. PubMed CrossRef
- Van de Water AT, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography—a systematic review. J Sleep Res. 2011;20(1 Pt 2):183–200. PubMed CrossRef
- Swanson LM, Burgess HJ, Zollars J, et al. An open-label pilot study of a home wearable light therapy device for postpartum depression. Arch Women Ment Health. 2018;21(5):583–586. PubMed CrossRef
- Bais B, Kamperman AM, van der Zwaag MD, et al. Bright light therapy in pregnant women with major depressive disorder: study protocol for a randomized, double-blind, controlled clinical trial. BMC Psychiatry. 2016;16(1):381. PubMed CrossRef
- Epperson CN, Terman M, Terman JS, et al. Randomized clinical trial of bright light therapy for antepartum depression: preliminary findings. J Clin Psychiatry. 2004;65(3):421–425. PubMed CrossRef
- Oren DA, Wisner KL, Spinelli M, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry. 2002;159(4):666–669. PubMed CrossRef
- Wirz-Justice A, Bader A, Frisch U, et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry. 2011;72(7):986–993. PubMed CrossRef
- Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: meta-analysis of clinical trials. J Affect Disord. 2016;198:64–71. PubMed CrossRef
- Pjrek E, Friedrich ME, Cambioli L, et al. The efficacy of light therapy in the treatment of seasonal affective disorder: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2020;89(1):17–24. PubMed CrossRef
- Youngstedt SD, Kripke DF. Does bright light have an anxiolytic effect? - an open trial. BMC Psychiatry. 2007;7(1):62. PubMed CrossRef
- Baxendale S, O’Sullivan J, Heaney D. Bright light therapy for symptoms of anxiety and depression in focal epilepsy: randomised controlled trial. Br J Psychiatry. 2013;202(5):352–356. PubMed CrossRef
- Bennett S, Alpert M, Kubulins V, et al. Use of modified spectacles and light bulbs to block blue light at night may prevent postpartum depression. Med Hypotheses. 2009;73(2):251–253. PubMed CrossRef
- Esaki Y, Takeuchi I, Tsuboi S, et al. A double-blind, randomized, placebo-controlled trial of adjunctive blue-blocking glasses for the treatment of sleep and circadian rhythm in patients with bipolar disorder. Bipolar Disord. 2020;22(7):739–748. PubMed CrossRef
- Esaki Y, Kitajima T, Takeuchi I, et al. Effect of blue-blocking glasses in major depressive disorder with sleep onset insomnia: a randomized, double-blind, placebo-controlled study. Chronobiol Int. 2017;34(6):753–761. PubMed CrossRef
This PDF is free for all visitors!