The Art of Sharing the Diagnosis and Management of Alzheimer’s Disease With Patients and Caregivers: Recommendations of an Expert Consensus Panel
Objective: To develop a set of recommendations for primary care physicians (PCPs) suggesting how best to communicate with patients, caregivers, and other family members regarding the diagnosis and management of Alzheimer’s disease (AD).
Participants: A national roundtable of 6 leading professionals involved in treating or advocating for patients with AD was convened on March 14, 2008. This roundtable included 4 leading academic physicians with diverse backgrounds (a geriatric psychiatrist, a neuropsychiatrist, a neurologist, and a geriatrician) from geographically diverse regions of the United States, who were invited on the basis of their national reputation in the field and experience working with minority populations with dementia; the executive director of a national AD advocacy organization; the executive director of a national advocacy organization for caregivers; and a medical correspondent with expertise in interviewing and small group leadership.
Evidence: Expert opinion supported by academic literature (search limited to PubMed, English language, 1996-2008, search terms: Alzheimer’s disease, primary care, diagnosis, management, caregiver, family, patient-physician relationship).
Consensus Process: Moderated dialogue aimed at generating consensus opinion; only statements endorsed by all authors were included in the final article.
Conclusions: Diagnosis and management of AD by PCPs, utilizing specialist consultation as needed, may contribute to earlier diagnosis and treatment, improved doctor-patient and doctor-caregiver communication, increased attention to caregiver needs, and better clinical and quality-of-life outcomes for patients and caregivers. A set of expert panel recommendations describing practical strategies for achieving these goals was successfully developed.
Prim Care Companion J Clin Psychiatry 2010;12(1):e1-e9
© Copyright 2010 Physicians Postgraduate Press, Inc.
Submitted: May 4, 2009; accepted July 23, 2009.
Published online: February 25, 2010 (doi:10.4088/PCC.09cs00833oli).
Corresponding author: George T. Grossberg, MD, Department of Psychiatry, St Louis University School of Medicine, 1438 S. Grand Blvd, St Louis, MO 63104 ([email protected]).
Alzheimer’s disease (AD) is a neurodegenerative disorder that causes cognitive and functional disability and affects an estimated 5.1 million Americans.1 Although available treatments do not cure or reverse the course of AD, clinical studies have demonstrated that the cholinesterase inhibitors (ChEIs) and an N-methyl-D-aspartic acid (NMDA) antagonist can delay or slow the progression of cognitive and functional impairment, with greatest benefits achieved when medication is started early and utilized persistently.1,2 Research also shows that psychosocial interventions providing support, education, and counseling to patients and caregivers can improve the quality of life of those afflicted as well as their families.3 Moreover, early diagnosis of AD provides time for patients and families to prepare for the future and obtain necessary services.4,5
Clinical Points
- Making and sharing the diagnosis of Alzheimer’s disease may best be accomplished by the patient’s primary care physician.
- Skillful communication with patients, caregivers, and family members is of paramount importance in the diagnosis and treatment of Alzheimer’s disease.
- Treatments “work” mostly by slowing progression of the disease.
Despite the potential benefits of early diagnosis of AD, patients are often diagnosed later in the course of the disease and frequently by a consultant rather than the primary care physician (PCP).6 A recent Internet survey of 539 caregivers of AD patients conducted by Harris Interactive on behalf of the Alzheimer’s Foundation of America found that the typical patient experiences symptoms for more than 2 years (26.1 months) and visits an average of 2.3 doctors before being diagnosed with AD.7 At the time of diagnosis, only 43% of patients were in the mild stage of the disease, whereas 23% were in the moderate stage, and the remaining patients (34%) had severe AD.7
The diagnosis and treatment of AD in the primary care setting may already be occurring with increasing frequency. According to GfK Market Measures’ 2007 Alzheimer’s Disease Caregiver Study, 68% of caregivers identified PCPs as key health care providers for both diagnosing and managing their loved one’s AD.8 In fact, PCPs are often in the best position to both make the diagnosis and share it with the patient and the family because of the often long-term, ongoing relationship that the PCP has with the patient and family. Optimal involvement of primary care physicians in making and sharing the diagnosis of AD should result in patients being diagnosed at an earlier stage of the disease, allowing the patients themselves to make decisions about their own health care and express preferences regarding choices that they will be unable to make in the future.3,5
Effectively sharing the diagnosis of AD with patients and their families and communicating with them over the course of the illness concerning the myriad issues that arise present a formidable challenge for many PCPs. Not surprisingly, significant gaps in communication between PCPs and patients with AD and their caregivers have been identified.9 Although guidelines have been issued by several organizations for sharing the diagnosis of AD and for providing education to patients and caregivers,2,10 there is a need for more practical and detailed guidance concerning how to accomplish this challenging task, particularly in a primary care setting.
Accordingly, a national roundtable was convened on March 14, 2008, with the principal goal of arriving at a consensus on a set of recommendations to be offered to PCPs suggesting how best to communicate with patients and families regarding the diagnosis and ongoing management of AD. This roundtable included 4 leading academic physicians with diverse backgrounds (a geriatric psychiatrist, a neuropsychiatrist, a neurologist, and a geriatrician) from geographically diverse regions of the United States, who were invited on the basis of their national reputation in the field and experience working with minority populations with dementia; the executive director of a national AD advocacy organization; the executive director of a national advocacy organization for caregivers; and a medical correspondent with expertise in interviewing and small group leadership.
This article, based on those proceedings and intended to be a practical resource for PCPs, will present these recommendations (Table 1), highlighting their potential to improve the quality of medical care for patients with AD, the quality of life for their caregivers, and satisfaction with the medical encounter for the PCP. Expert opinion is supported by the academic literature (search limited to PubMed, English language, 1996-2008, search terms: Alzheimer’s disease, primary care, diagnosis, management, caregiver, family, patient-physician relationship). Only statements endorsed by all authors were included in this article.
Sharing the Diagnosis of AD
Overcoming Barriers to Making the Diagnosis in the Primary Care Setting
It has been well documented that PCPs are often reluctant to make the diagnosis of AD.6,11 Barriers include uncertainty about the need for, and utility of, a specific diagnosis; time constraints; reimbursement issues; and limited treatment options.6,11 In addition, many physicians may be uncomfortable making the diagnosis of AD in the absence of clinically available laboratory, neurologic examination, or neuroimaging findings to confirm the diagnosis.
Recognizing the reality of these concerns, there are equally compelling factors that favor the PCP as the primary diagnostician and medical caregiver of the patient with AD. Most important in this regard is the assertion that by following recommended diagnostic guidelines,12 including the DSM-IV criteria and the National Institute of Neurologic and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria, PCPs can diagnose AD with acceptable accuracy (66%-85%), depending on the criteria utilized.11,12 Moreover, standard medical evaluations required for ruling out reversible causes of dementia are clearly stated in the most recent American Academy of Neurology guidelines,2 and the Alzheimer’s Association Web site provides excellent information regarding the diagnostic workup and differential diagnosis of AD (http://www.alz.org).
In one study comparing the accuracy of AD diagnosis between PCPs and tertiary care neurologists, both groups performed similarly well (84% accuracy for PCPs, 78% for neurologists) when initial diagnosis was compared with postmortem pathological diagnosis.13 The greater complexity of the cases seen by the neurologists may explain the nonsignificantly lower accuracy rate among neurologists. Further, the diagnostic burden need not rest entirely on the PCP, as there is always opportunity for consultation or referral when the diagnosis is unclear. Even in this circumstance, sharing the diagnosis may still be best accomplished by the PCP.
Recommendations for Sharing the Diagnosis
There is a broad-based consensus that sharing the diagnosis of AD is an important component of good medical care.4,14,15 Furthermore, it is clear from patient surveys of unaffected elderly5,16 and patients with memory complaints17 that the vast majority of older individuals would want to know their diagnosis if they developed AD, and most would favor disclosure for a spouse with AD.5,16,17
A patient’s expressed preference not to be told the diagnosis should, however, be respected. In some cases, caregivers may indicate that the patient is not ready to receive a diagnosis of AD, and this should also be taken into consideration. It is also necessary to consider each patient’s capacity to understand the diagnosis. Even in those situations in which it is deemed preferable not to share the diagnosis of AD, it is important to ensure that the patient and caregivers understand that there is a problem that can be addressed by medical treatment and that will require additional resources to manage appropriately.
The clinical diagnosis of AD should be a process rather than an event and can best be accomplished over the course of several visits.4,14,15 This strategy allows for more time to gather diagnostic information, assess the needs and capabilities of the patient and family (or other caregivers), and introduce the possibility of a diagnosis of AD gradually. Often, it is the family and not the patient that first brings the issue of memory difficulty to the attention of the physician. In any case, PCPs should encourage patients to have a family member present who can validate, amend, or correct the patient’s history. In fact, many diagnostic clinics require that a family member or reliable informant be present to provide corroboration. In addition, a patient with a memory problem may not remember clearly what the physician says and having family members present may ensure that the diagnosis is effectively communicated.
When first disclosing the diagnosis of AD, those family members or others who are likely to provide care during the course of the disease should be present; it is also important to include them at subsequent visits. If there is a large family, it may be beneficial to have a single person become the information spokesperson (ie, attends visits and passes information on to others). It is recommended that the primary caregiver not also be the information spokesperson because functioning in both capacities may be too burdensome. For patients who are still independent and capable, it is essential to obtain their consent to discuss the diagnosis with family.
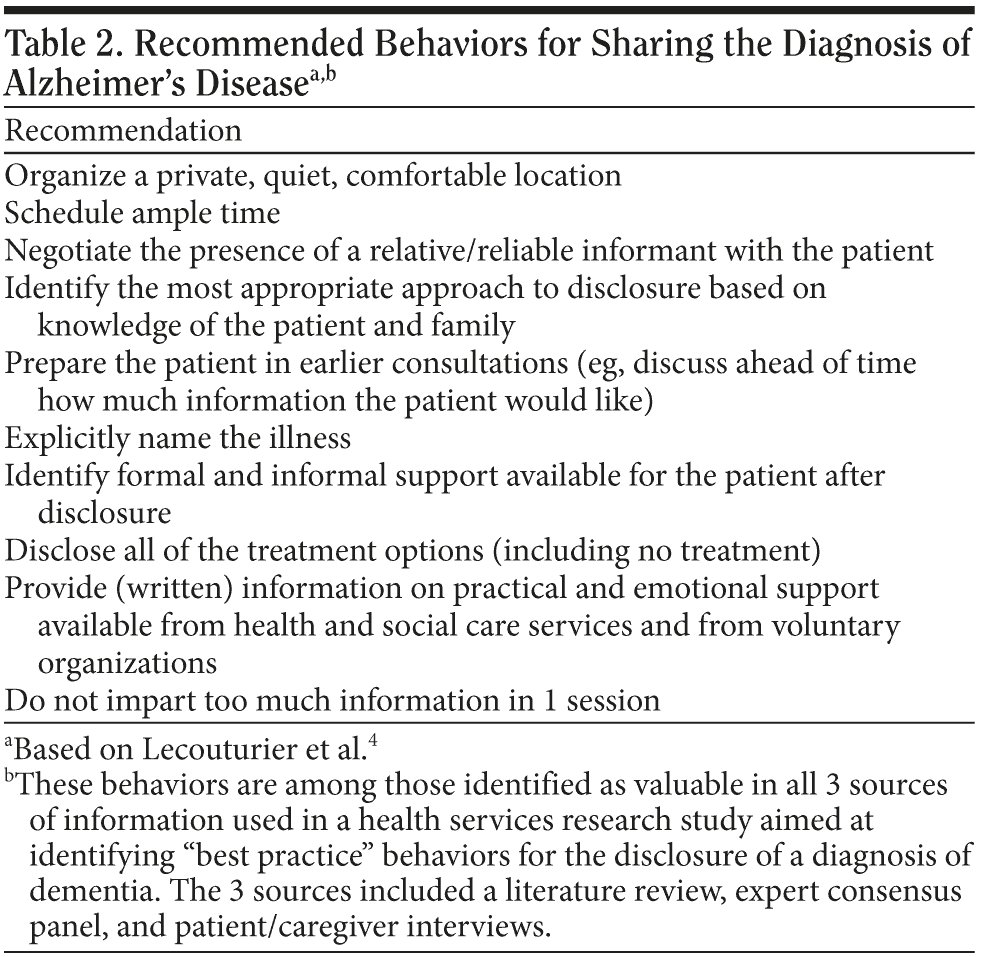
Heightened sensitivity is required when delivering a diagnosis of AD. A recent article identified “best practices” for disclosing a diagnosis of dementia4; among these, key practices include providing a private, quiet, comfortable location and scheduling ample time for the visit (Table 24). A trusting and supportive relationship with the patient and family is always beneficial, and this relationship is most likely to be present with the PCP. As much as possible, one should try to avoid all distractions for the duration of the visit (eg, telephone calls or pages).
The word Alzheimer’s should be used in the diagnosis, except in those cases, as noted above, for which an exception needs to be made. Cultural sensitivity and biases should also be taken into account when communicating the diagnosis. For example, in the Spanish-speaking community, dementia is associated more with mental illness than memory loss. Because the term Alzheimer’s usually carries understandably negative emotional connotations, there should be a hopeful emphasis on current capabilities and on maintaining and supporting functional abilities for as long as possible. To this end, there can be a discussion about the capabilities that the patient still has and the likelihood that they can be preserved for a reasonable amount of time. This is especially true, and most significant, for those patients who have been diagnosed early.
When sharing the diagnosis, it is recommended that the physician position himself or herself as a partner of, and advocate for, the patient and caregivers. He or she should let the patient and family know that the office staff is there to help them with educational resources and referral for necessary services. It is critically important to take a positive approach to the treatment of the disorder, emphasizing the value of pharmacotherapy, lifestyle changes, and other psychosocial interventions. Further, the concerned parties should be informed that there is a vast amount of ongoing research into both the causes of AD and treatments and that participation in clinical trials can be of benefit to both themselves and others.
Sharing of the diagnosis of AD should be tailored to each individual and family. The most appropriate approach to disclosing the diagnosis will vary depending on personalities, educational background, and culture. Some families are well informed about the diagnosis and treatment of AD, while others are not. The life circumstances of patients and their families shape the implications of the diagnosis as well. For example, a patient about to apply for long-term care insurance may be concerned about the potential financial consequences of a specific diagnosis. A patient still functioning in a leadership role in his or her job, however, must confront the issue of when and how to step down.
The consequences for the patient and the family of receiving a diagnosis of AD are immense. It is therefore entirely unrealistic to expect that 1 person (the doctor) or 1 encounter (the office visit) can satisfy their needs. Nonetheless, the physician is often in the best position to deal with certain issues. Perhaps most important is for the physician to elicit and address any misconceptions or myths about AD that the patient or family may harbor (eg, that it is just a part of normal aging or that treatment is futile). There may be an aura of stigma and shame associated with the diagnosis as well, primarily as a consequence of insufficient knowledge about the disease.
Caregivers may also have questions about what the diagnosis means for them and how they can reduce their risk of developing AD. This is a good opportunity to educate families about the current state of knowledge of disease risk and prevention, and many of the recommendations PCPs make for the at-risk family members will be similar to those recommended for the patient (eg, evidence regarding the potential protective effects of specific dietary factors18 or exercise19).
The PCP should emphasize to the patient and family that the sharing of the diagnosis is the beginning of an ongoing conversation. It is helpful to document that a discussion about diagnosis has occurred with the patient/family, particularly if the evaluation was conducted over several visits. Such documentation can also serve as an aid in keeping track of which issues have been adequately addressed and which issues may need to be raised at a future visit. It is best to recommend a meeting every 3 months, rather than scheduling appointments based only on acute medical problems. Finally, the availability of resources other than those provided by the PCP, such as respite care, adult day care, or support groups, should be made clear to the patient and the family/caregivers. Office staff should be able to arrange any consultations or referrals that are indicated (Table 3).
Communicating With Patients and Caregivers During Management of Mild to Moderate AD
Pharmacotherapy
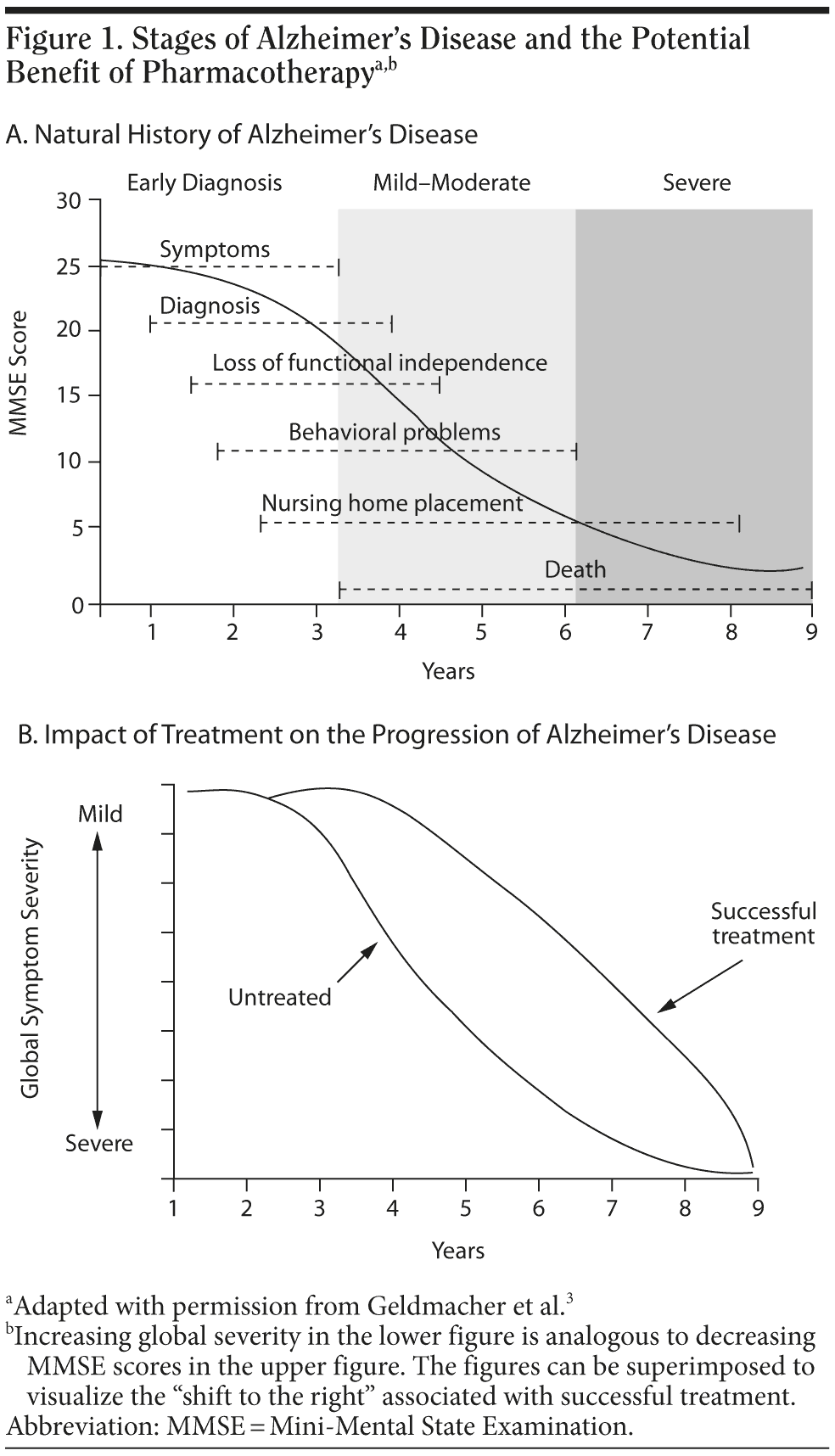
Among both PCPs and the public, there is often a perception that medications for AD are not useful because they do not cure the disease or that they may prolong the patient’s suffering by extending life in a severely demented state. Neither of these perceptions is accurate. Rigorously conducted clinical trials show that current treatments commonly reduce symptoms, behavioral problems, and functional decline.2,3 If the patient or family has unrealistic expectations, they may discontinue medication prematurely because of an erroneous assessment that the medication is “not working.” Although slowing cognitive, functional, and behavioral decline may not look like improvement to caregivers, clinical trials have shown that, on average, the course of disease is improved in treated patients compared with those who are not treated (Figure 1).3
The need to communicate this message clearly is supported by an Alzheimer’s Association survey, which found that, although 80% of PCPs believed that AD could be stabilized for a period if treated early, only 32% of caregivers recalled that the PCP informed them of this possibility.9 Furthermore, comprehensive treatment may provide sufficient improvement or stabilization to allow patients to remain appropriate candidates for new treatments as they become available.
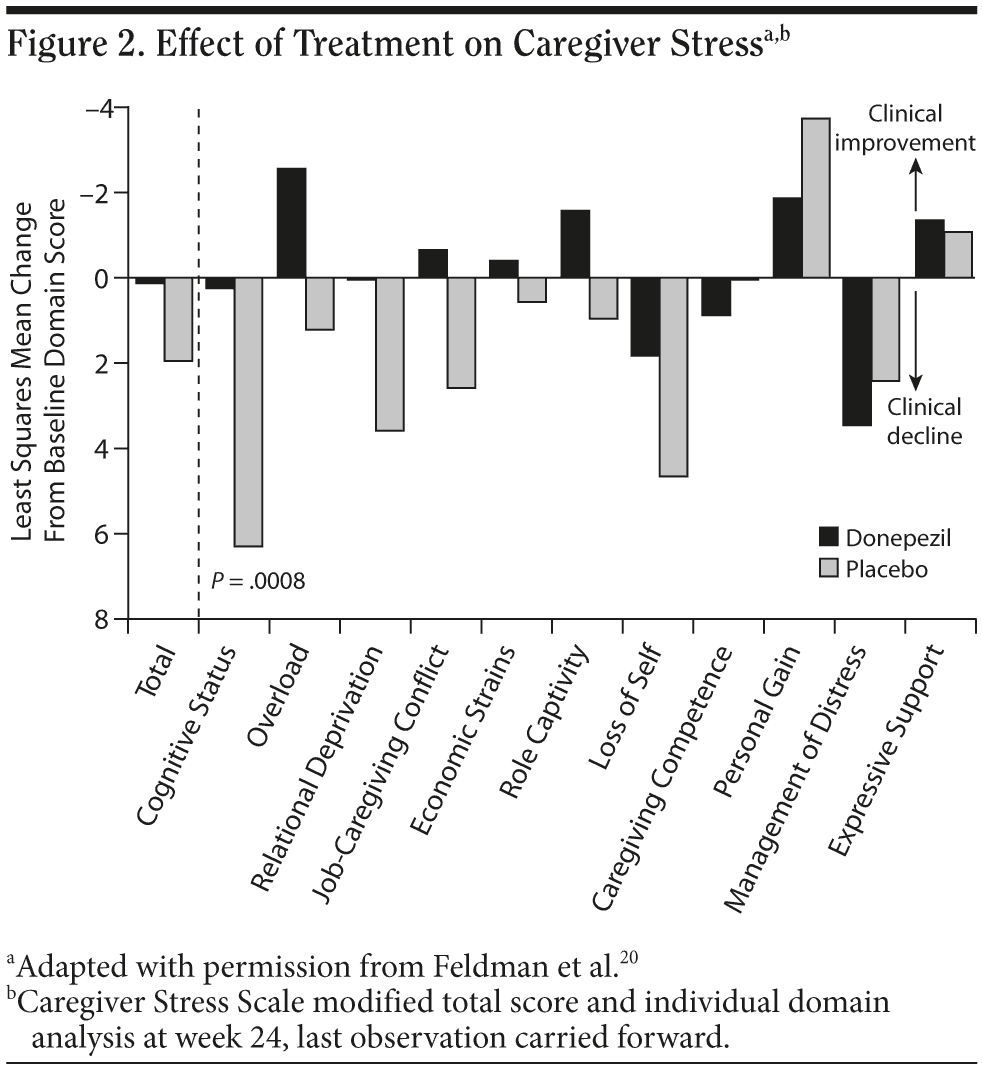
Early and persistent acetyl-cholinesterase inhibitor (ChEI) treatment may also reduce caregiver burden (Figure 2).20 For example, donepezil treatment over 1 year has been associated with a reduction in caregiver time of about 1 hour per day compared with placebo,21 and this finding has been replicated in studies with other ChEIs and with memantine, an NMDA receptor antagonist.3 Treatment with galantamine has been shown to reduce caregiver distress by means of a reduction in the severity of existing or emergent behavioral disturbances.22 Initiating sustained pharmacotherapy early in the disease may also delay nursing home placement.3,23,24
Lifestyle and Socialization
Some patients may limit socialization following diagnosis of AD because of embarrassment or shame, and some caregivers may encourage isolation in a desire to protect the patient. However, socialization should be part of the overall treatment plan because it may be valuable in combating the progression of the disease and in improving quality of life. Adult day care programs can reduce caregiver stress as well as provide education about managing problem behaviors.25 A comprehensive family educational intervention program was shown in a randomized, controlled trial to reduce the likelihood of institutional placement by one-third, especially in the early and middle stages of AD.26,27
Providing Education and Educational Resources on AD
Patients and their caregivers are usually very concerned about what to expect as AD progresses. It is important to tailor one’s communication to the individual and caregiver; some families may ask many questions, whereas others may ask few questions or indicate that they do not really want to know how the disease will progress. At a minimum, discuss with caregivers the stage the patient is currently at, what is likely to happen in the next 6 months, and which resources may be helpful.
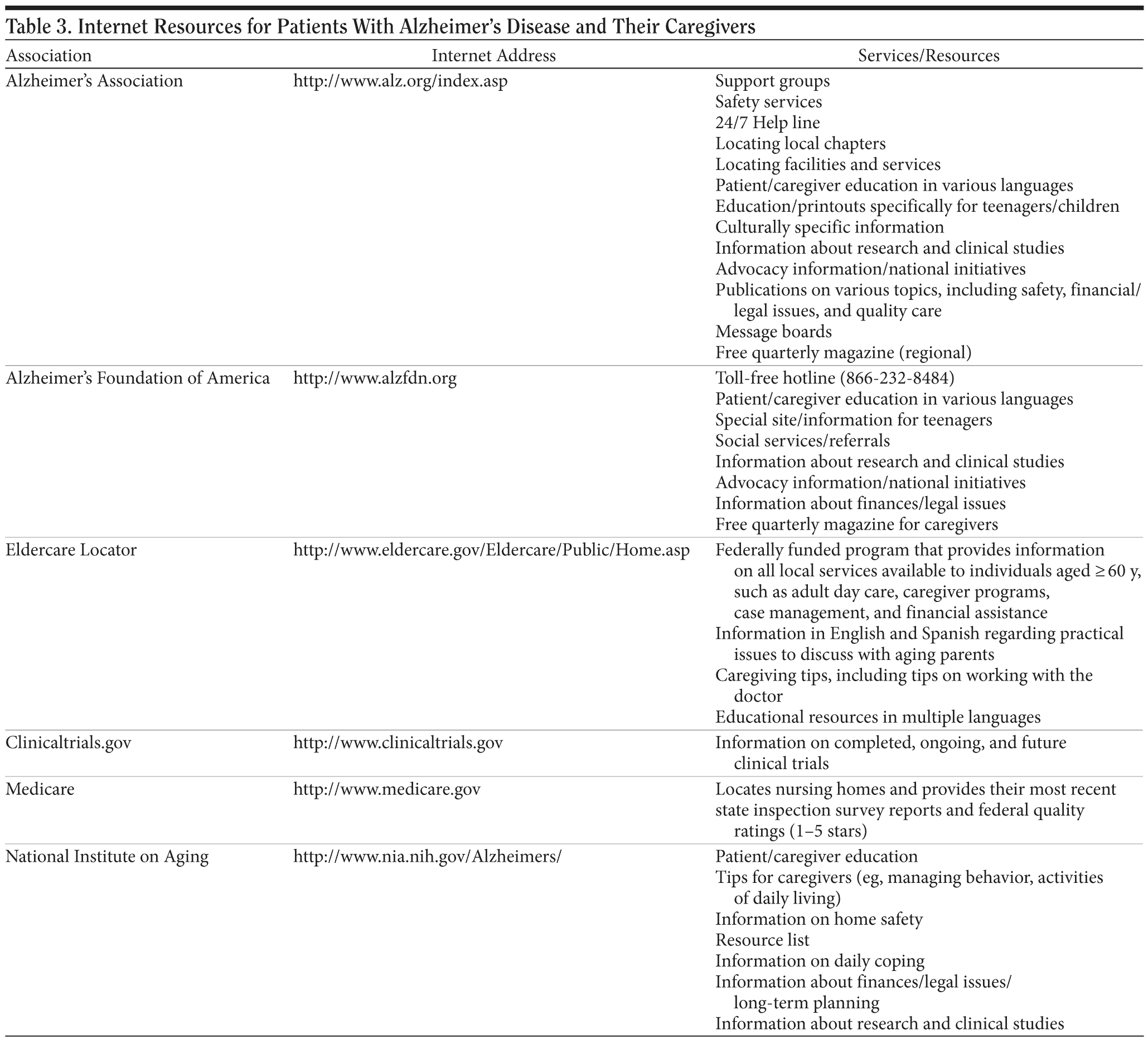
Patients and caregivers rely on providers of primary medical care for information about AD. It is strongly recommended that PCPs provide some basic literature on AD in the office. Today, there is a wealth of AD information available; the PCP can be an important filter to vet some of these resources and point people in the right direction. Table 3 lists several Internet resources and some of the types of information provided at these sites; in particular, the Alzheimer’s Association comprises more than 300 chapters and offers a range of information and services. For older caregivers who are not comfortable with online resources, written information on community resources may be provided, preferably in large print. The Eldercare Locator toll-free telephone number (1-800-677-1116) can be called to identify local resources for caregivers and patients aged 60 years and older. Magazines can also serve as an excellent source of patient/caregiver education. One example is Advantage (Alzheimer’s Foundation of America, www.alzfdn.org), a free quarterly magazine for caregivers of patients with AD and related illnesses.
Addressing Caregiver Needs
Addressing caregiver needs should be considered an integral part of the services provided for the family of a patient with AD. Caregivers of patients with AD, particularly spousal caregivers, often suffer from depression or other stress-related psychiatric disorders,28 experience a reduced quality of life,29 and may develop impairment in immune system functioning.30 For many caregivers, the emotional impact of caring for a loved one with AD is the most difficult aspect of the disease and outweighs more practical hardships, such as the financial burden or not having enough time for oneself.7 Almost half of caregivers report that the hardest thing for them is the emotional strain of seeing their loved ones lose the ability to function.7
Throughout the course of the illness, it is important to routinely assess how well the caregiver is functioning. General health questions that are also useful for identifying depression, such as those about appetite and sleep, as well as more specific inquiry about how the caregiver is coping, can help identify those caregivers who require additional support services and/or referral to professional sources of care. An excellent self-assessment tool for caregivers is also available on the American Medical Association Web site (http://www.ama-assn.org/ama/pub/physician-resources/public-health/promoting-healthy-lifestyles/geriatric-health/caregiver-health/caregiver-self-assessment.shtml).31
Caregivers who take better care of themselves are more likely to provide better care for their loved ones. According to a survey of 539 US caregivers, the most-often mentioned types of help that would make caregiving easier were help with day-to-day caring activities (20%), greater financial support (16%), more emotional support (15%), and more personal time (13%).7 To facilitate caregiver access to services that address these needs, the PCP should maintain a referral network of resources. Since many caregivers are employed, some services may be available through the workplace (eg, support groups, education programs, Family and Medical Leave Act).
It is important to emphasize to caregivers that there are other people in situations very similar to theirs. To this end, local support groups can provide an opportunity for caregivers to educate, console, and assist each other. In addition, for those who can afford it, direct care workers can provide valuable assistance with activities of daily living (ADLs) and other aspects of daily caregiving. Some caregivers, however, may feel that caring for their loved one is their personal responsibility and therefore will not seek or accept help. Under such circumstances, it may be beneficial to explicitly give the caregiver permission to utilize help by saying that it is medically indicated (ie, “doctor’s orders”). Clinical research has documented significant benefits to caregivers of supportive interventions.32
Special Issues in Moderate to Severe AD
Caregivers need to be prepared for the changes that their loved ones will undergo as AD progresses. Moderate and severe stages of AD are demarcated by more significant impairments of ADLs, as well as the evolution of more serious behavioral symptoms (eg, wandering, delusions, and misidentification of loved ones). Thus, the caregiver’s role becomes more focused on managing behavior and assisting with basic ADLs. During this time, it is important to set the expectation of decline and to educate caregivers regarding how they might address some of the anticipated milestones (eg, inability to manage finances, driving, or bowel/bladder control). Long-distance family members may also be included in these conversations via speakerphone.
Caregivers and other family members should be told that, when behavioral changes arise, there may be potentially treatable medical causes contributing to the problem, such as a urinary tract infection, pain,2 or depression.33 In addition to diagnosing and treating such conditions, the PCP can also provide some suggestions for how best to deal with the behavioral issues (eg, strategies for dealing with wandering and common sleep/wake cycle disturbances and behavioral modification approaches to inappropriate or aggressive behavior). A good source of practical information on this topic is The 36-Hour Day: A Family Guide to Caring for People with Alzheimer Disease, Other Dementias, and Memory Loss in Later Life by Mace and Rabins.34
Safety is a prevailing issue throughout the course of this disease and no less so in the later stages. At each visit, stage-specific safety issues should be addressed proactively. One should not wait until a safety issue occurs before addressing it (eg, do not wait until there is a medication mistake to discuss the use of a pill box). Patients and their caregivers may benefit from new technologies focused on home safety. One example is passive in-home health status monitoring that promotes the use of wearable sensors and sensors embedded in the home environment to monitor physiologic and behavioral activity (eg, getting in and out of bed, turning the stove on and off, falling).35
Driving is often a contentious issue. Patients with AD are at an increased risk of auto accidents. They commonly forget where they are going and may become confused while driving. It is helpful to begin a discussion about driving early in the course of the disease and to address the issue at subsequent visits. It is also highly recommended that the patient take an older adult driving evaluation test, which is available through the occupational therapy departments of many hospitals and at state Departments of Motor Vehicles.
Useful suggestions for driving include avoiding driving at night, on freeways, or without a licensed driver in the passenger seat; limiting trips to familiar routes, at low traffic times, and in good driving conditions; and having a cell phone programmed with speed dial to reach family caregivers. Limiting the patient’s driving via a staged withdrawal rather than an abrupt prohibition may reduce the patient’s resistance to complying with necessary restrictions. For adults who can no longer drive but who are very attached to their cars, it may be helpful to suggest disabling the car but keeping it in the garage. Finally, state-to-state differences exist regarding whether the PCP is legally obligated to report that a patient has AD if his or her driving safety is in question; the PCP must be familiar with the law in his or her own state and act accordingly.
Financial safety is a key concern as well. When appropriate, families should be encouraged to enlist the assistance of bank personnel in monitoring the financial safety of the patient. Financial planners may also need to be alerted regarding the patient’s diagnosis. If needed, an elder law attorney can be located via the National Academy of Elder Law Attorneys (http://www.naela.org).
Another common occurrence in later stages of AD is a query about stopping antidementia medication. While acknowledging the progression of the illness, it is important to discuss the likelihood that the medications may continue to benefit behavior and functioning.20
Despite optimal medical care and devoted caregiving, almost all patients with AD reach a state in which placement in a long-term care facility may become necessary. As noted previously, addressing this probability early in the course of the disease will allow the patient to have more meaningful input regarding his or her preferences.36 If such discussions have not yet occurred, this vexing topic will need to be raised and alternatives explored. There is a wealth of material available to assist patients and families with this decision-making process37; the primary role of the physician is to ensure that such resources are utilized in a timely fashion.
Similarly, end-of-life issues, including a living will or health care proxy, should also be addressed during earlier stages of the disease, and decisions made previously should be reviewed and confirmed. Here, too, utilization of external resources for information, support, and professional guidance should be encouraged.
CONCLUSION
PCPs are increasingly playing an integral role in the diagnosis and management of AD. Timely diagnosis and initiation of therapy for AD are important to optimize treatment response and provide the patient and family with sufficient time for planning. Early diagnosis of AD may also enable the patient to fully contribute to treatment and practical decisions. While the manner in which a diagnosis of AD is disclosed to the patient and caregivers should be tailored to the individual family, it is recommended that a specific diagnosis be communicated and that as many caregivers as possible be involved. It is important to focus on the positive aspects of the patient’s current capabilities and the value of maintaining function.
As the disease progresses, the focus shifts toward managing behaviors and accommodating functional decline. It is important to communicate with caregivers about these issues and to recommend resources for managing the day-to-day care of the patient and his or her own well being. Numerous resources are available to physicians, patients, and caregivers; PCPs can help direct patients and caregivers to these resources in order to ensure that they obtain the educational, social, and emotional support that they need.
Drug names: donepezil (Aricept), galantamine (Razadyne), memantine (Namenda).
Author affiliations: Department of Psychiatry, St Louis University School of Medicine, Missouri (Dr Grossberg); Departments of Psychiatry, Neurology, and Pharmacology, Neuropsychiatric Institute, University of Utah School of Medicine, Salt Lake City (Dr Christensen); Department of Internal Medicine, Meharry Medical College, Nashville, Tennessee (Dr Griffith); Division of Geriatrics and Gerontology, Medical College of Wisconsin, Milwaukee (Dr Kerwin); National Alliance for Caregiving, Bethesda, Maryland (Ms Hunt); and Alzheimer’s Foundation of America, New York, NY (Dr Hall).
Study participants: The authors comprise all the members of the Consensus Panel.
Potential conflicts of interest: Dr Grossberg has served as a consultant to Forest, Medivation, Novartis, and Pam Lab; has received grant/research support from Elan, Forest, National Institutes of Health, Novartis, and Wyeth; and has received honoraria from Eisai and Pfizer. Dr Christensen has served as a consultant to Bayer, Bristol-Myers Squibb, Designer Genes Inc, Eisai, GlaxoSmithKline, Janssen, Eli Lilly, Medivation, Myriad Genetics, Novartis, NPS, Pfizer, RiboMed, Solvay, and Wyeth-Ayerst; has received grant/research support from Abbott, Bristol-Myers Squibb, Designer Genes, Eccles Institute of Human Genetics, GlaxoSmithKline, Janssen, Myriad Genetics, Novartis, NPS, Organon, Pfizer, RiboMed, Solvay, and Wyeth-Ayerst; has received honoraria from Eisai and Pfizer; and has served on the speakers’ or advisory boards of Abbott, Bayer, Bristol-Myers Squibb, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Jazz, Medivation, Myriad, Novartis, Pfizer, Solvay, Upjohn, and Wyeth-Ayerst. Dr Griffith has served as a consultant to Eisai and Pfizer; has received grant/research support from ADCS; has received honoraria from Eisai, Novartis, and Pfizer; and has served on the speakers’ or advisory boards of Medivation and Novartis. Dr Kerwin has received honoraria from Eisai and Pfizer; has served on the speakers’ or advisory boards of Forest, Medivation, Novartis, and Pfizer; and has served as a medico-legal expert witness. Mr Hall and Ms Hunt have received honoraria from Eisai and Pfizer.
Funding/support: The roundtable was funded by a grant from Pfizer Inc and Eisai Inc; the authors received an honorarium for their participation. The panel was convened on March 14, 2008 in Orlando, Florida.
Acknowledgments: Editorial assistance was provided by Bill Kadish, MD, at PPSI (Stamford, Connecticut) and was funded by Eisai Inc and Pfizer Inc.
REFERENCES
1. Alzheimer’s Association. Alzheimer’s disease facts and figures 2007. http://www.alz.org/alzheimers_disease_publications_reports.asp. Accessed June 27, 2008.
2. Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review).Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1154-1166. PubMed
3. Geldmacher DS, Frolich L, Doody RS, et al. Realistic expectations for treatment success in Alzheimer’s disease. J Nutr Health Aging. 2006;10(5):417-429. PubMed
4. Lecouturier J, Bamford C, Hughes JC, et al. Appropriate disclosure of a diagnosis of dementia: identifying the key behaviours of ‘ best practice.’ BMC Health Serv Res. 2008;8(1):95. PubMed doi:10.1186/1472-6963-8-95
5. Ouimet MA, Dendukuri N, Dion D, et al. Disclosure of Alzheimer’s disease: senior citizens’ opinions. Can Fam Physician. 2004;50:1671-1677. PubMed
6. Boise L, Camicioli R, Morgan DL, et al. Diagnosing dementia: perspectives of primary care physicians. Gerontologist. 1999;39(4):457-464. PubMed
7. Alzheimer’s Foundation of America. I CAN: investigating caregivers’ attitudes and needs. http://www2f.biglobe.ne.jp/~boke/ican2996.pdf. Accessed March 5, 2009.
8. Role in diagnosis of Alzheimer’s disease patients shifts from neurologists to primary care physicians and psychiatrists in long-term care market. Medical News Today. http://www.medicalnewstoday.com/articles/85548.php. Accessed October 29, 2008.
9. Alzheimer’s Association. Alzheimer’s disease study: communication gaps between primary care physicians and caregivers. Alzheimer’s Association. http://www.alz.org/national/documents/ report_communicationgap.pdf. Accessed June 27, 2008.
10. Gattman R, Seleski M, eds. Diagnosis, Management and Treatment of Dementia: a Practical Guide for Physicians. Chicago, IL: American Medical Association; 1999.
11. Carter RE, Rose DA, Palesch YY, et al. Alzheimer’s disease in the family practice setting: assessment of a screening tool. Prim Care Companion J Clin Psychiatry. 2004;6(6):234-238. PubMed doi:10.4088/PCC.v06n0603
12. Jobst KA, Barnetson LP, Shepstone BJ. Accurate prediction of histologically confirmed Alzheimer’s disease and the differential diagnosis of dementia: the use of NINCDS-ADRDA and DSM-III-R criteria, SPECT, X-ray CT, and Apo E4 in medial temporal lobe dementias. Oxford Project to Investigate Memory and Aging. Int Psychogeriatr. 1998;10(3):271-302. PubMed doi:10.1017/S1041610298005389
13. Mok W, Chow TW, Zheng L, et al. Clinicopathological concordance of dementia diagnoses by community versus tertiary care clinicians. Am J Alzheimers Dis Other Demen. 2004;19(3):161-165. PubMed doi:10.1177/153331750401900309
14. Holmes SB, Adler D. Dementia care: critical interactions among primary care physicians, patients and caregivers. Prim Care. 2005;32(3):671-682, vi [vi]. PubMed
15. Wald C, Fahy M, Walker Z, et al. What to tell dementia caregivers: the rule of threes. Int J Geriatr Psychiatry. 2003;18(4):313-317. PubMed doi:10.1002/gps.828
16. Turnbull Q, Wolf AM, Holroyd S. Attitudes of elderly subjects toward “truth telling” for the diagnosis of Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2003;16(2):90-93. PubMed doi:10.1177/0891988703016002005
17. Elson P. Do older adults presenting with memory complaints wish to be told if later diagnosed with Alzheimer’s disease? Int J Geriatr Psychiatry. 2006;21(5):419-425. PubMed doi:10.1002/gps.1485
18. Burgener SC, Buettner L, Coen Buckwalter K, et al. Evidence supporting nutritional interventions for persons in early stage Alzheimer’s disease (AD). J Nutr Health Aging. 2008;12(1):18-21. PubMed doi:10.1007/BF02982159
19. Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55(2):158-165. PubMed doi:10.1111/j.1532-5415.2007.01035.x
20. Feldman H, Gauthier S, Hecker J, et al. Donepezil MSAD Study Investigators Group. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer’s disease and the effect on caregiver burden. J Am Geriatr Soc. 2003;51(6):737-744. PubMed doi:10.1046/j.1365-2389.2003.51260.x
21. Wimo A, Winblad B, Shah SN, et al. Impact of donepezil treatment for Alzheimer’s disease on caregiver time. Curr Med Res Opin. 2004;20(8):1221-1225. PubMed doi:10.1185/030079902125004349
22. Cummings JL, Schneider L, Tariot PN, et al. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. Am J Psychiatry. 2004;161(3):532-538. PubMed doi:10.1176/appi.ajp.161.3.532
23. Beusterien KM, Thomas SK, Gause D, et al. Impact of rivastigmine use on the risk of nursing home placement in a US sample. CNS Drugs. 2004;18(15):1143-1148. PubMed doi:10.2165/00023210-200418150-00008
24. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51(7):937-944. PubMed doi:10.1046/j.1365-2389.2003.51306.x
25. Pinquart M, Sörensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006;18(4):577-595. PubMed doi:10.1017/S1041610206003462
26. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer disease: a randomized controlled trial. JAMA. 1996;276(21):1725-1731. PubMed doi:10.1001/jama.276.21.1725
27. Weimer DL, Sager MA. Early identification and treatment of Alzheimer’s disease: social and fiscal outcomes. Alzheimers Dement. 2009;5(3):215-226. PubMed doi:10.1016/j.jalz.2009.01.028
28. Covinsky KE, Newcomer R, Fox P, et al. Patient and caregiver characteristics associated with depression in caregivers of patients with dementia. J Gen Intern Med. 2003;18(12):1006-1014. PubMed doi:10.1111/j.1525-1497.2003.30103.x
29. Markowitz JS, Gutterman EM, Sadik K, et al. Health-related quality of life for caregivers of patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17(4):209-214. PubMed doi:10.1097/00002093-200310000-00003
30. Mills PJ, Yu H, Ziegler MG, et al. Vulnerable caregivers of patients with Alzheimer’s disease have a deficit in circulating CD62L-T lymphocytes. Psychosom Med. 1999;61(2):168-174. PubMed
31. American Medical Association. Caregiver self-assessment. AMA Web site. http://www.ama-assn.org/ama/pub/physician-resources/ public-health/promoting-healthy-lifestyles/geriatric-health/ caregiver-health/caregiver-self-assessment.shtml. Accessed March 5, 2009.
32. Belle SH, Burgio L, Burns R, et al. Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II Investigators. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med. 2006;145(10):727-738. PubMed
33. Shim YS, Yang DW. Depression as prognostic factor: 6 months follow-up in a geriatric institution. Arch Gerontol Geriatr. 2006;43(2):277-283. PubMed doi:10.1016/j.archger.2005.11.002
34. Mace NL, Rabins PV. The 36-Hour Day: a Family Guide to Caring for People With Alzheimer Disease, Other Dementias, and Memory Loss in Later Life. 4th ed. Baltimore, MD: Johns Hopkins University Press; 2006.
35. Alwan M, Mack D, Dalal S, et al. Impact of passive in-home health status monitoring technology in home health: outcome pilot. Presented at: Proceeding of the 1st Distributed Diagnosis and Home Healthcare (D2H2) Conference; Arlington, VA; April 2-4, 2006.
36. Agency for Healthcare Research and Quality. Research in action: advance care planning: preferences for care at the end of life. March 2003. http://www.ahrq.gov/research/endliferia/endria.pdf. Accessed March 3, 2009.
37. Alzheimer’s Association CareFinder. http://www.alz.org/carefinder/index.asp. Accessed March 3, 2009.