As Reviva Pharmaceuticals barrels toward a “do or die” fourth quarter meeting with the U.S. Food and Drug Administration, company executives are confident in brilaroxazine’s ability to treat schizophrenia.
“The successful completion of our global OLE 1-year trial marks a major milestone for the brilaroxazine program as we advance toward potential registration,” Reviva Founder, President, and CEO Laxminarayan Bhat, PhD, said on the company’s latest earnings call. “We believe all key clinical data required for NDA are completed and we are preparing for an End-of-Phase 3 meeting with the FDA planned in the fourth quarter of the year to discuss our future NDA submission based on the current data package and excluding the planned Phase 3 RECOVER-2 trial. Pending favorable feedback from the FDA, we will target an NDA submission in the second quarter of 2026.”
A Persistent Problem
The company first revealed the promising results of its phase 3 open-label study at the Schizophrenia International Research Society (SIRS) 2025 conference back in June.
Brilaroxazine is a novel serotonin-dopamine signaling modulator with multimodal activity across several receptors implicated in schizophrenia. It also reduces pro-inflammatory cytokines, which are increasingly recognized as drivers of neuroinflammation in psychiatric illness.
The data showed that taking brilaroxazine once a day offered sustained, broad-spectrum efficacy while maintaining a favorable safety profile. The findings added to earlier short-term results and positioned the drug for regulatory review.
While the current generation of medications can treat schizophrenia’s hallmark positive symptoms, such as hallucinations or delusions, persistent negative symptoms, such as cognitive impairment, still frustrate clinicians (and patients).
Brilaroxazine Study Specifics
The OLE trial followed patients who’d completed Reviva’s pivotal four-week RECOVER study and enrolled additional participants with stable schizophrenia. In total, researchers treated 435 patients with a range of daily doses – from as low as 15 mg to as high as 50 mg.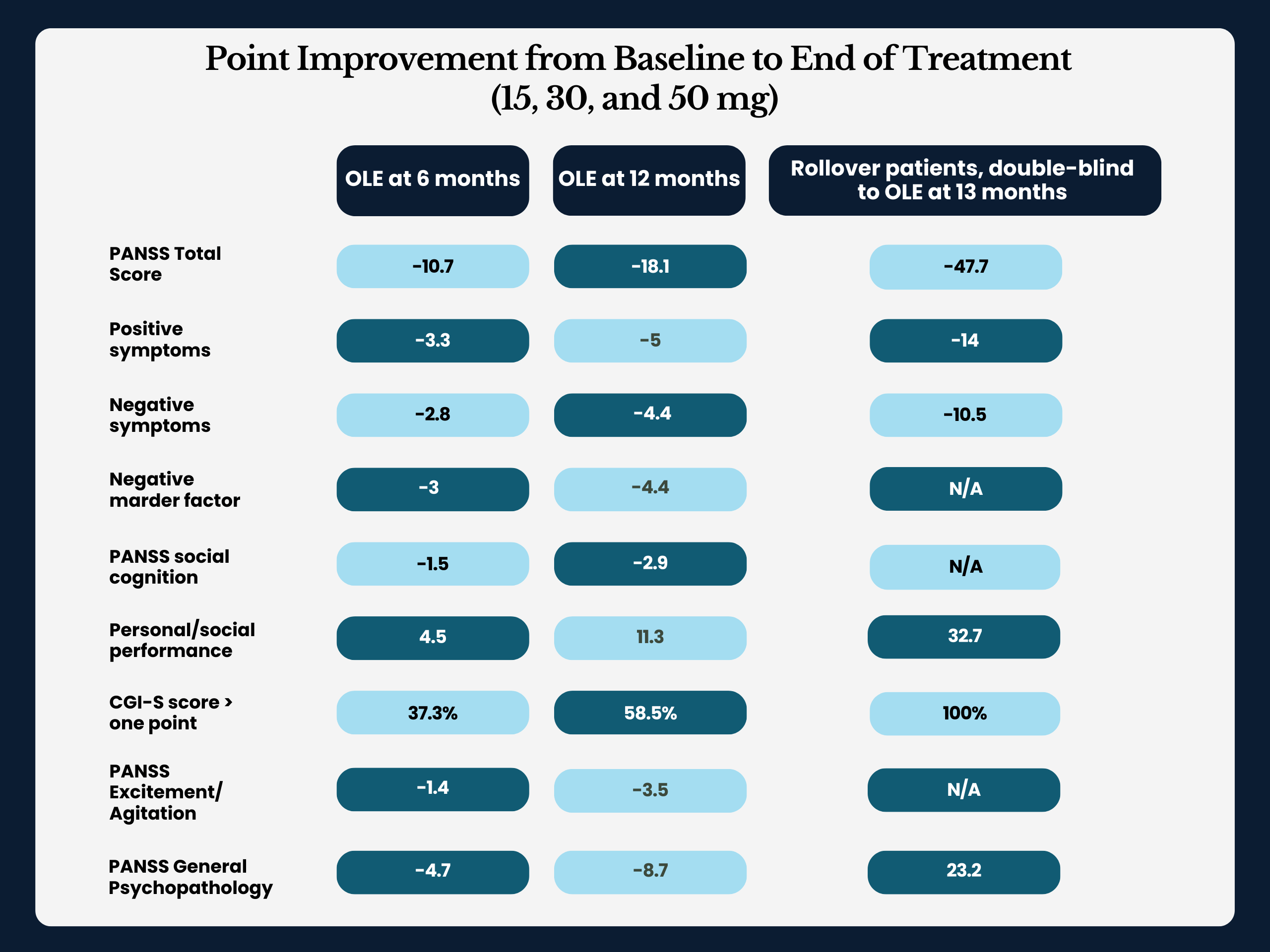
The team evaluated efficacy primarily through the Positive and Negative Syndrome Scale (PANSS), a widely accepted assessment of schizophrenia symptoms. Other measures included the Clinical Global Impression-Severity (CGI-S) scale and Personal and Social Performance (PSP) scores.
At the 52-week mark, pooled results across doses illustrated sustained, clinically meaningful improvements, including:
- PANSS total score: -18.6 points from baseline.
- Positive symptoms: -5.2.
- Negative symptoms: -4.5.
- Social cognition: -2.9.
- PSP (functioning): +11.3.
Patients rolling over from the double-blind trial demonstrated even more dramatic improvements, with PANSS reductions exceeding 30 to 50 points for many participants.
“These long-term data reinforce brilaroxazine’s consistent, wide-spectrum efficacy and well-tolerated safety profile,” Bhat added. “Importantly, the additional biomarker data serve as independent measures of efficacy that further support improvements across all major symptom domains of schizophrenia.”
Brilaroxazine Safety and Tolerability
The 12-month study also confirmed an encouraging safety profile. A little more than 15% of patients reported at least one treatment-related adverse event, although most appeared to be mild or moderate – as well as transient. The most common reported side effects included modest weight gain, insomnia, and somnolence.
Additionally, the researchers found:
- A lack of drug-related serious adverse events after one year.
- No noteworthy changes in movement disorder scales, avoiding motor side effects like akathisia or extrapyramidal symptoms.
- Minimal weight gain (average 1.5 kg), not dose dependent.
- Improved lipid and endocrine profiles, including lower prolactin and thyroid levels.
“The impact of reduced inflammation on symptoms may result in improved patient adherence and clinical outcomes,” Larry Ereshefsky, MD, a retired University of Texas professor, said in a press release. “The low discontinuation rates observed in both the double-blind and OLE trials are consistent with this beneficial treatment profile.”
Overall discontinuation across the OLE trial hovered around 35%, but only 1.6% of those attributed it to adverse events. Most left for other reasons.
Beyond schizophrenia, Reviva execs are evaluating the drug for other neuropsychiatric conditions, such as bipolar disorder, major depressive disorder, and ADHD. Preclinical research has also hinted at potential applications in inflammatory and fibrotic diseases, such as psoriasis, pulmonary arterial hypertension, and idiopathic pulmonary fibrosis.
Completion of the OLE study is a critical component of the company’s planned New Drug Application (NDA) submission to the FDA later this year.
Further Reading
Could Hepatitis C Be Hiding A Schizophrenia Connection?
Antipsychotic Adherence Halves Crash Risk for Drivers with Schizophrenia
Global Experts Unveil New Schizophrenia Treatment Guidelines



