ABSTRACT
Objective: Fluoxetine, paroxetine, sertraline, topiramate, and venlafaxine have previously shown efficacy for posttraumatic stress disorder (PTSD) in randomized clinical trials. Two prior studies using Department of Veterans Affairs (VA) medical records data show these medications are also effective in routine practice. Using an expanded retrospective cohort, we assessed the possibility of differential patterns of response based on patient and clinical factors.
Methods: We identified 6,839 VA outpatients with clinical diagnoses of PTSD between October 1999 and September 2019 who initiated one of the medications and met pre-specified criteria for treatment duration and dose, combined with baseline and endpoint PTSD checklist (PCL) measurements. We compared 12-week changes in PCL score within clinical subgroups defined by sex, race and ethnicity, and military exposures, as well as comorbidities. Comorbidities were identified using International Classification of Diseases diagnostic codes and grouped according to major diagnostic classifications in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (eg, Psychotic Disorders, Depressive Disorders). We used a propensity score weighting approach to balance covariates among medication arms within each clinical subgroup. In our exploratory analyses using unweighted data for the overall cohort, we built penalized logistic regression models to identify covariates that predicted meaningful improvement.
Results: There were no significant differences between medications in our weighted subgroup analyses. In unweighted exploratory analyses, higher baseline PCL scores and concurrent receipt of evidence-based psychotherapy predicted meaningful improvement, while high levels of disability predicted not realizing meaningful improvement.
Conclusions: In the largest real-world study of medications for PTSD to date, we did not observe a pattern of differential response among clinical subgroups. All patients taking medications for PTSD, especially those with the highest levels of disability, should consider combined treatment with evidence-based psychotherapy.
J Clin Psychiatry 2021;82(6):21m13913
To cite: Shiner BR, Gui J, Rozema L, et al. Patient and clinical factors associated with response to medications for posttraumatic stress disorder. J Clin Psychiatry. 2021;82(6):21m13913.
To share: https://doi.org/10.4088/JCP.21m13913
© Copyright 2021 Physicians Postgraduate Press, Inc.
aNational Center for PTSD, White River Junction, Vermont
bVeterans Affairs Medical Center, White River Junction, Vermont
cDepartments of Psychiatry and The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire
dDepartments of Biomedical Data Science, Community & Family Medicine, and The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire
eNational Center for PTSD, White River Junction, Vermont
fDepartment of Psychiatry, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire
gOffice of Systems Redesign and Improvement, Washington, DC
*Corresponding author: Brian R. Shiner, MD, MPH, VA Medical Center; 215 North Main St, 116D, White River Junction, VT 05009 ([email protected]).
Randomized controlled trials (RCTs) show that effective treatments for posttraumatic stress disorder (PTSD) include both pharmacologic and psychotherapeutic approaches.1,2 Several individual medications have shown efficacy as PTSD treatment in placebo-controlled RCTs,1,2 but there have not been head-to-head prospective comparisons of the most effective agents. Two prior retrospective studies using Department of Veterans Affairs (VA) data to examine 5 of these medications—fluoxetine, sertraline, paroxetine, topiramate, and venlafaxine—indicate that they are also effective in routine clinical practice.3,4
There is a great deal of variation among patients with PTSD in terms of demographic characteristics and comorbidities that appear to drive medication selection and may influence outcomes.5,6 For example, patients with PTSD and comorbid pain disorders, headache disorders, and alcohol use disorder (AUD) are increasingly likely to receive anticonvulsants such as topiramate.5 While expert opinion has focused on factors such as comorbidity in the selection of specific medications for PTSD,7 there is a lack of definitive data to support such an approach.8 In the absence of RCTs examining treatment effectiveness in specific clinical scenarios, retrospective studies using health care data can inform clinical decision making based on patient and clinical factors.9
Building on our prior work,3,4 we conducted a retrospective comparative effectiveness study of the same 5 medications for PTSD in clinically important subgroups defined by sex, race and ethnicity, and military service characteristics, as well as comorbidities. In addition to examining these pre-identified groups, we conducted exploratory analyses to identify other potential predictors of meaningful improvement in symptoms. As there has been no prior research comparing these agents within clinical subgroups, we did not have a hypothesis. Rather, our goal was to provide clinicians with preliminary information about how to best select a medication based on demographic characteristics and comorbidities. While we did not have formal hypotheses, we expected to find differences in the pattern of response related to differences between the medications. For example, this might include superior PTSD symptom reduction in patients with comorbid headache disorders when they receive topiramate, presumably representing a synergistic effect of addressing both problems.
METHOD
Data Sources
This was a retrospective medical record review. We used the VA Corporate Data Warehouse (CDW) to identify all VA users with a clinical diagnosis of PTSD (309.81, F43.1x) from October 1, 1999–September 30, 2019. We obtained information on services use, clinical diagnoses, prescription fills, and patient-reported outcome measures (PROMs) from the CDW for these patients. This study was approved by the Veterans Institutional Review Board of Northern New England.
Cohort Selection
We identified patients who initiated a course of fluoxetine, sertraline, paroxetine, topiramate, or venlafaxine. The study sample was further restricted to those who met our criteria for adequate acute phase medication management. Patients receiving continuous treatment with sertraline, fluoxetine, paroxetine, venlafaxine, or topiramate daily for ≥ 12 weeks at an adequate dose were considered to have received an adequate medication trial (AMT). Adequate doses, which were required for the final 8 weeks only to allow for titration, were as follows: fluoxetine ≥ 20 mg, paroxetine ≥ 20 mg, sertraline ≥ 100 mg, topiramate ≥ 100 mg, and venlafaxine ≥ 150 mg. We further restricted to those who received baseline PTSD symptom measurement within 4 weeks prior to or 2 weeks after treatment initiation, received follow-up symptom measurement within 2 weeks prior to or 4 weeks after the 12-week point, and met our criterion for PTSD severity at baseline (defined below).
PTSD Symptoms
In order to maximize sample size within our clinical subgroups, we integrated 2 different versions of a PROM for PTSD, captured from up to 2 data sources within the CDW, to obtain our baseline and follow-up symptom measurements. This included scores obtained from structured data produced by psychometric assessment software in the VA medical record and scores documented by clinicians in their treatment notes. We used a previously published natural language processing (NLP) algorithm with 98% precision in identifying the correct score and version of the PCL to abstract scores from clinical notes.10,11 Scores abstracted from structured data and from NLP of clinical notes were integrated into a single dataset.
The two PROMs were the PTSD Checklist (PCL) versions aligned to the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fourth and Fifth Editions,12,13 which we will henceforth call the PCL-IV and the PCL-5.14,15 Validation work shows a correlation of 0.87 between PCL versions in a large sample of Veterans.16 We used a validated crosswalk (intraclass correlation coefficient = 0.96) to convert all values to PCL-5 scoring17 and required a baseline severity score of ≥ 31 out of 80 to classify participants as having PTSD.16 We did not examine individual symptomatic criteria for PTSD both because individual item scores were not available when abstracting PCL data from note text using NLP and because the PCL version scoring crosswalk we used was based on total scores. We created a covariate for whether the original score was from the PCL-IV or PCL-5 due to prior findings that venlafaxine may have superior effects on PTSD as assessed using DSM-5, but not DSM-IV, which may be a result of additional items related to negative alterations in cognitions and mood.3 In addition to calculating continuous change from baseline to follow-up, we assessed a categorical outcome of clinically meaningful improvement, which was a decrease of 15 points or more from baseline to follow-up.18
Our a priori power calculations were based on a random sample of 200 patients from our first published study of the comparative effectiveness of evidence-based medications for PTSD in routine VA practice who had 5 repeated PCL measurements over their initial 8 weeks of an AMT (correlation, ρ = 0.7).4 We modeled between group differences in effect size per 2-week period for change in PCL with a power of 80% and a Bonferroni-corrected 2-sided type I error rate of 0.005 to account for 10 potential comparisons at each time point using a generalized estimating equation. We found minimum cell sizes of 288, 104, and 41 for small, medium, and large effects (d = 0.3, 0.5, and 0.8, respectively). Therefore, we eliminated subgroups with less than 41 AMTs in any of the 5 medication cells, as greater effect size differences were implausible based on a prior meta-analysis of RCT results.2
Independent Variables
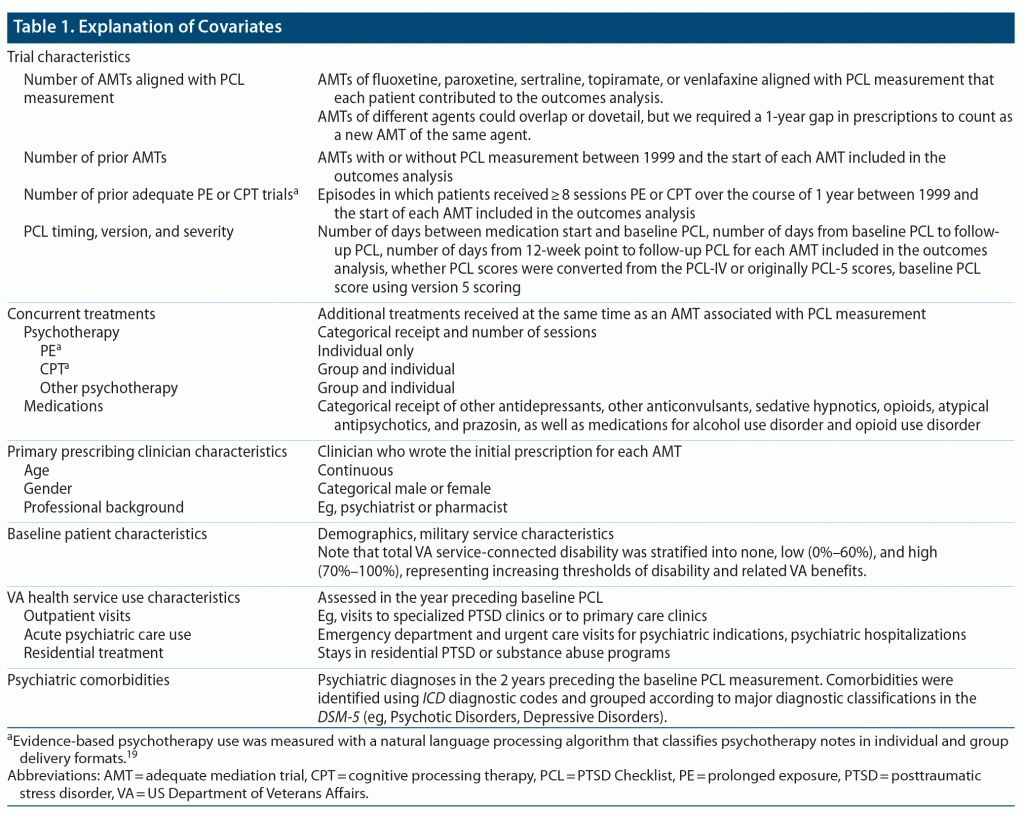
We measured 6 groups of covariates that could plausibly affect the relationship between treatment and outcome. See Table 1 for details.
Analysis
To conduct our primary analysis, we were guided by expert opinion in dividing the sample into putative clinical subgroups based on sex, race and ethnicity, military service era, military exposures, and comorbidities.7 We defined comorbidities by the presence of 2 or more outpatient diagnoses or 1 or more inpatient diagnosis in the year prior to medication initiation. We repeated 3 analytic steps described below separately for each clinical subgroup. As point of reference for subgroup results, we also conducted analyses in the overall group.
The first step in our primary analysis was to account for differences in covariate profile among trials of each of the 5 medications. We used the RAND Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG).20 The TWANG package supports causal modeling of observational data through the estimation and evaluation of propensity scores and associated weights. In our application, the propensity score represented the probability that a particular trial would be of each medication.21 We estimated propensity scores with multinomial logistic regression using generalized booster effects,22 in which the dependent variable is an indicator for each of the 5 medications and the independent variables are an antiparsimonious specification of variables that have a plausible correlation with the outcome (ie, our 6 groups of covariates).21,22 Using these propensity scores, we weighted participants in order to balance the covariate distributions across medications.
The second step in our primary analysis was to compare continuous and categorical outcomes among the 5 medications with weighted regression analyses, using medication received as the sole independent variable. In general, weighted means can have greater sampling variance than unweighted means. Therefore, we used survey commands, which account for the weights, to perform the outcomes analyses when comparing the weighted medication groups. These weighted medication groups were defined by the inverse of the propensity scores and adjusted covariates unbalanced at the P < .01 level after TWANG weighting. In balancing our extensive list of covariates, a Bonferroni correction would indicate a corrected α of P < .001. However, we conservatively maintained an α threshold of P < .01 for significant differences to avoid type II error. For our continuous outcome of change in total PCL score, we used weighted linear regression analysis, whereby the coefficient of the variable tests the hypothesis that each of the 5 psychotropic medications has the same mean change from baseline to follow-up. For our categorical outcome of clinically meaningful improvement, we used weighted logistic regression analysis, whereby the coefficient of the variable tests the hypothesis that each of the 5 psychotropic medications results in the same percentage of patients achieving clinically meaningful improvement. P values were calculated from Wald test in the propensity score weighted regression models.
The third step in our primary analysis was to the potential contribution of unmeasured confounding on significant baseline to follow-up differences by calculating E-values, which indicate the minimum strength of association on the risk ratio scale that an unmeasured confounder would need to have with both the exposure and the outcome, conditional on the measured covariates, to fully explain away a specific exposure-outcome association.23,24
For our exploratory analyses, we conducted penalized logistic regression models to identify the strongest predictors of meaningful improvement and remission using least absolute shrinkage and selection operators (LASSO).25 As we were interested in predictors within (rather than between) comparison groups, we used raw (rather than propensity-score weighted) covariates described in Table 1. We chose LASSO because it provides information about predictors that are most important when many covariates are available. We set the tuning parameter to select the most regularized model such that error is within 1 standard error of the cross-validated minimum. We evaluated the robustness of our feature selection using 100 bootstrapped samples. At the extreme ends of the distribution of bootstrapped replications, some features that are important in the full model are dropped by LASSO. We ran LASSO models in 6 groups: overall (including an indicator for medication received) and patients who received each of the 5 medications. We performed data management in SAS version 9.4 (SAS Institute) and statistical modeling in R version 4.0.2 (R core team).
RESULTS
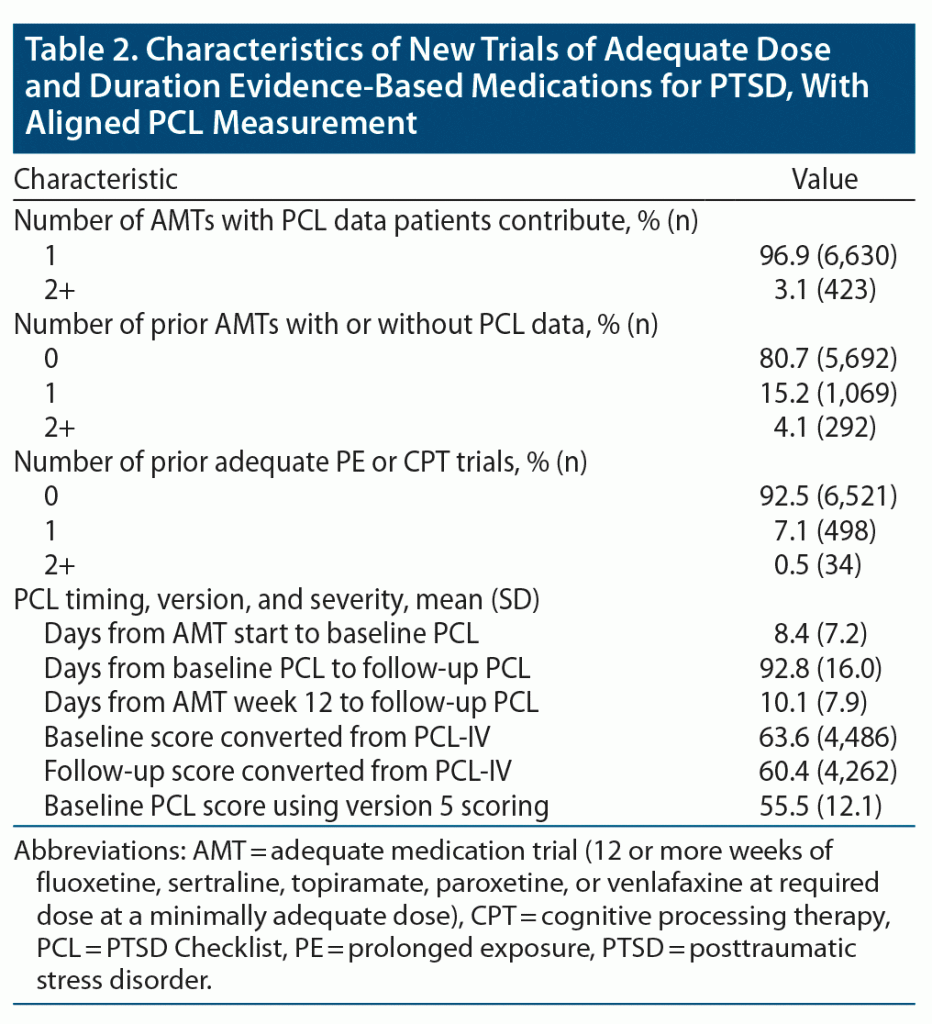
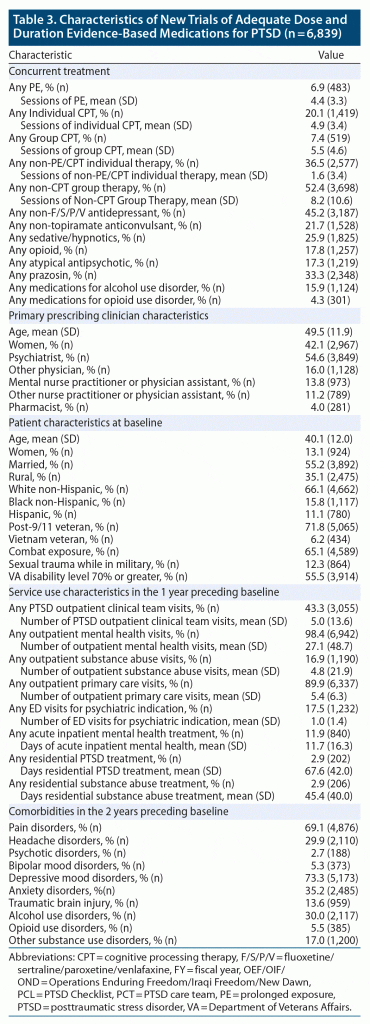
There were 7,053 AMTs aligned with PCL measurement, including 2,093 of fluoxetine, 889 of paroxetine, 2,399 of sertraline, 594 of topiramate, and 1,078 of venlafaxine. Roughly 3% of patients contributed more than 1 AMT with PCL measurement to the analysis (Table 2), although almost 20% of patients had 1 or more prior AMTs with or without associated PCL measurement. Most PCL scores were converted from PCL-IV values, and mean baseline symptom severity was high. While less than 10% had an adequate trial of PE or CPT prior to the start of their AMT, almost 7% received concurrent PE, over 20% received concurrent individual CPT, and over 7% received concurrent group CPT (Table 3). Patients also commonly received other antidepressants, other anticonvulsants, sedative-hypnotics, and prazosin concurrently with their AMT. Patients had a high degree of VA primary care, mental health, and substance abuse service use in the year preceding their AMT. There was generally good representation across our pre-defined demographic and diagnostic subgroups. However, cell sizes were below our sample size threshold for Vietnam Veterans and comorbid psychotic disorders. Because cell sizes were also too small for comorbid opioid use disorder, this group was combined with other non-AUD substance use disorders for a general drug use disorder category in subgroup analyses.
While there were differences among the medication treatment groups, our weighting procedure allowed us to balance covariates in the overall group and in almost all subgroup analyses. The exception was a higher percentage of non-psychiatrist physicians prescribing topiramate in the subgroup analyses for women, military sexual trauma, and headache. Therefore, this covariate was retained in the relevant subgroup analyses.
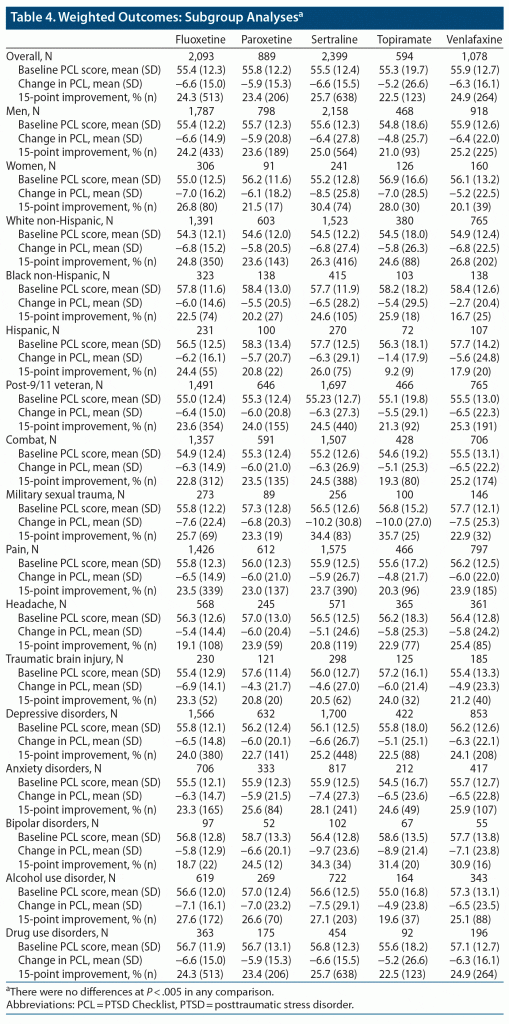
All 5 of the medications were associated with an approximately 10% improvement in PTSD symptoms in the overall analysis (Table 4). The mean improvement in total PCL score ranged from 5.2 points for the topiramate group to 6.6 points for the sertraline and fluoxetine groups; clinically meaningful improvement rates ranged from 22.5% in the topiramate group to 25.7% in the sertraline group. There were no significant differences between medication arms in our overall analysis or in our clinical subgroups at our a priori threshold for significance of P < .005. As there were no significant differences, there were no findings to assess with the E-value.
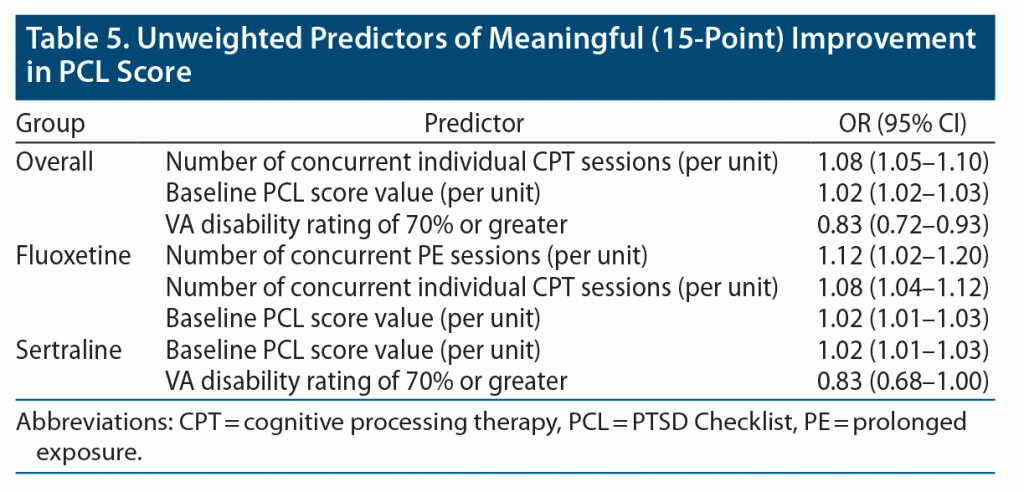
In our penalized regression analysis, each session of concurrent individual CPT was associated with 8% increased odds of clinically meaningful improvement, while a VA disability rating of 70% or greater was associated with 17% decreased odds of clinically meaningful improvement in the overall group (Table 5). Both PE and individual CPT sessions were associated with increased odds of clinically meaningful improvement among patients in the fluoxetine arm, and a VA disability rating of 70% of greater was associated with decreased odds of clinically meaningful improvement among patients in the sertraline arm. A higher baseline PCL score was associated with increased odds of improvement in the overall group as well as the fluoxetine and sertraline arms. No covariates predicted clinically meaningful improvement in the paroxetine, topiramate, or venlafaxine arms.
DISCUSSION
Our data do not support the idea that patient and clinical factors predict differential treatment response among effective medications for PTSD. Across all clinical subgroups, a quarter to a fifth of patients had a meaningful improvement of 15 points or more on the PCL. Our results showed extremely consistent response of PTSD to the 5 treatments across the multiple subgroups. There is meaningful heterogeneity among the treatments we examined; fluoxetine, sertraline, and paroxetine are selective serotonin reuptake inhibitors, while venlafaxine is a serotonin-norepinephrine reuptake inhibitor, and topiramate is an anticonvulsant.26
In our prior work using only PCL-5 data, venlafaxine was associated with superior remission rates compared to the other agents, possibly due to new items in the negative alterations in cognitions and mood cluster.3 While it might have been reasonable to expect a signal for venlafaxine in the clinical subgroup with comorbid depression based on our prior finding, we did not see one. This may be because most PCL values in the venlafaxine arm originated from PCL-IV data, which do not represent these new items. Additionally, we did not have individual item data for all patients, as this larger analysis involved combining all possible PCL data sources. Therefore, we could not assess symptomatic remission and used a clinically meaningful improvement threshold instead. It is possible that we would have seen differences had we considered other outcomes. We were similarly disappointed in the lack of any signal for topiramate. For example, topiramate is FDA-approved for prophylaxis of migraine headaches and is also recommended for prevention of several other headache types,27,28 yet we did not observe superior PTSD outcomes among patients receiving topiramate in the clinical subgroup with headache disorders. While we were unable to assess potentially mediating changes in headache symptoms, it is possible that headache response is unassociated with PTSD response even if clinical trial data indicate that topiramate can be helpful in both disorders. These two examples speak more broadly to a weakness in our approach, namely that we did not include other relevant outcomes such as pain, depression, or anxiety apart from our overall PTSD symptom scale. Therefore, it remains possible that specific agents do have an advantage in populations with particular comorbidities because they provide benefit for important symptoms other than PTSD such as depression or chronic pain. Future work should include as many measures as possible of these relevant comorbidity symptoms.
Our exploratory analysis similarly did not lead to new information about which patients will have a clinically meaningful improvement to individual medications despite the inclusion of an extensive list of patient and treatment-related covariates as potential predictors. In the penalized regression model for the overall group, no indicator for medication arm emerged as a predictor. While concurrent evidence-based psychotherapy sessions emerged as a predictor of clinically meaningful improvement in the fluoxetine arm and VA disability of 70% or greater emerged as a predictor of not achieving clinically meaningful improvement in the sertraline arm, other medication groups were far smaller and there were similar predictors in the overall model. Therefore, it is unlikely that these effects are particular to individual agents. It is our belief that concurrent treatment with evidence-based psychotherapy for PTSD predicts better treatment outcomes and severe disability predicts worse outcomes across medication groups. Notably, however, we performed our exploratory analysis using unweighted data, so it is possible that patients receiving evidence-based psychotherapy or with VA disability of 70% or greater could be different in other ways that account for differences in outcomes seen in these groups. Consistent with our findings, several prior studies have found poorer PTSD treatment outcomes for patients who are service connected or in the process of applying for service connection.29–31 One possibility is that some patients may underreport improvements due to fear of losing disability benefits,32 and this factor could have influenced our findings.
As noted in our prior work, there are several major limitations inherent in using an uncontrolled retrospective cohort design to emulate an RCT using VA data.3,4 The chief differences compared to an RCT are that this work was not prospective, randomized, or placebo-controlled. As a result, limitations include significant differences in concurrent treatment receipt between those with and without PCL measurement; lack of information about treatment adherence, patient preference, and expectations; and uncertainty about whether these results would apply to users of other health systems. VA patients with sufficient PCL data to contribute to outcomes analysis are generally receiving very high-intensity care compared to those without PCL data.6 The need for such intensive care may indicate a degree of treatment resistance,33 and our outcomes must be considered in this context. Improved clinical data collection in the VA as well as replication in other health systems could address these concerns in future studies. A new limitation particular to this analysis is that PCL measurements were combined from multiple versions of the tool in order to generate enough sample size for subgroup analyses. While the versions are well-correlated and we used a validated crosswalk, the PCL-5 includes additional items representing the contemporary case definition of PTSD that are not included in the PCL-IV. While we accounted for this by including a covariate for PCL version in our propensity weighting model, subgroup analyses using exclusively PCL-5 data may be possible in the future if the VA’s effort to increase use of PROM through the measurement-based care initiative are successful.34 Lastly, our lack of measures for severity of important and common comorbid disorders was a clear deficiency.
In conclusion, we have conducted the largest real-world study of medications for PTSD to date and did not observe a pattern of differential response among clinical subgroups. Thus, it is appropriate to question the conventional clinical wisdom that factors such as patient comorbidities can guide clinicians and patients in selecting among effective medications for PTSD. Our work continues to show that all patients taking medications for PTSD should be encouraged to pursue combined treatment with evidence-based psychotherapy.
Submitted: February 1, 2021; accepted April 26, 2021.
Published online: October 5, 2021.
Potential conflicts of interest: The authors report no conflicts of interest.
Funding/support: This study was funded by the Department of Defense Peer Reviewed Medical Research Program (PR160203), Congressionally Directed Medical Research Program, Fort Dietrich, MD (Dr Shiner).
Role of the sponsor: The sponsors did not have any role in the study design, methods, analysis, and interpretation of results, or in preparation of the manuscript and the decision to submit it for publication.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the position or policy of the US Department of Veterans Affairs or US Department of Defense.
Additional information: The VA Corporate Data Warehouse (CDW) contains electronic medical record data compiled from individual VA facilities and is described at https://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm. Data are stored on geographically dispersed server farms. To access the CDW, researchers generally need to have an employment relationship with the VA. After local institutional review board approval, requests for data are submitted to VA National Data Systems using the Data Access Request Tracker. Datasets are then built and analyzed in secure virtual project workspaces within the VA Informatics and Computing Infrastructure environment. Researchers with VA network access can obtain descriptions of CDW data at http://vaww.virec.research.va.gov/.
Clinical Points
- While expert opinion has focused on factors such as comorbidity to guide the selection of specific medications for PTSD, there is a lack of definitive data to support such an approach.
- In a real-world study of almost 7,000 patients with PTSD, we did not observe any patterns of differential response to medications in clinical subgroups defined by demographics and comorbidities.
- To improve their chances of meaningful symptomatic improvement, patients taking medications for PTSD should be encouraged to pursue combined treatment with evidence-based psychotherapy.
References (34)

- Jonas DE, Cusack K, Forneris CA, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder (PTSD). Agency for Healthcare Research and Quality; 2013.
- Watts BV, Schnurr PP, Mayo L, et al. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541–e550. PubMed CrossRef
- Shiner B, Leonard CE, Gui J, et al. Comparing medications for DSM-5 PTSD in routine VA practice. J Clin Psychiatry. 2020;81(6):20m13244. PubMed CrossRef
- Shiner B, Westgate CL, Gui J, et al. A retrospective comparative effectiveness study of medications for posttraumatic stress disorder in routine practice. J Clin Psychiatry. 2018;79(5):18m12145. PubMed CrossRef
- Shiner B, Westgate CL, Bernardy NC, et al. Anticonvulsant medication use in veterans with posttraumatic stress disorder. J Clin Psychiatry. 2017;78(5):e545–e552. PubMed CrossRef
- Shiner B, Gui J, Leonard Westgate C, et al. Using patient-reported outcomes to understand the effectiveness of guideline-concordant care for post-traumatic stress disorder in clinical practice. J Eval Clin Pract. 2019;25(4):689–699. PubMed CrossRef
- Schoenfeld FB, Marmar CR, Neylan TC. Current concepts in pharmacotherapy for posttraumatic stress disorder. Psychiatr Serv. 2004;55(5):519–531. PubMed CrossRef
- Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD). Int J Neuropsychopharmacol. 2012;15(6):825–840. PubMed CrossRef
- Shiner B, Watts BV. Invited commentary: baby steps to a learning mental health-care system-can we do the work? Am J Epidemiol. 2021;190(7):1220–1222. PubMed CrossRef
- Holder N, Shiner B, Li Y, et al. Cognitive processing therapy for veterans with posttraumatic stress disorder: what is the median effective dose? J Affect Disord. 2020;273:425–433. PubMed CrossRef
- Holder N, Shiner B, Li Y, et al. Determining the median effective dose of prolonged exposure therapy for veterans with posttraumatic stress disorder. Behav Res Ther. 2020;135:103756. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
- Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489–498. PubMed CrossRef
- Weathers FW, Litz BT, Herman DS, et al. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Trauma, coping, and adaptation. International Society for Traumatic Stress Studies, 9th Annual Meeting; 1993; San Antonio, Texas.
- Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379–1391. PubMed CrossRef
- Moshier SJ, Lee DJ, Bovin MJ, et al. An empirical crosswalk for the PTSD Checklist: translating DSM-IV to DSM-5 using a veteran sample. J Trauma Stress. 2019;32(5):799–805. PubMed CrossRef
- Lee DJ, Bovin MJ, Weathers FW, et al. Reliable Change Index and Clinically Significant Margins for the CAPS-5 and PCL-5 among Veterans. International Society for Traumatic Stress Studies, 35th Annual Meeting; 2019; Boston, MA.
- Maguen S, Madden E, Patterson OV, et al. Measuring use of evidence based psychotherapy for posttraumatic stress disorder in a large national healthcare system. Adm Policy Ment Health. 2018;45(4):519–529. PubMed CrossRef
- Ridgeway G, McCaffrey DF, Morral AR, et al. Toolkit for Weighting and Analysis of Nonequivalent Groups: A Tutorial for the TWANG Package. RAND Corporation; 2017.
- Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21. PubMed CrossRef
- McCaffrey DF, Griffin BA, Almirall D, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–3414. PubMed CrossRef
- Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. PubMed CrossRef
- VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. PubMed CrossRef
- Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. PubMed CrossRef
- Asnis GM, Kohn SR, Henderson M, et al. SSRIs versus non-SSRIs in post-traumatic stress disorder: an update with recommendations. Drugs. 2004;64(4):383–404. PubMed CrossRef
- Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;(6):CD010610. PubMed CrossRef
- May A, Leone M, Afra J, et al; EFNS Task Force. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066–1077. PubMed CrossRef
- Sripada RK, Blow FC, Rauch SAM, et al. Examining the nonresponse phenomenon: factors associated with treatment response in a national sample of veterans undergoing residential PTSD treatment. J Anxiety Disord. 2019;63:18–25. PubMed CrossRef
- Crawford EF, Wolf GK, Kretzmer T, et al. Patient, therapist, and system factors influencing the effectiveness of prolonged exposure for veterans with comorbid posttraumatic stress disorder and traumatic brain injury. J Nerv Ment Dis. 2017;205(2):140–146. PubMed CrossRef
- Goodson JT, Helstrom AW, Marino EJ, et al. The impact of service-connected disability and therapist experience on outcomes from prolonged exposure therapy with veterans. Psychol Trauma. 2017;9(6):647–654. PubMed CrossRef
- Walter KH, Varkovitzky RL, Owens GP, et al. Cognitive processing therapy for veterans with posttraumatic stress disorder: a comparison between outpatient and residential treatment. J Consult Clin Psychol. 2014;82(4):551–561. PubMed CrossRef
- Sippel LM, Holtzheimer PE, Friedman MJ, et al. Defining treatment-resistant posttraumatic stress disorder: a framework for future research. Biol Psychiatry. 2018;84(5):e37–e41. PubMed CrossRef
- Resnick SG, Hoff RA. Observations from the national implementation of measurement based care in mental health in the Department of Veterans Affairs. Psychol Serv. 2020;17(3):238–246. PubMed CrossRef
This PDF is free for all visitors!