In 2003, the US Food and Drug Administration (FDA) issued a boxed bolded warning for selective serotonin reuptake inhibitor (SSRI) use in children and adolescents based on findings from clinical trials conducted by developers.1 Concerns were raised regarding the risk of suicidal thoughts and behaviors, which led to the potential avoidance of prescribing antidepressant medications to children, adolescents, and young adults.2 Questions remain regarding the potential of behavioral activation in this population.3
Treatment-Emergent Suicidality
Treatment-emergent suicidality was assessed in several studies, including the Treatment of Adolescents with Depression Study (TADS),4 Treatment of Resistant Depression in Adolescents (TORDIA),5 and Treatment of Adolescent Suicide Attempters (TASA).6 During the acute phase, TADS demonstrated a reduction in suicidal events (SEs; defined as worsening suicidal ideation or attempts) across all 4 treatment groups. At the end of 36 weeks, 44 events were reported. Fluoxetine with cognitive behavioral therapy (CBT) was statistically superior to all other treatment groups in addressing SEs. The rate of SEs was higher during the acute phase compared to the follow-up period (61.4% vs 38.6%).4 In the TORDIA trial, there was no discernible advantage of combined treatment over medication alone for SEs. However, during the 12-week follow-up, 14% of participants experienced an SE and 9% had a nonsuicidal self-injury event. The median time to an SE was 3 weeks.5 Like TORDIA contrary to TADS, the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT) in the United Kingdom7 did not find evidence for SSRI-associated treatment-emergent suicidality or the protective effects of CBT. The median time to an SE in the TASA trial was 44 days, with 40% occurring within 4 weeks of treatment initiation.6
Most recently, a network meta-analysis reviewed 26 placebo-controlled antidepressant trials that also included (besides SSRIs) novel therapeutics such as selective norepinephrine reuptake inhibitors, norepinephrine reuptake inhibitors, norepinephrine and dopamine reuptake inhibitors, norepinephrine and dopamine disinhibitors, and tetracyclic antidepressants. These newer antidepressants were associated with both smaller reductions in depressive symptoms and “small and unimportant” differences between classes of antidepressants compared to the placebo.
SE was frequently an exclusion criterion in these trials, which challenges the overall analysis. Escitalopram emerged as the safer option (odds ratio [OR], 0.89; 95% CI, 0.43–1.84), whereas venlafaxine (OR, 13.84; 95% CI, 1.79–106.90) was associated with increased odds of suicide-related outcomes compared to the placebo.8 Another meta-analysis reported that sertraline was associated with reduced risks of suicidal behavior/ideation in the treatment of anxiety disorders.9
The wider discussion about small group differences, high placebo response rates, variability in efficacies for other disorders, failed or negative trials, and small effect sizes of antidepressants is beyond the scope of this review.
How Do Autism Spectrum Disorder Phenotypes Intersect With SSRI- Associated Activation?
The empirical literature linked to individuals with autism spectrum disorder (ASD) has several nuanced findings. First, 2 meta-analyses studying the effects of SSRIs (2006 and 2013)10,11 indicated marked variability in clinical response (for repetitive, restricted behaviors and interests, or RRBIs)12 ranging from no effects,13,14 to increased likelihood of behavioral activation,3,15 to the potential for harm.16 Second, the 2023 epidemiological trends suggest an increase in the prevalence (1:36 under the age of 8) attributed to many factors including late or missed diagnosis.17 This raises the question that amidst myriad diagnostic issues in children and adolescents with ASD,18,19 there is a likelihood of selection bias in the inclusion and exclusion criteria of the earlier studies. Third, a growing body of evidence supports a distinct phenomenology of suicidal thoughts and behaviors relative to typically developing children, and substantially higher rates of SEs.20,21
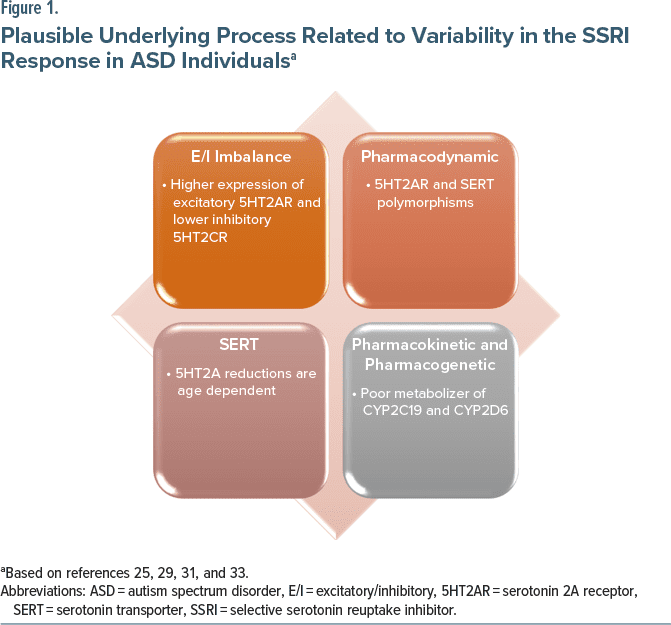
What are the biological underpinnings? A study demonstrated a statistically significant decrease in serotonin transporter (SERT) within the anterior cingulate cortex (ACC), but not in the prefrontal cortex (PFC) and fusiform gyrus. Notably, a reduction in serotonin 2A receptor (5HT2AR) density in the ACC was observed in the adult cohort, but not in postmortem cases of children with ASD, suggesting that the reductions in 5HT2AR density are age-dependent.22
To understand its correlation with SSRI response in individuals with ASD as a function of genetic variation, researchers have focused on the serotonin-transporter-linked polymorphic region (5HTTLPR) of SLC6A4 (a single SERT gene located on the long arm of chromosome 17q11-17q12) and the serotonin receptor 2A gene (HTR2A). The SS and LL genotypes are associated with decreased and increased responses to SSRI medications, respectively. Two studies report lesser responses in irritability measured on the Aberrant Behavior Checklist-Irritability and Clinical Global Impressions-Improvement Scale in the 5HTTLPR SS group, respectively.23,24 Another group studied both SLC6A4 and HTR2A alleles in treatment response to escitalopram for 6 weeks in an open-label trial but could not find associations.25 They postulated that the downregulation of SERT is a mechanism to offset central hyperserotonemia; however, many SERT polymorphism forms are unable to perform this function.22 As a result, increased central serotonin (5-HT) is associated with severe ASD symptoms.
In 2003, studies found both an increase in the expression of 5HT2AR in the PFC of postmortem brains of adolescents who died by suicide and abnormal editing of the serotonin (5-HT)2C receptors (5HT2C receptors).26,27 In 2011, a study found how the expression of mRNA from excitatory 5HT2AR follows a developmental pattern, increasing from infancy through toddlerhood and school age, peaking during adolescence, and then declining in the young adult years until reaching levels observed in adulthood. Concurrently, mRNA expression from inhibitory 5HT2CR follows a similar pattern of upregulation, maintaining a level two-thirds of 5HT2AR expression and achieving equilibrium in adulthood. It suggests that the activating form of 5HT2AR expression predominates from the school-age years through adolescence.28 The most significant difference in expression occurs during adolescence. This pattern aligns with the well-known phenomenon that behavioral activation associated with using SSRIs is more likely to occur in younger children and adolescents.29 These ontological delays may correlate with the observed behavioral activation associated with SSRIs in nearly a third of individuals with ASD, with younger children seeming to be more affected than adolescents.29 The in vivo studies suggest that disrupting the excitatory/inhibitory (E/I) balance in cortical circuits underlies the pathophysiology, particularly in the PFC.30 A 2017 proton magnetic resonance spectroscopic study reported hypo-γ-aminobutyric acid (GABAergic) alterations in children and adolescents with ASD, but normal glutamate markers suggest that the ACC may contribute to E/I imbalance.31 The developmental aspects of multiple monoaminergic systems9 and prefrontal-amygdala circuits that regulate arousal29 continue to confound these findings requiring further research.
Finally, besides the pharmacodynamic (PD) factors associated with 5HTTLPR and HTR2A gene polymorphism, the pharmacokinetic (PK) factors like poor metabolism of CYP450 and sudden peaks in plasma concentration due to rapid medication titration are linked to variability in responses and behavioral activation. The dose adjustments are recommended for citalopram, escitalopram, and sertraline based on CYP2C19 metabolizer status and for fluvoxamine and paroxetine based on CYP2D6 metabolizer status.32,33
Should SSRIs Be Prescribed for Individuals With ASD, and How?
The pharmacologic interventions in ASD individuals are complex, attributed to multiple factors not limited to heterotypic continuity, phenotypic variabilities linked with gender, cognitive abilities, ethnicities, and comorbidities. The developmental phenomenology, the validity of diagnostics, the differentiation between normative and psychopathological symptoms, receptor ontogeny, and the intricate PK, PD, and pharmacogenomic (PGx) profiles represent only a subset of the multifaceted and challenging variables.
In children with ASD, there are numerous inconclusive SSRI trials with insignificant small effect sizes. Therefore, as a rule, irrespective of the ASD diagnosis in children and adolescents, the SSRIs must be initiated at a low dose, with slow gradual titration. The critical step is a frequent follow-up during the initiation period given that treatment-emergent suicidality is typically observed after 2–3 weeks. Not only is it crucial to discontinue SSRI at that point, a second diagnostic workup to rule out ASD may also be indicated. It is also reasonable to discern (given widespread diagnostic overshadowing) that developing ASD phenotypes have a higher likelihood of activation. Psychoeducation and shared decision-making are recommended if another trial with a different class of SSRI is considered given that the risk for treatment-emergent suicidality increases substantially. Therefore, if there is a clinical suspicion of ASD, particularly in school-age children and adolescents, then caution is required while using SSRIs. Paroxetine and venlafaxine were 2 identified therapeutics during research trials that were linked with treatment-emergent suicidality9,34 and must be avoided. At the same time, there are off-label options with atypical mechanisms of action like buspirone and mirtazapine.35
Lastly, an overwhelming preponderance of nuanced contrarian evidence calls for reinvestigating the boxed warning for SSRIs.1 While boxed warnings served their purpose, incorporating emerging evidence to develop improved guidance would foster an alliance among stakeholders critical for public health partnerships.
Conclusion
These findings correspond to boxed bolded warnings contained in antidepressant drug labels and discourage the use of SSRIs in people with developmental disorders. Therefore, the utility of SSRIs in this population remains limited given equivocal empirical evidence with a higher risk of behavioral activation and treatment-emergent suicidality. The clinicians must reaffirm ASD diagnostics and when psychopharmacological treatment is indicated may consider off-label therapeutic options.
Article Information
Published Online: April 3, 2024. https://doi.org/10.4088/JCP.24ac15280
© 2024 Physicians Postgraduate Press, Inc.
J Clin Psychiatry 2024;85(2):24ac15280
To Cite: Gupta M. SSRI behavioral activation, the boxed bolded warning, and neurodevelopmental disorders. J Clin Psychiatry. 2024;85(2):24ac15280.
Author Affiliation: Southwood Psychiatric Hospital, Pittsburgh, Pennsylvania.
Corresponding Author: Mayank Gupta, MD, Southwood Psychiatric Hospital, 2575 Boyce Plaza Rd, Pittsburgh, PA 15241 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.

The ASCP Corner, edited by Leslie L. Citrome, MD, MPH, is a collection of brief peer-reviewed, evidence-based articles, authored by American Society of Clinical Psychopharmacology members, that examine the practice of psychopharmacology through the lens of clinical experience. The information contained herein only represents the opinion of the author(s). See more ASCP Corner Articles at Psychiatrist.com/ASCP-Corner
References (35)

- Gupta M, Gupta N. FDA black box warning for SSRI: reexamining the role of high-functioning autism as a confounder. Adv Neurodev Disord. Published online October 26, 2022. doi: 10.1007/s41252-022-00301-6 CrossRef
- Noel C. Antidepressants and suicidality: history, the black-box warning, consequences, and current evidence. Ment Health Clin. 2015;5(5):202–211. CrossRef
- Amitai M, Chen A, Weizman A, et al. SSRI-induced activation syndrome in children and adolescents—what is next? Curr Treat Options Psychiatry. 2015;2:28–37. CrossRef
- March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292(7):807–820. PubMed CrossRef
- Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901–913. PubMed CrossRef
- Brent DA, Greenhill LL, Compton S, et al. The Treatment of Adolescent Suicide Attempters study (TASA): predictors of suicidal events in an open treatment trial. J Am Acad Child Adolesc Psychiatry. 2009;48(10):987–996. PubMed CrossRef
- Goodyer IM, Dubicka B, Wilkinson P, et al. A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial. Health Technol Assess. 2008;12(14):iii–iv, ix–60. PubMed CrossRef
- Hetrick SE, McKenzie JE, Bailey AP, et al. New generation antidepressants for depression in children and adolescents: a network meta-analysis. Cochrane Database Syst Rev. 2021;5(5):CD013674. PubMed CrossRef
- Boaden K, Tomlinson A, Cortese S, et al. Antidepressants in children and adolescents: meta-review of efficacy, tolerability and suicidality in acute treatment. Front Psychiatry. 2020;11:717. PubMed CrossRef
- Kolevzon A, Mathewson KA, Hollander E. Selective serotonin reuptake inhibitors in autism: a review of efficacy and tolerability. J Clin Psychiatry. 2006;67(3):407–414. PubMed CrossRef
- Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;(8):CD004677. PubMed CrossRef
- Mouti A, Reddihough D, Marraffa C, et al. Fluoxetine for Autistic Behaviors (FAB trial): study protocol for a randomized controlled trial in children and adolescents with autism. Trials. 2014;15:230. PubMed CrossRef
- King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583–590. PubMed CrossRef
- Alolaby RR, Jiraanont P, Durbin-Johnson B, et al. Molecular biomarkers predictive of sertraline treatment response in young children with autism spectrum disorder. Front Genet. 2020;11:308. PubMed CrossRef
- King BH. Fluoxetine and repetitive behaviors in children and adolescents with autism spectrum disorder. JAMA. 2019;322(16):1557–1558. PubMed CrossRef
- Herscu P, Handen BL, Arnold LE, et al. The SOFIA study: negative multi-center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord. 2020;50(9):3233–3244. PubMed CrossRef
- Gupta M, Gupta N, Moll J. Duration of untreated autism in rural America: emerging public health crisis. CNS Spectr. 2023;28(3):271–274. PubMed CrossRef
- Gupta N, Gupta M. Diagnostic overshadowing in high-functioning autism: mirtazapine, buspirone, and modified cognitive behavioral therapy (CBT) as treatment options. Cureus. 2023;15(5):e39446. PubMed CrossRef
- Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry-and NIMH-funded studies. AJP. 2017;174(5):430–437. PubMed CrossRef
- Kirby AV, Bakian AV, Zhang Y, et al. A 20-year study of suicide death in a statewide autism population. Autism Res. 2019;12(4):658–666. PubMed CrossRef
- Kõlves K, Fitzgerald C, Nordentoft M, et al. Assessment of suicidal behaviors among individuals with autism spectrum disorder in Denmark. JAMA Netw Open. 2021;4(1):e2033565. PubMed CrossRef
- Brandenburg C, Blatt GJ. Differential serotonin transporter (5-HTT) and 5-HT2 receptor density in limbic and neocortical areas of adults and children with autism spectrum disorders: implications for selective serotonin reuptake inhibitor efficacy. J Neurochem. 2019;151(5):642–655. PubMed CrossRef
- Sugie Y, Sugie H, Fukuda T, et al. Clinical efficacy of fluvoxamine and functional polymorphism in a serotonin transporter gene on childhood autism. J Autism Dev Disord. 2005;35(3):377–385. PubMed CrossRef
- Owley T, Brune CW, Salt J, et al. A pharmacogenetic study of escitalopram in autism spectrum disorders. Autism Res. 2010;3(1):1–7. PubMed CrossRef
- Najjar F, Owley T, Mosconi MW, et al. Pharmacogenetic study of serotonin transporter and 5HT2A genotypes in autism. J Child Adolesc Psychopharmacol. 2015;25(6):467–474. PubMed CrossRef
- Pandey GN, Dwivedi Y, Rizavi HS, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159(3):419–429. PubMed CrossRef
- Schmauss C. Serotonin 2C receptors: suicide, serotonin, and runaway RNA editing. Neuroscientist. 2003;9(4):237–242. PubMed CrossRef
- Lambe EK, Fillman SG, Webster MJ, et al. Serotonin receptor expression in human prefrontal cortex: balancing excitation and inhibition across postnatal development. PLoS One. 2011;6(7):e22799. PubMed CrossRef
- Luft MJ, Lamy M, DelBello MP, et al. Antidepressant-induced activation in children and adolescents: risk, recognition and management. Curr Probl Pediatr Adolesc Health Care. 2018;48(2):50–62. PubMed CrossRef
- Saitow F, Takumi T, Suzuki H. Change in serotonergic modulation contributes to the synaptic imbalance of neuronal circuit at the prefrontal cortex in the 15q11-13 duplication mouse model of autism. Neuropharmacology. 2020;165:107931. PubMed CrossRef
- Ito H, Mori K, Harada M, et al. A proton magnetic resonance spectroscopic study in autism spectrum disorder using a 3-tesla clinical magnetic resonance imaging (MRI) system: the anterior cingulate cortex and the left cerebellum. J Child Neurol. 2017;32(8):731–739. PubMed CrossRef
- Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127–134. PubMedCrossRef
- Milosavljević F, Bukvić N, Pavlović Z, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(3):270–280. PubMed CrossRef
- Smith EG. Association between antidepressant half-life and the risk of suicidal ideation or behavior among children and adolescents: confirmatory analysis and research implications. J Affect Disord. 2009;114(1-3):143–148. PubMed CrossRef
- Gupta N, Gupta M. Off-label psychopharmacological interventions for autism spectrum disorders: strategic pathways for clinicians. CNS Spectr. Published online August 4, 2023. doi: 10.1017/S1092852923002389 PubMed CrossRef
This PDF is free for all visitors!