Those living with attention-deficit/hyperactivity disorder (ADHD) could have something new to be thankful for soon.
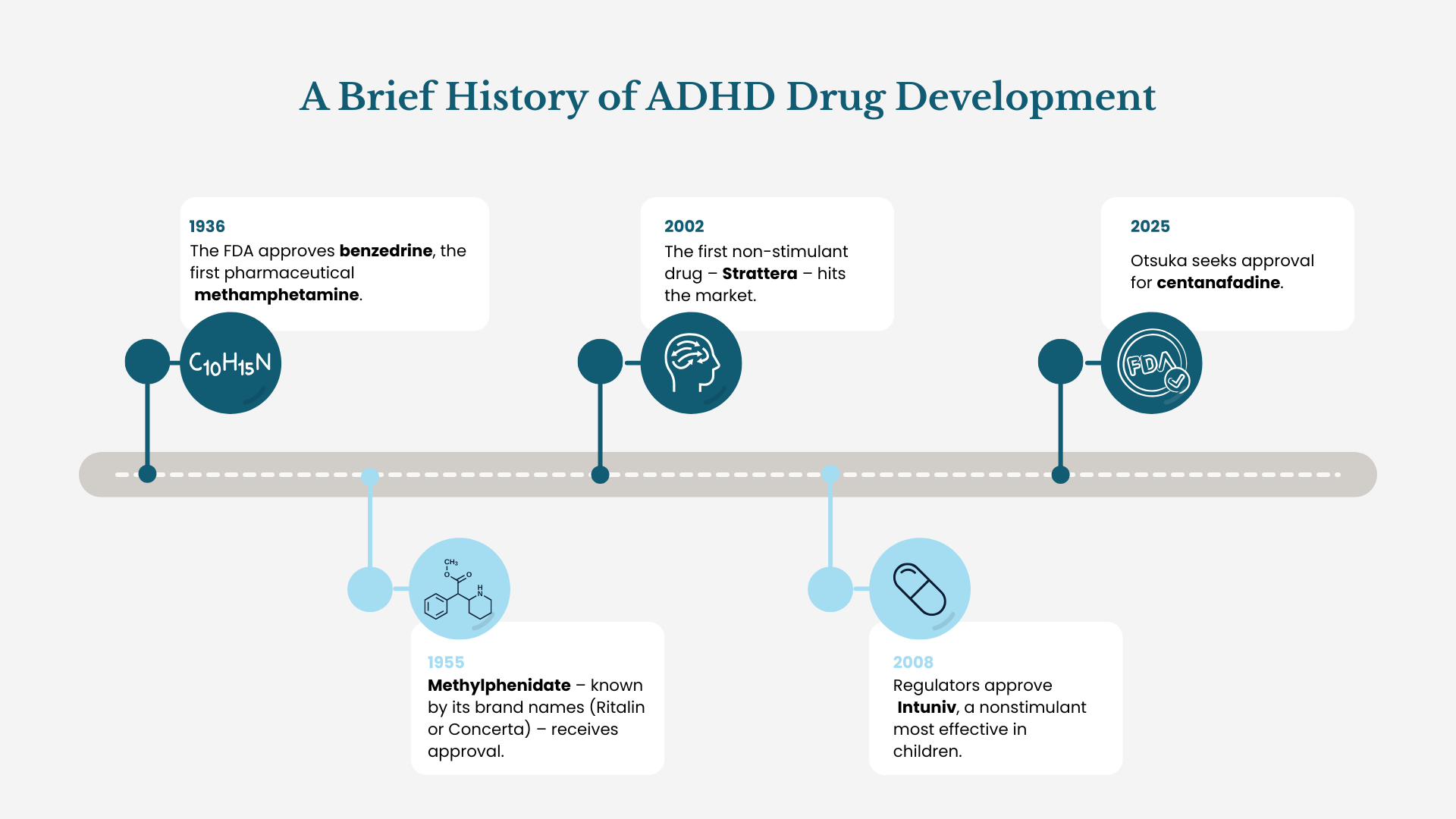
Otsuka Pharmaceutical has submitted a New Drug Application (NDA) to the U.S. Food & Drug Administration (FDA) for centanafadine. The announcement – lost among the clutter of a government shutdown and a national holiday – marks a potential novel ADHD treatment.
Company officials insist that this latest development could open the door to more therapeutic options.
“Centanafadine represents a first-in-class mechanism of action among available ADHD therapies, and if approved, may expand the range of options available to those managing this complex condition,” Otsuka executive vice-president and chief medical officer John Kraus, M.D., Ph.D., explained.
 Studies Offer Strong Support
Studies Offer Strong Support
Kraus describes centanafadine as a norepinephrine, dopamine and serotonin reuptake inhibitor (NDSRI). Four pivotal Phase 3 trials support the FDA submission with evidence that company officials claim backs up the efficacy and safety in both pediatric and adult populations.
Among the pediatric studies, one trial enrolled children between the ages of 4 and 12 while another looked at adolescents between 13 and 17. In both trials, a high fixed-dose of centanafadine achieved statistically significant improvement on the ADHD-Rating Scale-5 (ADHD-RS-5) compared with the placebo, while the low-dose arm in children failed to make a difference.
For adults between 18 and 55, two randomized, double-blind placebo-controlled studies using sustained-release centanafadine tablets (at 200 mg/day and 400 mg/day) showed statistically significant reductions in the Adult ADHD Investigator Symptom Rating Scale (AISRS) versus placebo.
The research also revealed a generally favorable safety and tolerability profile. Among the younger trial participants, researchers noted that the most common adverse events appeared to be a subdued appetite, nausea, rash, fatigue, abdominal pain and somnolence.
Among the adult participants, the researchers reported side effects such as decreased appetite and headaches. But maybe the most important revelation was a much lower potential for abuse and dependence.
Hope for a New Approach
If regulators sign off on centanafadine, the drug would introduce a novel pharmacological profile into the ADHD treatment landscape by targeting all three monoamine reuptake systems (norepinephrine, dopamine and serotonin) in a singly, sustained-release daily formulation.
Otsuka’s case rests on a robust dataset spanning children, adolescents and adults. But the approval now rests in the hands of regulators. And for clinicians and patients, centanafadine’s approval could mean an expanded – and more robust – arsenal for managing ADHD.
Further Reading
ADHD’s Fluctuating Nature Challenges What We Think We Know
ADHD Might Have Been an Early Evolutionary Edge
Study Reveals the Most Accurate Estimate of Adult ADHD to Date