Lessons Learned at the Interface of Medicine and Psychiatry
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2025;27(5):25f03958
Author affiliations are listed at the end of this article.
Have you been unsure about when and how to use antihistamines? Have you been uncertain about whether dose adjustments are necessary in those with medical conditions? Have you wondered whether you could administer an antidote in the event of an antihistamine overdose? If you have, the following case vignette and discussion should prove useful. With this case and subsequent review, we aim to describe the pearls and pitfalls of antihistamine use in psychiatric conditions and symptoms. We will cover the safe use of antihistamines to manage anxiety and insomnia (as well as other conditions including nausea and reduced appetite), with a focus on navigating use of these medicines in patients with medical illness.
CASE VIGNETTE
Mr A, a 67-year-old man with a history of metabolic dysfunction and alcohol-associated liver disease, end-stage renal disease (on hemodialysis), and a 2-year history of hepatic encephalopathy, was admitted to the hospital for a liver transplant. After undergoing a successful liver transplant, his postoperative course was complicated by delirium, insomnia, and intermittent anxiety associated with his prolonged hospital stay. The primary team ordered hydroxyzine as needed for sleep and quetiapine 50 mg nightly for insomnia as well as mirtazapine 15 mg nightly to help with residual insomnia symptoms and to improve his appetite.
After Mr A’s anxiety and insomnia failed to improve, the psychiatry department was consulted. On the morning of the consultation, Mr A was unarousable. In the afternoon, he was groggy and complained of dry mouth. He continued to feel anxious and felt as though he was now sleeping too much, which interfered with his physical therapy.
DISCUSSION
What Is Histamine?
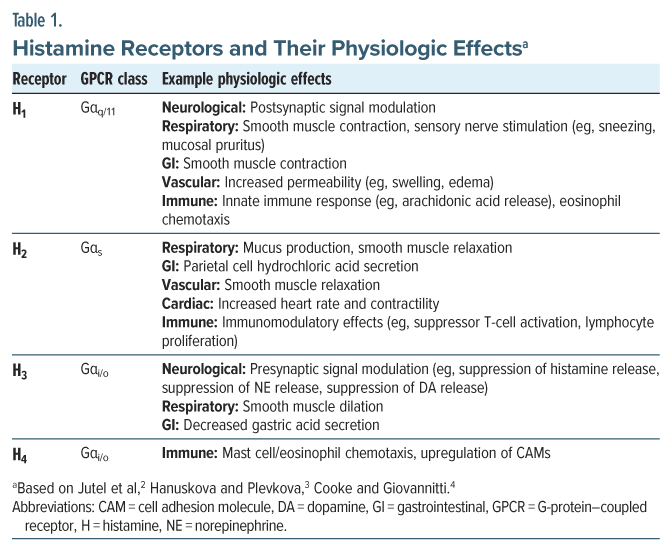
Primary care physicians and psychiatrists alike should be aware of histamine’s role in mental health treatment, especially with multiple psychopharmacologic agents acting to antagonize histamine receptors to produce certain symptomatic relief. Histamine is a biologically active amine, synthesized in multiple tissues—namely, granulocytes (eg, mast cells, basophils) and histaminergic neurons—that express the histidine decarboxylase (HDC) enzyme.1 The myriad effects of histamine are mediated by 4 histamine receptors, members of the G-protein–coupled receptor (GPCR) superfamily (Table 1).5
In the central nervous system (CNS), histamine is a highly conserved monoamine neuromodulator, like dopamine and norepinephrine.1 Histamine release is mediated by the tuberomammillary nucleus, originating in the posterior hypothalamus and projecting to almost all the human brain, including the cerebral cortex, amygdala, and striatum.6 While specific mechanisms remain to be elucidated, histamine plays a critical role in several key neurophysiological processes, such as sleep-wake cycle regulation, satiety signaling, and cognitive functioning.7
Outside of the nervous system, histamine functions as a local signaling molecule, playing a role in the innate and adaptive immune responses as well as gastrointestinal (GI) system function.8 When immunoglobulin E (IgE) antibodies bind to the high-affinity IgE receptors (eg, FcεRI) present on mast cells and basophils, proinflammatory agents are released, including histamine, tryptase, leukotrienes, and prostaglandins.9 Peripheral histamine release plays a role in the anaphylaxis response, characterized by generalized urticaria, angioedema, bronchospasm, hypotension, syncope, nausea, cramping, and other symptoms.10 Moreover, the chronic systemic release of histamine can lead to generalized symptoms (eg, fatigue, weight loss, malaise), cardiovascular signs (eg, hypotension, tachycardia), and neurological effects (eg, anxiety, insomnia, impaired memory).11 Histamine signaling inhibition has wide-ranging physiological effects that depend on the cell type, organ system, and specific receptor(s) involved.12
Which Medical Conditions Involve Histamine?
Histamine has been implicated in both acute and chronic immunological conditions (eg, allergic rhinitis, atopic dermatitis, conjunctivitis, urticaria, asthma, and anaphylaxis). Cutaneous mast cell degranulation and histamine-mediated angioedema and pruritic wheels have been described in cases of chronic urticaria.13 In addition, histamine release has been implicated in the visceral hypersensitivity found in some patients with irritable bowel syndrome.14 Similarly, conditions that involve the overproduction of mast cells, eg, mastocytosis, or the overactivation of mast cells, eg, mast cell activation syndrome, involve histamine signaling.11
What Are Antihistamines, and How Do They Work?
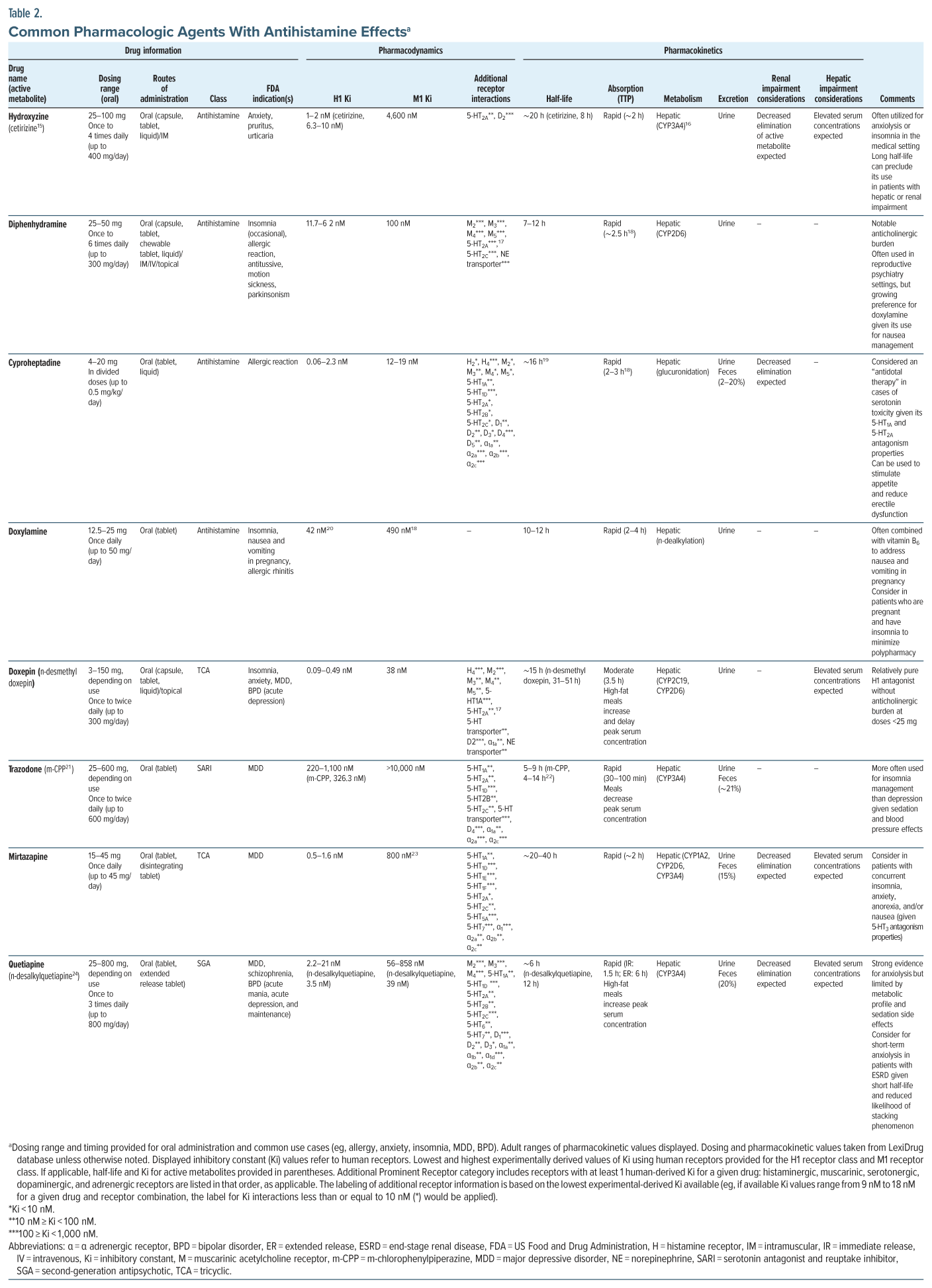
Antihistamines are a diverse class of pharmacologic agents that stabilize 1 or more of the histamine receptors.12 Antihistamines are largely classified as inverse agonists: akin to receptor antagonists but with further ability to prevent baseline activation of the receptor.12 The term antihistamine is largely a misnomer; instead, “pharmacologic agents with antihistaminergic properties” may be more accurate. Many pharmacologic agents (including antidepressants and antipsychotics) demonstrate antihistaminergic properties. While the reason for prescribing an agent may be for its antihistaminergic properties, it is important to consider its broader pharmacodynamic and pharmacokinetic profile, which varies widely between agents (Table 2).
Most of the antihistamines on the market today, more than 40, act on the histamine 1 (H1) receptor.25 Three histamine 2 (H2) receptor antihistamines remain on the market to decrease gastric acid secretion.26 The histamine 3 (H3) receptor is an autoreceptor, inhibiting histamine signaling with activation, in addition to impacting the transmission of other neuromodulators (eg, norepinephrine, dopamine).6 Accordingly, H3 receptor antihistamines are currently being investigated for use in promoting wakefulness, cognitive processing, and weight loss.6 A single H3 receptor antihistamine, pitolisant, gained US Food and Drug Administration (FDA) approval in 2019 for the treatment of excessive daytime sleepiness and cataplexy in those with narcolepsy.27
The first-generation antihistamines (eg, hydroxyzine, diphenhydramine) readily cross the blood-brain barrier, allowing for their CNS-mediated sedative and anxiolytic effects, as well as weight gain, impairment of attention and working memory, and diminished motor coordination.7,12 The development of second-generation and third-generation antihistamines (eg, cetirizine, fexofenadine, loratadine) that failed to cross the blood-brain barrier readily allowed for widespread and over-the-counter use of these drugs for the control of allergic symptoms, without the CNS effects of histamine blockade.12
What Are the Signs and Symptoms of Antihistaminergic Activity?
For antihistamines that cross the blood-brain barrier, the main manifestations are sedation and impaired motor coordination.20 In addition, many antihistamines share a propensity for muscarinic receptor antagonism at higher doses, which leads to signs and symptoms of anticholinergic toxicity (eg, xerostomia, reduced secretions, reduced GI motility, mydriasis, tachycardia, hyperthermia, sedation, urinary retention, and confusion).28
Which Drugs Can Interact With Histamine and Affect Histamine Levels?
Several drugs can interact with the histamine system. Glucocorticoids (GCs), commonly used to address inflammation, affect histamine through multiple mechanisms. GCs can inhibit histamine release from mast cells directly (often in minutes) through their effects on intracellular calcium levels, which are necessary for mast cell degranulation.29 They also reduce histamine synthesis through downregulation of HDC activity and further reduce histamine content in tissues through reduction in mast cell count directly.30,31 Beta-adrenergic agonists (eg, terbutaline) inhibit histamine release from mast cells.32 Diamine oxidase (DAO) supplements also enhance degradation of histamine in the GI system. DAO is an enzyme involved in the degradation of histamine found in various foods. Supplementation of DAO has been proposed as a treatment for patients with histamine intolerance.33,34
Several tricyclic antidepressants (TCAs) are potent antagonists at H1 receptors, which commonly leads to sedation (and is why doxepin is an agent utilized for insomnia). TCAs also influence histamine metabolism directly. Amitriptyline, a tertiary amine TCA sometimes used to help with functional GI syndromes, affects histamine-N-methyltransferase and DAO activity in vivo.35 Amitriptyline works to enhance the activity of these enzymes and leads to subsequent histamine degradation and overall lower histaminergic tone.35 At higher doses, TCAs inhibit histamine release from mast cells directly.36 Numerous antipsychotics (eg, olanzapine, clozapine) have complex interactions with histamine. H1 antagonism by certain antipsychotics is associated with sedation and increased appetite through its actions in the hypothalamus.37 However, the weak H3 antagonism of second-generation antipsychotics (SGAs) has been associated with increased neuronal histamine levels, with potential procognitive effects and mitigation of adverse metabolic effects.38,39
How Can Ki Values (Of Agents With Antihistaminergic Activity) Be Used to Guide Clinical Practice?
Antihistaminergic compounds bind to a wide variety of other GPCRs, including those associated with the other major monoaminergic neurotransmitters. The inhibitory constant (Ki) is a pharmacodynamic parameter that conveys useful information on the off-target effects of psychotropics. Ki has been used in conjunction with clinical data to inform the creation of the anticholinergic burden (ACB) score, an estimation of the off-target antimuscarinic receptor effects of commonly used medications.40 The constant represents the concentration (nM) of a molecule that leads to the occupation of half of the applicable receptor sites. When evaluating cytochrome P450 inhibition, the FDA utilizes a guideline: if the ratio of the maximum total plasma concentration of a molecule at steady state at the highest clinical dose ([I]) to an in vitro inhibitory constant (Ki) at a given receptor or enzyme exceeds 0.1 (eg, [I]/Ki > 0.1), a molecule requires further in vivo inhibition testing.41
In practice, the variability in interpersonal pharmacodynamic and pharmacokinetic parameters, including blood-brain barrier diffusion, makes estimating medication concentration and Ki values for a given tissue and receptor combination challenging. As such, Ki values can be used to provide a directional estimate of the likelihood of a drug interacting with a receptor, with smaller values increasing the likelihood of an interaction. For antihistamines specifically, clinicians should consider off-target activity. For example, hydroxyzine may be chosen for its consideration as a “purer” antihistamine given its relatively low propensity for antimuscarinic activity compared to diphenhydramine (which has a stronger affinity for muscarinic receptors). Similarly, if a patient had been delirious and was managed with low-dose quetiapine at night, once the delirium resolved, the clinician could consider switching from quetiapine to hydroxyzine to maintain the sleep-promoting effects of the antihistamine, while limiting the deleterious effects of quetiapine’s anticholinergic and metabolic actions.
What Conditions Can Benefit From Antihistamines?
Insomnia. One of the primary effects of centrally acting antihistamines is sedation.20 Animal model evidence of the importance of histamine signaling in the regulation of sleep-wake states includes the presence of histaminergic neurons being necessary for normal arousal, attention, and interest and the firing of histaminergic neurons that increases during waking hours and diminishes during sleep.6,42 Histamine is also necessary for orexin’s effect on sleep-wake cycles, further establishing its potential to promote sleep.6
Despite widespread use, clinical data that support the use of antihistamines for insomnia are limited. Three clinical trials have evaluated the use of diphenhydramine for insomnia; each found no or limited improvement in primary outcome measures, including sleep diary results and next-day measures of sleepiness and reaction time.43 However, TCAs (such as doxepin) have known hypnotic effects that can be attributed, primarily, to the antihistaminergic activity of the agents. For example, doxepin is FDA-approved for insomnia and is a second-line option after cognitive-behavioral therapy for insomnia.44 Moreover, the H1 receptor antihistamine effects of low-dose doxepin have been shown in clinical trials to reduce awakenings in later hours of sleep, without the lingering drowsiness that is associated with nonbenzodiazepine sleep medications (eg, zolpidem).20 The off-label use of trazodone for sleep has more mixed data, with 2 systematic reviews finding an overall improvement in subjective sleep quality amidst insignificant results (eg, no difference in sleep efficiency, sleep latency, or total sleep time).45,46
Anxiety. The specific anxiety-reducing effects of H1 receptor antihistamines are an area of active investigation.20 Animal model experiments reveal that histamine is a sensitive indicator of stress, and histamine blockade has anxiolytic effects.6,47 More recent mice experiments have shown that histamine blockade in the bed nucleus of the stria terminalis, an extended amygdala structure, alleviates the anxiogenic effects of acute stressors.47 However, early animal model experiments found a diminished anxiety response with hydroxyzine administration, but not with similar H1 antihistamines imipramine and chlorpheniramine, suggesting anxiolysis being separate from the 3 medications’ shared histaminergic and serotonergic receptor antagonism.48 Therefore, histamine blockade appears to have both sedative and anxiety-relieving effects and impacts the interconnected neuromodulatory systems that help coordinate vigilance and sleep.49 Of the current treatments (used on-label and off-label) for anxiety disorders (eg, generalized anxiety disorder, social anxiety disorder, panic disorder), 4 (hydroxyzine, mirtazapine, olanzapine, and quetiapine) have H1 receptor inverse agonist effects.50 However, their anxiolytic mechanisms are likely spread between multiple monoamine receptor interactions, including dopaminergic, serotonergic, and adrenergic receptors. For example, the H1 receptor antihistamine hydroxyzine weakly antagonizes serotonin 2A (5-HT2A) and dopaminergic (D1) receptors, confounding the exact pharmacological mechanisms of the medication that lead to anxiolysis (see Table 2).
Hydroxyzine is currently FDA approved for the treatment of anxiety; a meta-analysis of 39 studies demonstrated superiority over placebo with a similar efficacy to benzodiazepines and buspirone.50 Preliminary analysis indicated that quetiapine is highly effective for anxiety and can be utilized as a stand-alone agent for anxiety.50 However, studies also found that it had low patient tolerability due to sedation and weight gain.50 Olanzapine and mirtazapine have more limited clinical data, and these medications are currently recommended as potentially helpful adjunct medications for anxiety disorders.50 In the absence of manic symptoms, mirtazapine is more commonly used to manage anxiety than is olanzapine. Both medications have metabolism-related adverse effects, and clinicians should be cautious when prescribing them to patients at high risk for metabolic syndrome.
Weight gain. Centrally acting H1 antihistamines predispose to weight gain; this side effect may be desired or undesired depending on the aim when prescribing these medications (eg, use of mirtazapine in patients with cancer complicated by depression and anorexia). The metabolic adverse effects of common psychotropic medications (eg, TCAs and SGAs) are at least partially mediated by the blockade of the H1 receptor.37 While still being elucidated, animal models suggest that central histamine signaling is necessary for leptin-mediated satiety signaling.51 A proposed mechanism for these effects is the disruption of the histamine receptor–mediated AMP-kinase pathway, a signaling pathway that integrates satiety signals provided by hormones (eg, leptin and agouti-related peptide) and nutrient signals (insulin, glucose) to mediate food intake.52,53 Interestingly, H3 receptor antihistamines (eg, pitolisant) may help to prevent the weight gain that is associated with a high-calorie diet and are still under study.54
Nausea. Centrally acting antihistamines (eg, meclizine, promethazine, dimenhydrinate) can be used to mitigate nausea and vomiting. This proves useful in pregnancy, the postoperative period, with motion sickness, and during chemotherapy protocols25 as the blockade of histamine signaling from the vestibular nucleus to the area postrema of the medulla (the region of the brain that mediates the nausea response) facilitates these uses.25 In addition, many first-generation antihistamines have prominent anticholinergic properties. The additional blockade of muscarinic receptor signaling to the vestibular nucleus also helps to prevent nausea.55
Allergic reactions. When allergens are introduced to mucosal and connective tissue (eg, the nasal passage), subsequent binding to the IgE receptors on the surface of tissue-resident mast cells lead to their degranulation: vesicles containing histamine, prostaglandins, and other proinflammatory mediators are released into the local environment.2 Histamine binding to the H1 receptor leads to the classic symptoms of allergic reaction (eg, congestion, rhinorrhea, pruritus). Likewise, H1 receptor antihistamines blunt the allergic response. The use of these newer antihistamines for the symptomatic treatment of urticaria, allergic conjunctivitis, and allergic rhinitis is supported by longitudinal clinical data.25
Peptic ulcer disease. In the GI tract, histamine release from enterochromaffin-like cells, and subsequent binding to H2 receptors on the basolateral surface of parietal cells, leads to the increased secretion of hydrochloric acid.26 After their development in the 1970s, H2 receptor–specific antihistamines (eg, cimetidine, ranitidine, famotidine) became the standard treatment for peptic ulcer disease.5
How and When Should Antihistamines Be Used to Improve Sleep and Appetite and Reduce Anxiety and Allergic Reactions?
Antihistamines are considered relatively safe in everyday practice, and as a result, they are commonly used in patients with insomnia or anxiety. As with all psychotropics, clinicians should aim to reduce polypharmacy by selecting an agent that can serve more than 1 function and address patients’ complaints. In a patient who presents with anxiety or depression, along with low appetite and insomnia, mirtazapine can target multiple symptoms (addressing mood and anxiety symptoms via serotonergic modulation, insomnia with antihistaminergic activity, and appetite with antihistaminergic and anti–5-HT2C activity). Similarly, in a patient with mild hyperactive delirium, standing doses of quetiapine may improve insomnia and reduce agitation, while as-needed low doses may address intermittent distress and anxiety.
Antihistamines may also temporarily reduce symptoms in patients with psychiatric conditions while other interventions are beginning to take effect. For example, in a patient with generalized anxiety or panic disorder, while a selective serotonin reuptake inhibitor may be the first-line treatment, in conjunction with psychotherapy, more immediate symptomatic improvement is often desired. Antihistamines can reduce anxiety and promote sleep. Hydroxyzine, given its relatively clean pharmacodynamic profile and its ability to serve as both an anxiolytic and sleep-promoting agent, may be selected.
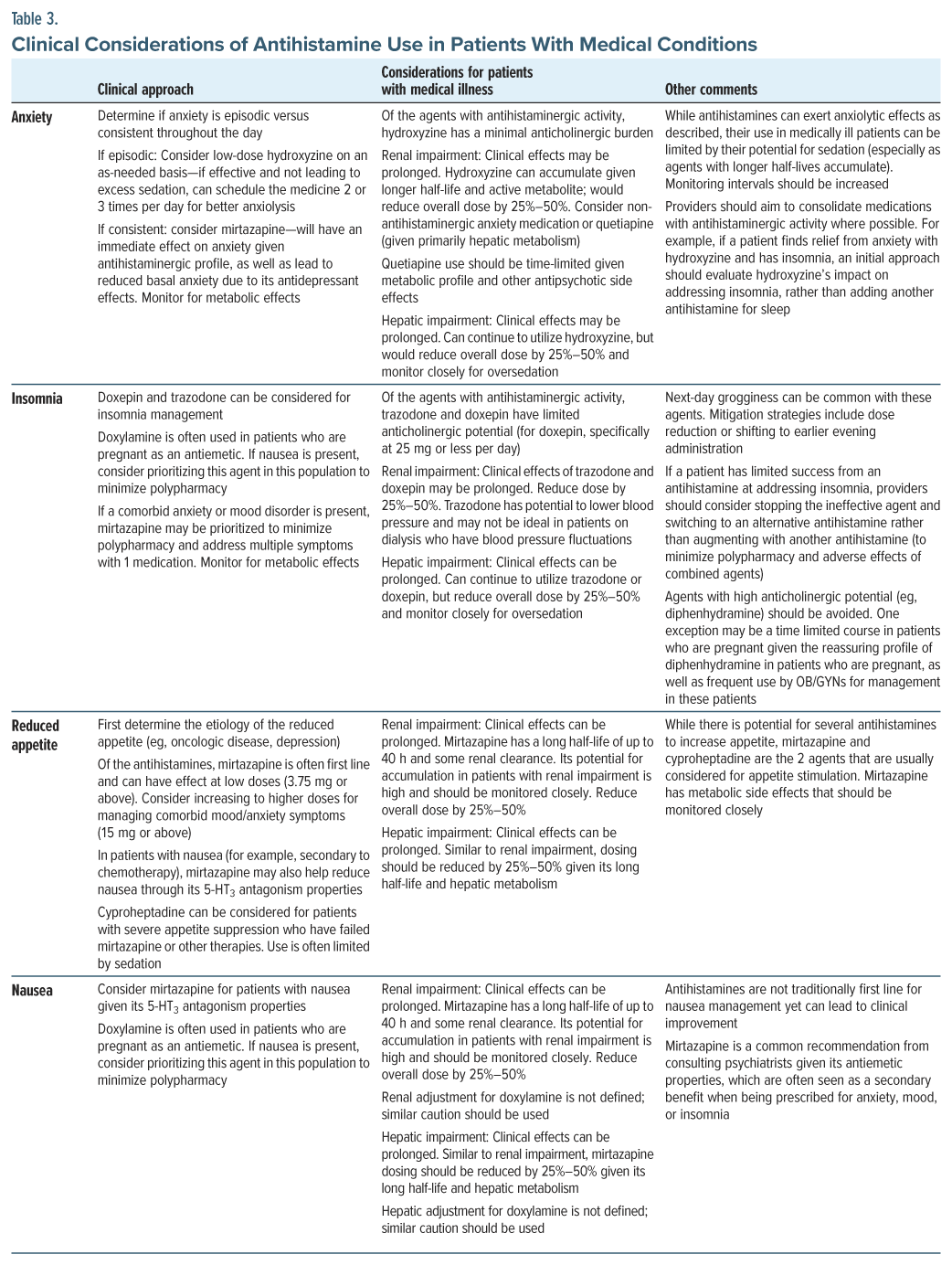
Clinicians should be mindful about the risks, and potential futility, of coadministering multiple antihistaminergic medications. Some patients attain minimal relief from anxiety with antihistamines and instead feel groggy rather than relaxed; therefore, these patients may respond to medications having a different pharmacodynamic profile rather than making dose adjustments or using alternative antihistamines. See Table 3 for guidance on the clinical use of antihistamines in patients with medical conditions.
Can Antihistamines Be Coadministered Safely With Anticholinergic Agents?
Medications with antihistaminergic properties often have varying degrees of anticholinergic properties. For example, clozapine is highly antihistaminergic and anticholinergic, while hydroxyzine has minimal activity at the antimuscarinic receptor.19,56 Research on the adverse impact of anticholinergic medications typically describes the idea of “anticholinergic accumulation.” This refers to medicines exerting a cumulative effect on a person based on their combined anticholinergic profiles. ACB scales can help clinicians determine the cumulative anticholinergic potential of commonly described medications.23,57
When using antihistamines concurrently or an antihistamine with an anticholinergic agent, clinicians should be mindful of acute and chronic side effects. Acutely, systemic anticholinergic effects include dry mouth, constipation, and blurred vision. More concerning side effects include constipation, urinary retention, and delirium. Cognitive decline and anticholinergic medication use have been well described in the literature, eg, the amount of anticholinergic exposure has been associated with an increased risk of dementia.23 Depending on the patient’s cognitive health, even a small amount of an anticholinergic agent can be deleterious to cognition and physical health; some patients with mild cognitive impairment and biomarkers for Alzheimer disease are highly susceptible to anticholinergic medication.58
Because of the concept of ACB, clinicians should coprescribe antihistamines cautiously, especially when medications target multiple receptors. These medications are often prescribed to patients for multiple issues yet are maintained for extended periods beyond what may be indicated and are often not deprescribed. If a patient is not responding to a medication having strong antihistamine properties, clinicians should question if additional antihistamine activity is beneficial or detrimental. Clinical situations vary; it is often best to augment medications with agents having alternative mechanisms, rather than “stacking” antihistamines to target anxiety or insomnia.
What Are Relative and Absolute Contraindications to the Use of Antihistamines?
Contraindications to use of antihistamines are primarily related to the anticholinergic potential of these agents, as well as their propensity to induce sedation. Absolute contraindications include hypersensitivity to or anaphylactic reactions from specific antihistamines. Relative contraindications are broader and involve the antihistamine’s anticholinergic side effects on a variety of organ systems, including the CNS. Antihistamines should be used with caution in the context of urinary retention, especially in those with prostatic hypertrophy or other urinary bladder defects. Caution should also be used with patients prone to slow gastric motility, constipation, or a history of ileus.
While sedation may be a desired effect of antihistamines, some individuals become over-sedated. This is especially true for those who are using other sedating medications (eg, opioids or gabapentinoids), and all patients should undergo careful medication reconciliation before being prescribed an antihistamine. Use of alcohol and other substances should be considered given the potential for synergistic sedation.
Which Patients Require Dose Adjustments of Antihistamines?
Antihistamine prescriptions should account for the patient’s risk factors and medical comorbidities. For example, the elderly are vulnerable to the anticholinergic effects of antihistamines. Therefore, the tactic of “starting low and going slow” in geriatric medicine applies to use of antihistamines. Beyond anticholinergic effects, clinicians should consider how the sedating effects of antihistamines might exacerbate fall risks in the elderly. Similarly, caution should be used in those with cardiac disease or other risk factors for QTc prolongation, as antihistamines contribute to QT prolongation.59 First-generation antihistamines, eg, hydroxyzine and diphenhydramine, may pose a higher risk than second-generation antihistamines (eg, cetirizine).59 Cases of torsades de pointes from first-generation antihistamines have been reported, but they typically occur in the setting of toxicity or overdose.59,60 Mirtazapine is generally thought to convey a lower risk of QTc prolongation.61 Trazodone, a commonly prescribed medication for insomnia, may pose a higher risk than initially thought. A recent study of QTc prolongation indicated that there was a dose-dependent QT increase of 19.8 ms with doses of 140 mg.62 Clinicians should also be mindful of additive QT-prolonging effects when patients are on multiple medications and consider alternative medications or routine electrocardiogram (ECG) monitoring in patients at high risk of QTc prolongation.
Dosage adjustments are required in individuals with organ dysfunction; antihistamines that undergo hepatic metabolism (eg, quetiapine, trazodone, doxepin, and hydroxyzine) should be dose adjusted in the context of hepatic impairment; starting at half of the normal starting dose, taking extra time in between dose increases, and adjusting based on clinical response are recommended. While most antihistamines are hepatically metabolized, in those with renal impairment, decreasing elimination of antihistamines and their metabolites can lead to prolonged clinical effects.
Antihistamines are commonly used in those with serious medical conditions to target anxiety and insomnia. Not only are patients with organ dysfunction and complex medical illness sensitive to sedation, but often patients in intensive care units (ICUs) receive multiple as-needed medications that have sedating properties. A well-meaning recommendation of an antihistamine for anxiolysis may lead to over-sedation and hypercarbia, hindering progress in weaning from a ventilator and prolonging their ICU stay. See Table 2 for a review of commonly used antihistamines in the medical setting.
What Are the Manifestations of an Antihistamine Overdose?
Antihistamine overdoses vary depending on the medication involved. CNS depression (ranging from drowsiness to coma) or excitement (including frank agitation) can arise, as can delirium. Hallucinations are also common, even when therapeutic doses of antihistamines have been administered.63 When taken to excess, antihistamines can induce dilated/fixed pupils, nausea, vomiting, diarrhea, and fever. The mnemonic “red as a beet, dry as a bone, hot as a hare, blind as a bat, mad as a hatter, and plugged like a pump” can help clinicians remember the effects of anticholinergic toxicity, which can be seen with antihistamine overdose. Severe adverse effects include seizure, hypotension, and cardiorespiratory collapse. Although rare, rhabdomyolysis and renal impairment can develop.64 Because of downstream effects of antihistamines on other neurotransmitters, patients are also at risk for serotonin toxicity.
Is There an Antidote for Antihistamine Overdoses?
Prompt evaluation is essential for effectively managing an antihistamine overdose. The patients’ airway, breathing, and circulation should be monitored. Activated charcoal can be administered, especially if the patient presents shortly after their overdose. Cardiac monitoring with an ECG is recommended. While care is primarily supportive, if anticholinergic toxicity is severe and life-threatening, use of physostigmine should be considered. Outcomes after antihistamine overdose are primarily good, depending on the amount ingested, the time to evaluation, and the presence of severe cardiac or neurological toxicity.
Future Directions
Further studies are needed to explore the use of antihistamines for managing psychiatric symptoms in patients with complex medical conditions. For example, in the critical care setting, consultation-liaison psychiatrists often recommend the use of antihistamines to manage anxiety secondary to dyspnea in weaning ventilatory support despite little evidence. Complications then arise in terms of potential oversedation leading to prolonged weans or organ dysfunction, leading to increased pharmacodynamic effects. Some research is emerging in this area, including a recent retrospective study on the use of hydroxyzine to manage delirium, but more data are needed on the safety profile of these medications in patients with critical illness.65
What Happened to Mr A?
The psychiatric consultant recommended stopping the scheduled quetiapine and the as-needed hydroxyzine and reducing mirtazapine (to 7.5 mg) to account for his renal impairment and over-sedation. Mirtazapine administration was moved from 11:00 pm to 8:00 pm to prevent morning grogginess and ensure proper sleep. Within 2 days, Mr A was more alert and awake, although he reported intermittent anxiety and persistent low appetite. Cautious titration of mirtazapine (to 15 mg) was recommended, and a spiritual care consultation was arranged. With bedside psychoeducation, the psychiatry team worked with Mr A to practice breathing techniques for anxiety management. Mr A’s appetite gradually improved over several weeks, and his sleep patterns stabilized. He was discharged to a rehabilitation facility on mirtazapine (15 mg nightly) with planned follow-up with a therapist to work on skill building for anxiety management.
CONCLUSION
Histamine has potent biological effects, being integral for not only the innate and adaptive immune response but also key nervous system functions, such as sleep and cognition. The myriad effects of histamine are mediated by the 4 histamine receptors, which can be bound and inhibited by a wide variety of pharmacologic agents (including antidepressants and antipsychotics) with antihistaminergic activity. These agents mitigate insomnia, anxiety, nausea, and allergic reactions, as histamine plays a central role in adaptive immune responses and GI function. The first-generation antihistamines (eg, hydroxyzine, diphenhydramine) readily cross the blood-brain barrier, allowing for their CNS-mediated sedative and anxiolytic effects, as well as inducing weight gain, impaired attention, working memory, and motor coordination.
Article Information
Published Online: September 16, 2025. https://doi.org/10.4088/PCC.25f03958
© 2025 Physicians Postgraduate Press, Inc.
Submitted: March 6, 2025; accepted May 27, 2025.
To Cite: Gunther M, Valido A, Jiang S, et al. Antihistamines: indications, interactions, and adverse effects. Prim Care Companion CNS Disord 2025;27(5):25f03958.
Author Affiliations: Department of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University, Stanford, California (Gunther, Valido); Department of Psychiatry, College of Medicine, University of Florida (Jiang); Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts (Stern).
Corresponding Author: Matthew Gunther, MD, Department of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University, 401 Quarry Rd, #2317, Stanford, CA 94305 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Clinical Points
- Histamine plays a critical role in several key neurophysiological processes (eg, sleep-wake cycle regulation, satiety signaling, and cognitive function).
- Histamine binding to the H1 receptor leads to the classic symptoms of allergic reaction (eg, congestion, rhinorrhea, pruritus), and it has been implicated in acute and chronic immunological conditions (eg, allergic rhinitis, atopic dermatitis, conjunctivitis, urticaria, asthma, anaphylaxis).
- Many antihistamines share a propensity for muscarinic receptor antagonism at higher doses, which leads to signs and symptoms of anticholinergic toxicity, and clinicians should be mindful about the risks of coadministering multiple antihistaminergic medications.
- Contraindications to use of antihistamines are primarily related to the anticholinergic potential of these agents, as well as their propensity to induce sedation.
- Dosage adjustments of antihistamines are advised when hepatic metabolism is impaired; start at half of the normal starting dose, take extra time between dose increases, and adjust the dose based on clinical response.
References (65)

- Goulty M, Botton-Amiot G, Rosato E, et al. The monoaminergic system is a bilaterian innovation. Nat Commun. 2023;14(1):3284. PubMed CrossRef
- Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy. 2009;39(12):1786–1800. PubMed CrossRef
- Hanuskova E, Plevkova J. The role of histamine H4 receptors as a potential targets in allergic rhinitis and asthma. OJMIP. 2013;03(01):6–14. CrossRef
- Cooke MR, Giovannitti JA. Histamine and histamine antagonists. In: Pharmacology and Therapeutics for Dentistry. Elsevier; 2017:276–286. CrossRef
- Leurs R, Vischer HF, Wijtmans M, et al. En route to new blockbuster anti-histamines: surveying the offspring of the expanding histamine receptor family. Trends Pharmacol Sci. 2011;32(4):250–257. PubMed CrossRef
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4(2):121–130. PubMed CrossRef
- Panula P, Nuutinen S. The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci. 2013;14(7):472–487. PubMed CrossRef
- Thangam EB, Jemima EA, Singh H, et al. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol. 2018;9:1873. PubMed CrossRef
- Mahdy AM, Webster NR. Histamine and antihistamines. Anaesth Intensive Care Med. 2011;12(7):324–329. CrossRef
- Peavy RD, Metcalfe DD. Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8(4):310–315. PubMed CrossRef
- Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(2):163–172. PubMed CrossRef
- Church DS, Church MK. Pharmacology of antihistamines. World Allergy Org J. 2011;4(3 suppl):S22–S27. PubMed CrossRef
- Castells M, Butterfield J. Mast cell activation syndrome and mastocytosis: initial treatment options and long-term management. J Allergy Clin Immunol Pract. 2019;7(4):1097–1106. PubMed CrossRef
- Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376(26):2566–2578. PubMed CrossRef
- Simons KJ, Watson WT, Chen XY, et al. Pharmacokinetic and pharmacodynamic studies of the H1-receptor antagonist hydroxyzine in the elderly. Clin Pharmacol Ther. 1989;45(1):9–14. PubMed CrossRef
- Stahl SM. Prescriber’s Guide: Stahl’s Essential Psychopharmacology. 7th ed. Cambridge University Press; 2020. CrossRef
- Mansbach RP. 2.d.023 In vitro pharmacological profile of doxepin, a sleep-promoting histamine H1 antagonist. Eur Neuropsychopharmacol. 2008;18:S357–S358. CrossRef
- Paton DM, Webster DR. Clinical pharmacokinetics of H1-receptor antagonists (The Antihistamines). Clin Pharmacokinet. 1985;10(6):477–497. PubMed CrossRef
- Lieberman JA. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):8–13. PubMed
- Krystal AD, Richelson E, Roth T. Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications. Sleep Med Rev. 2013;17(4):263–272. PubMed CrossRef
- Odagaki Y, Toyoshima R, Yamauchi T. Trazodone and its active metabolite m-chlorophenylpiperazine as partial agonists at 5-HT1A receptors assessed by [35S]GTPgammaS binding. J Psychopharmacol. 2005;19(3):235–241. PubMed CrossRef
- Rotzinger S, Bourin M, Akimoto Y, et al. Metabolism of some “second”– and “fourth”–generation antidepressants: iprindole, viloxazine, bupropion, mianserin, maprotiline, trazodone, nefazodone, and venlafaxine. Cell Mol Neurobiol. 1999;19(4):427–442. PubMed CrossRef
- Coupland CAC, Hill T, Dening T, et al. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084–1093. PubMed CrossRef
- Jensen NH, Rodriguiz RM, Caron MG, et al. N-desalkylquetiapine, a potent norepinephrinereuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacol. 2008;33(10):2303–2312. PubMed CrossRef
- Simons FER, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128(6):1139–1150.e4. PubMed CrossRef
- Kuna L, Jakab J, Smolic R, et al. Peptic ulcer disease: a brief review of conventional therapy and herbal treatment options. J Clin Med. 2019;8(2):179. PubMed CrossRef
- Pitolisant [Package Insert]. Harmony Biosciences. LLC; 2024.
- Armstrong SC, Cozza KL. Antihistamines. Psychosomatics. 2003;44(5):430–434. PubMed CrossRef
- Zhou J, Liu DF, Liu C, et al. Glucocorticoids inhibit degranulation of mast cells in allergic asthma via nongenomic mechanism. Allergy. 2008;63(9):1177–1185. PubMed CrossRef
- Kitamura Y, Das AK, Murata Y, et al. Dexamethasone suppresses histamine synthesis by repressing both transcription and activity of HDC in allergic rats. Allergol Int. 2006;55(3):279–286. PubMed CrossRef
- Santoro T, Azevedo CT, E Silva PMR, et al. Glucocorticoids decrease the numbers and activation of mast cells by inducing the transactivation receptors of AGEs. J Leukoc Biol. 2019;105(1):131–142. PubMed CrossRef
- Wang X, Lau H. Beta-adrenoceptor-mediated inhibition of mediator release from human peripheral blood-derived mast cells. Clin Exp Pharma Physio. 2006;33(8):746–750. PubMed CrossRef
- Schnedl WJ, Enko D. Histamine intolerance originates in the gut. Nutrients. 2021;13(4):1262. PubMed CrossRef
- Nazar W, Plata-Nazar K, Sznurkowska K, et al. Histamine intolerance in children: a narrative review. Nutrients. 2021;13(5):1486. PubMed CrossRef
- Rajtar S, Irman-Florjanc T. Amitriptyline affects histamine-N-methyltransferase and diamine oxidase activity in rats and Guinea pigs. Eur J Pharmacol. 2007;574(2-3):201–208. PubMed CrossRef
- Ferjan I, Erjavec F. Changes in histamine and serotonin secretion from rat peritoneal mast cells caused by antidepressants. Inflamm Res. 1996;45(3):141–144. PubMed CrossRef
- Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacol. 2003;28(3):519–526. PubMed CrossRef
- Deng C, Weston-Green K, Huang XF. The role of histaminergic H1 and H3 receptors in food intake: a mechanism for atypical antipsychotic-induced weight gain?. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(1):1–4. PubMed CrossRef
- Morisset S, Sahm UG, Traiffort E, et al. Atypical neuroleptics enhance histamine turnover in brain via 5-Hydroxytryptamine2A receptor blockade. J Pharmacol Exp Ther. 1999;288(2):590–596. PubMed CrossRef
- Rudolph JL, Salow MJ, Angelini MC, et al. The Anticholinergic Risk Scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508–513. PubMed CrossRef
- Zhang L, Zhang Y, Zhao P, et al. Predicting drug–drug interactions: an FDA perspective. AAPS J. 2009;11(2):300–306. PubMed CrossRef
- Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake–sleep states in the mouse. J Neurosci. 2006;26(40):10292–10298. PubMed CrossRef
- Culpepper L, Wingertzahn MA. Over-the-counter agents for the treatment of occasional disturbed sleep or transient insomnia: a systematic review of efficacy and safety. Prim Care Companion CNS Disord. 2015;17(6).doi:10.4088/PCC.15r01798. PubMed CrossRef
- Morin CM, Buysse DJ. Management of insomnia. N Engl J Med. 2024;391(3):247–258. PubMed CrossRef
- Everitt H, Baldwin DS, Stuart B, et al. Antidepressants for insomnia in adults. Cochrane common mental disorders group. Cochrane Database Syst Rev. 2018;2018(5). doi:10.1002/14651858.CD010753.pub2. CrossRef
- YiNiGhadami XYSFMR, Meng HQ, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25–32. PubMed
- Li B, Chang L, Zhuang QX. Histamine signaling in the bed nucleus of the stria terminalis modulates stress-induced anxiety. J Affect Disord. 2023;335:195–203. PubMed CrossRef
- Lamberty Y, Gower A. Hydroxyzine prevents isolation-induced vocalization in Guinea pig pups: comparison with chlorpheniramine and immepip. Pharmacol Biochem Behav. 2004;79(1):119–124. PubMed CrossRef
- Nir Y, De Lecea L. Sleep and vigilance states: embracing spatiotemporal dynamics. Neuron. 2023;111(13):1998–2011. PubMed CrossRef
- Garakani A, Murrough JW, Freire RC, et al. Pharmacotherapy of anxiety disorders: current and emerging treatment options. Front Psychiatry. 2020;11:595584. PubMed CrossRef
- Jørgensen EA, Knigge U, Warberg J, et al. Histamine and the regulation of body weight. Neuroendocrinol. 2007;86(3):210–214.
- Kim SF, Huang AS, Snowman AM, et al. Antipsychotic drug-induced weight gain mediated by histamine H 1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci USA. 2007;104(9):3456–3459. PubMed CrossRef
- Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428(6982):569–574. PubMed CrossRef
- Mika K, Szafarz M, Bednarski M, et al. Metabolic benefits of novel histamine H3 receptor ligands in the model of excessive eating: the importance of intrinsic activity and pharmacokinetic properties. Biomed Pharmacother. 2021;142:111952. PubMed CrossRef
- Athavale A, Athavale T, Roberts DM. Antiemetic drugs: what to prescribe and when. Aust Prescr. 2020;43(2):49–56. PubMed CrossRef
- Kubo N, Shirakawa O, Kuno T, et al. Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay. Jpn J Pharmacol. 1987;43(3):277–282. PubMed CrossRef
- Hsu W, Huang S, Lu W, et al. Impact of multiple prescriptions with anticholinergic properties on adverse clinical outcomes in the elderly: a longitudinal cohort study in taiwan. Clin Pharmacol Ther. 2021;110(4):966–974. PubMed CrossRef
- Weigand AJ, Bondi MW, Thomas KR, et al. Association of anticholinergic medications and AD biomarkers with incidence of MCI among cognitively normal older adults. Neurology. 2020;95(16):e2295–e2304. PubMed CrossRef
- Ali Z, Ismail M, Khan F, et al. Association of H1-antihistamines with torsade de pointes: a pharmacovigilance study of the food and drug administration adverse event reporting system. Expert Opin Drug Saf. 2021;20(1):101–107. PubMed CrossRef
- Poluzzi E, Raschi E, Godman B, et al. Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe. PLoS ONE. 2015;10(3):e0119551. PubMed CrossRef
- Allen ND, Leung JG, Palmer BA. Mirtazapine’s effect on the QT interval in medically hospitalized patients. Ment Health Clin. 2020;10(1):30–33. PubMed CrossRef
- Tellone V, Rosignoli MT, Picollo R, et al. Effect of 3 single doses of trazodone on QTc interval in healthy subjects. J Clin Pharmacol. 2020;60(11):1483–1495. PubMed CrossRef
- Borowy CS, Mukherji P. Antihistamine toxicity. In: StatPearls. StatPearls Publishing; 2025. Accessed February 17, 2025. http://www.ncbi.nlm.nih.gov/books/NBK482318/
- Basha A, Karatas R, Taneja AK, et al. Rhabdomyolysis following diphenhydramine overdose-a case report. Skeletal Radiol. 2025;54(5):1159–1163. PubMed CrossRef
- Kubo K, Takehara M, Hirata M, et al. Effects of intravenous hydroxyzine versus haloperidol monotherapy for delirium: a retrospective study. J Clin Psychiatry. 2025;86(1):24m15569. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!