Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Memory Disorders Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows.
BANNER ALZHEIMER’ S INSTITUTE
The Banner Alzheimer’s Institute located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2012;14(4):doi:10.4088/PCC.12alz01431
© Copyright 2012 Physicians Postgraduate Press, Inc.
Received: June 27, 2012; accepted June 27, 2012.
Published online: August 30, 2012.
AUTHORS
Roy Yaari, MD, MAS, a neurologist, is associate director of the Memory Disorders Clinic of Banner Alzheimer’s Institute and a clinical professor of neurology at the College of Medicine, University of Arizona, Tucson.
Adam S. Fleisher, MD, MAS, is associate director of Brain Imaging at the Banner Alzheimer’s Institute, a neurologist at the Institute’s Memory Disorders Clinic, and an associate professor in the Department of Neurosciences at the University of California, San Diego.
James D. Seward, PhD, ABPP, is a clinical neuropsychologist at Banner Alzheimer’s Institute.
Anna D. Burke, MD, is a geriatric psychiatrist and dementia specialist at the Memory Disorders Clinic of Banner Alzheimer’s Institute.
Helle Brand, PA, is a physician assistant at the Memory Disorders Clinic of Banner Alzheimer’s Institute.
Pierre N. Tariot, MD, a geriatric psychiatrist, is director of Banner Alzheimer’s Institute and a research professor of psychiatry at the College of Medicine, University of Arizona, Tucson.
Corresponding author: Roy Yaari, MD, MAS, Banner Alzheimer’s Institute, 901 E. Willetta St, Phoenix, AZ 85006 ([email protected]).
CME Background
Original material is selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME activities on a variety of topics from volume to volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute Staff. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the material and go to PrimaryCareCompanion.com to complete the Posttest and Evaluation online.
CME Objective
After studying this case, you should be able to:
- Identify patients with mild cognitive impairment and be aware of new tests that assess the deposition of amyloid-β plaques
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Date of Original Release/Review
This educational activity is eligible for AMA PRA Category 1 Creditâ„¢ through August 31, 2015. The latest review of this material was July 2012.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for AstraZeneca, Pfizer, Takeda, and Trovis; and has been a member of the speakers/advisory boards for Forest and Merck. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
CASE 1
History of Presenting Illness
Mr A, a 70-year-old man with a strong family history of Alzheimer’s disease, presented to the Memory Disorders Clinic at the Banner Alzheimer’s Institute with his wife for a cognitive evaluation. Clinical history was obtained from both the patient and his wife. Mr A noted that 6 years prior to his initial visit to the Banner Alzheimer’s Institute, he began developing word-finding difficulties and misplacing items. His wife first noted subtle word-finding difficulties 2 years prior to this visit. Mr A stated that this is the first year that he is choosing not to do the taxes, as it is frustrating for him. Although he manages the other finances without difficulty, he has asked his wife to be more involved.
Mr A has had some navigational problems while driving and finding his way home from places but has never become lost. Mr A stated that he sometimes gets “turned around” and needs assistance. His wife has begun prompting him with directions and has taken over more of the driving. He is asking that she drive more. Mr A recently accidentally hit the median divider in a road causing damage to his tires, but he continues to drive. Neither the patient nor his wife reported significant driving safety concerns.
Mr A needs some reminders for appointments and sometimes repeats himself. He stated that previously he had a “stellar” memory and now is slowly “losing it.” Mr A feels that he is not as cognitively organized. When giving public speeches, which he used to do from memory, Mr A now finds himself reading more from notes. He generally is oriented to date and place. He is independent with medications and shopping. There are no problems with appliances, but Mr A is cooking less than he used to for unknown reasons. All personal activities of daily life are intact. Mr A is not overtly depressed or tearful, but his wife notes that he is quicker to anger than he has been in the past. There is irritability when he is corrected.
Past Medical History
Mr A has a history of a right knee replacement; cancer of the bladder in May 2006, which is in remission; and hypertension. He has venous insufficiency with mild lower extremity edema. Mr A also had “skin cancer” removed recently.
Medications
Mr A’s only medication is valsartan.
Allergies
Mr A has no known drug allergies.
Social History
Mr A has a master’s degree. He worked as an educator for many years and retired in 2004. He currently lives with his wife and does not drink alcohol or use tobacco.
Family History
Mr A’s father, 2 paternal aunts, and 1 paternal uncle were diagnosed with Alzheimer’s disease. He had 1 maternal aunt with Alzheimer’s disease. Polycystic kidney disease runs in his mother’s family.
Review of Systems
Review of systems was positive for lower extremity edema and weight gain. There was no report of gait/balance changes or urinary incontinence.
Physical Examination
Mr A’s general physical examination was unremarkable except for the possibility of very mild bilateral cataracts and bilateral lower extremity edema.
Neurologic Examination
The neurologic examination was unremarkable except for subtle broken smooth pursuits and a positive snout reflex.
Smooth pursuit can be tested by asking the patient to track a small moving target at a distance of about 1 m, while keeping his/her head stationary. Both horizontal and vertical smooth pursuit should be assessed. The target should be moved at a slow uniform speed, and the pursuit eye movements are observed to determine whether they are smooth or broken up by catch-up saccades or a fast movement of the eye. Because smooth pursuit requires the coordination of many brain regions, this is a nonspecific finding, but could be indicative of cerebral degeneration. Sudo et al (2010) reported that impaired smooth pursuit can be indicative of impaired intellectual and frontal lobe function and can be regarded as a primitive reflex (frontal release sign).
Different dementias may be associated with various physical examination findings. However, most often the physical examination is normal in the early stages. Some subtle general findings can include frontal release signs such as a positive snout, glabellar, or palmomental reflex (Links et al, 2010).
- The DSM-IV defines dementia as multiple cognitive deficits that include memory impairment and at least 1 of the following cognitive disturbances: aphasia, apraxia, agnosia, or a disturbance in executive functioning. The cognitive deficits must be sufficiently severe to cause impairment in social or occupational functioning and must represent a decline from a previously higher level of functioning. A diagnosis of dementia should not be made if the cognitive deficits occur exclusively during the course of a delirium (American Psychiatric Association, 2000).
Mild cognitive impairment (MCI) refers to cognitive impairment that does not meet the criteria for normal aging or dementia because the cognitive impairment does not impair activities of daily living. Several criteria for, and subtypes of, MCI have been proposed (Voisin et al, 2003). Originally, MCI emphasized memory impairment as a precursor state for Alzheimer’s disease (Petersen et al, 1999). It then became apparent that MCI is a heterogeneous entity that affects other cognitive domains and includes the prodromal stages of other dementias. The diagnostic criteria for MCI are not exact and require subjectivity in determining whether a cognitive impairment is present or what constitutes impairment in activities of daily living.
Based on the clinical history alone do you think?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. He meets criteria for dementia | 25% |
| B. He is most likely to be cognitively normal | 0% |
| C. He possibly has MCI | 75% |
| D. His cognitive issues are most likely due to an underlying psychiatric disorder | 0% |
The majority of conference attendees felt that Mr A has MCI, in that he has cognitive impairment that does not impair activities of daily living. Those who felt that he meets criteria for dementia pointed out the navigational problems while driving, cessation of doing the taxes himself this year, and a decrease in his cooking. None of those present felt that Mr A has normal cognition for his age or an underlying psychiatric disorder.
A MMSE score generally correlates with disease severity. Scores ≤ 9 points can indicate severe dementia, scores between 10-20 points can indicate moderate dementia, and a score > 20 can indicate mild dementia (Mungas, 1991). Although MMSE scores must be interpreted in light of both the patient’s age and education, education is the primary demographic factor that affects scores. Therefore, whereas a cutoff of ≤ 23 is widely used in distinguishing between normal and abnormal performance, this cutoff may have less predictive ability in poorly educated individuals (Folstein et al, 1975).
Based on the clinical history alone, what would you expect Mr A’s Mini-Mental State Examination (MMSE) score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 28-30 | 6% |
| B. 25-27 | 81% |
| C. 22-24 | 13% |
| D. 19-21 | 0% |
| E. 15-18 | 0% |
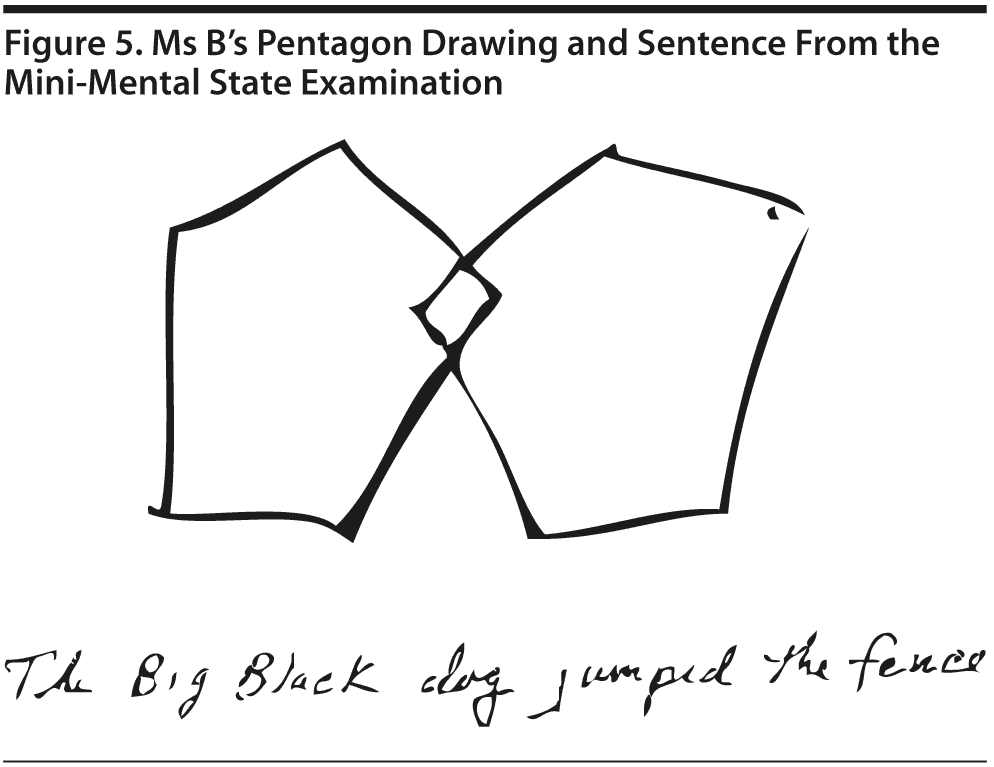
All conference attendees predicted a relatively high MMSE score of > 24, which is consistent with MCI or normal cognition. Indeed, Mr A scored a perfect 30 points on the MMSE. Figure 1 shows Mr A’s pentagon drawing and sentence from the MMSE.
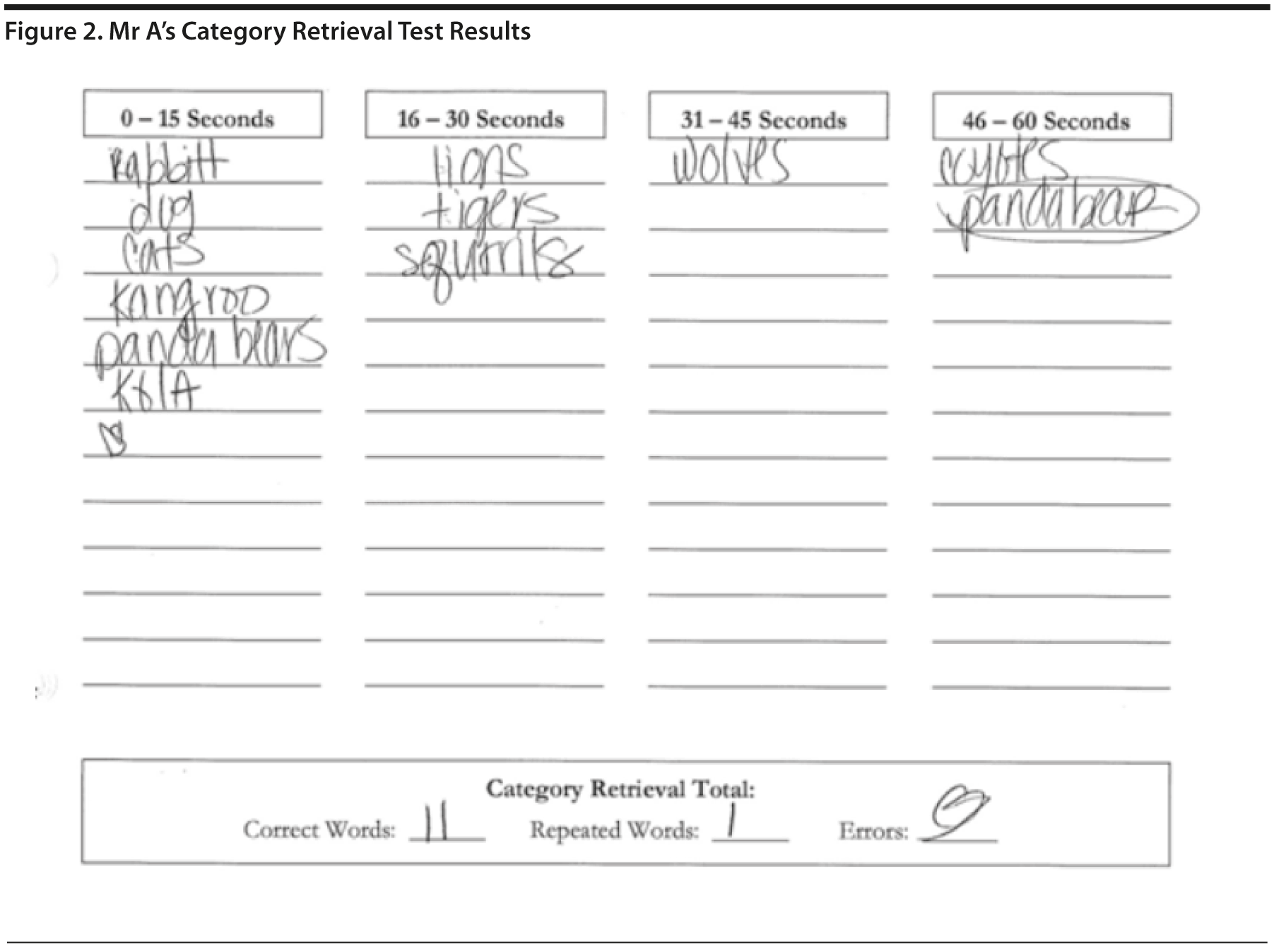
In the Category Retrieval Test, the examiner asks the patient to name as many animals as possible in 1 minute. The examiner records the responses. Performance on this measure is influenced by age; unimpaired people in their 60s should name about 18 animals, whereas people in their 80s should name about 15 (Mitrushina et al, 2005). There is no hard-and-fast cutoff for impairment. However, patients who name 4 or more animals less than expected raise concerns. Note that bilingual individuals are at a disadvantage on this and other measures of verbal fluency (Gollan et al, 2002).
Based on the information known so far, what would you expect Mr A’s Category Retrieval Test Score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 0-5 | 0% |
| B. 6-10 | 0% |
| C. 11-15 | 44% |
| D. 16-20 | 50% |
| E. 21-25 | 6% |
Mr A listed 11 animals, with 1 repetition. This result could indicate a mild impairment with language and/or executive functioning (Figure 2).
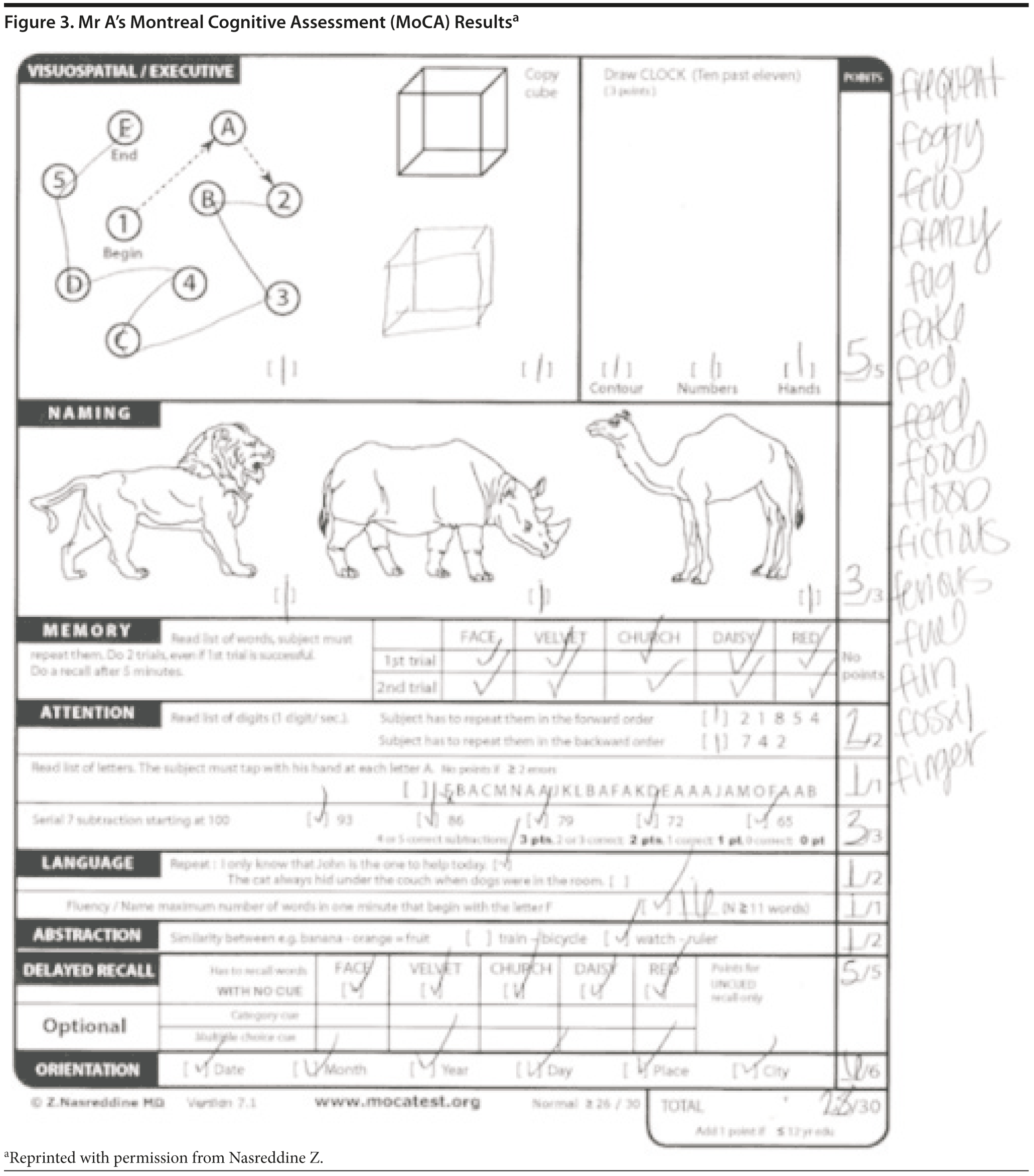
The MoCA is a 30-point test that assesses several cognitive domains. Because it is more challenging than the MMSE, the MoCA has greater sensitivity for MCI and early stages of dementia. With a cutoff score < 26, the sensitivity for detecting MCI (N = 94) was 90% and the specificity was 87% (Nasreddine et al, 2005). Research has demonstrated that MoCA scores are highly correlated with education. It is recommended that education be taken into account when interpreting MoCA performance, but there are no formal specific cutoff scores for lower education at this time (Johns et al, 2008). This test is available online at http://mocatest.org/.
Based on the information known so far, what would you expect Mr A’s MoCA score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 28-30 | 0% |
| B. 25-27 | 63% |
| C. 22-24 | 31% |
| D. 19-21 | 6% |
| E. 15-18 | 0% |
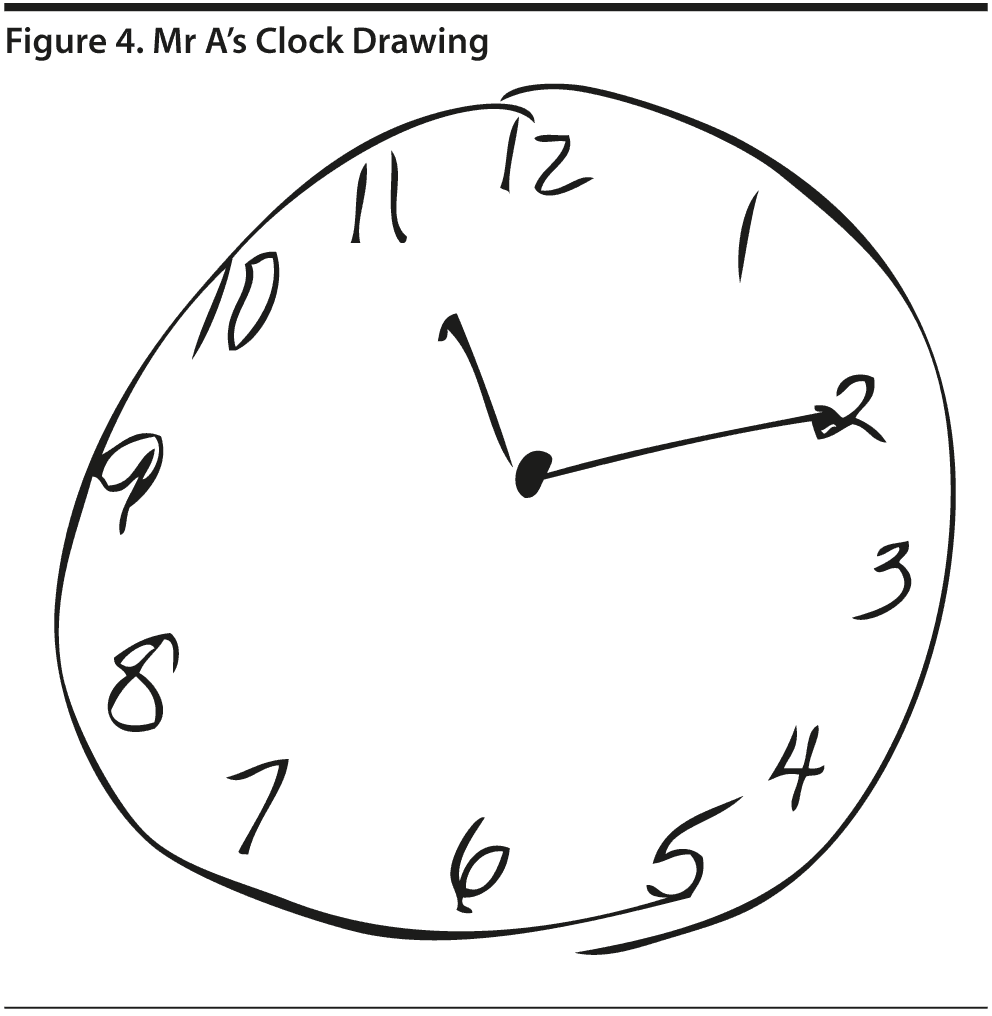
Given Mr A’s perfect MMSE score, most conference attendees predicted a relatively high MoCA score, but most predicted that the MoCA score would likely show cognitive impairment. Mr A scored 28 points, which is within the normal range. He missed 1 point on a language task and 1 point on abstraction. Figures 3 and 4 show Mr A’s MoCA results and clock drawing, respectively.
Laboratories/Radiology
Mr A had a recent complete blood count (CBC) and comprehensive metabolic panel (CMP), both of which were unremarkable.
What further laboratory, radiology, or other tests would you order at this time?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Structural brain scan, TSH, vitamin B12, and rapid plasma reagin | 0% |
| B. Structural brain scan, TSH, vitamin B12, neuropsychological testing | 88% |
| C. Neuropsychological testing | 12% |
| D. TSH and vitamin B12 | 0% |
| E. Structural brain scan | 0% |
| F. Structural brain scan and neuropsychological testing | 0% |
Most conference attendees believed that Mr A had MCI and thus opted to order a brain magnetic resonance image (MRI) and laboratory studies to complete the cognitive workup, which would include vitamin B12 and TSH levels in this case (since a recent CBC and CMP were completed prior). Guidelines for a routine dementia workup include a CBC, CMP, vitamin B12, TSH, and structural brain imaging with either an MRI or computed tomography (Knopman et al, 2001). All conference attendees voiced the opinion that formal neuropsychological testing should be ordered to help identify and clarify any pattern of cognitive strengths and weaknesses.
The treating physician, at this point, ordered only the neuropsychological testing to help establish whether Mr A has normal cognition versus MCI. Should the neuropsychological testing show cognitive impairment, then the MRI, TSH, and vitamin B12 would be ordered.
Should a cholinesterase inhibitor be started at this time?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Yes | 0% |
| B. No | 100% |
All attendees at the case conference did not think a cholinesterase inhibitor was indicated at this time. Some attendees wished to wait for the neuropsychological test results to help confirm whether Mr A has MCI, and if MCI is confirmed, to then discuss with Mr A whether a cholinesterase inhibitor should be started as an off-label agent.
Cholinesterase inhibitors are not US Food and Drug (FDA) approved for treatment of MCI. Several studies have investigated cholinesterase inhibitors in the treatment of MCI but did not show significant evidence to support their use (Doody et al, 2009; Feldman et al, 2007; Mayor, 2005; Petersen et al, 2005; Salloway et al, 2004). A donepezil study showed a lower rate of progression to Alzheimer’s disease during the first year, but this finding was not sustained over the 3-year duration (Petersen et al, 2005). Despite the paucity of data, cholinesterase inhibitors are often initiated in MCI in clinical practice. In the case of Mr A, the treating physician, after discussing the data with the patient, did not initiate a cholinesterase inhibitor. The treating physician and Mr A opted to discuss use of a cholinesterase inhibitor after the neuropsychological testing.
THE TREATING PHYSICIAN’ S IMPRESSION AND PLAN
Impression
Mr A is a 70-year-old man with a significant family history of Alzheimer’s disease who presents for a cognitive evaluation. The clinical history is consistent with MCI. Most cognitive screening tests today were within the normal range. Further testing is indicated to assess whether the patient has normal cognition for age versus MCI.
Plan
- Order neuropsychological testing to help clarify the pattern of cognitive strengths and weaknesses, to assist with the differential diagnosis, to serve as a basis for rehabilitative suggestions, and to provide a basis against which to measure future changes.
- Monitor the patient’s mood (short temper and irritability).
- Initiate a full cognitive workup including blood tests and brain imaging and discuss potential use of a cholinesterase inhibitor and research options if the neuropsychological testing indicates cognitive impairment.
- Follow up with the patient after the neuropsychological testing has been completed.
NEUROPSYCHOLOGICAL TESTING
Results
On neuropsychological tests assessing specific cognitive domains, Mr A displayed a pattern on memory testing that revealed difficulty with the learning and immediate recall of verbal material, with a generally intact ability to retain what he had learned. Mr A displayed a relative weakness in his word-finding abilities. Verbal fluency was essentially within normal limits. He also displayed intact executive functioning, although his abilities in this area may be a drop from higher premorbid levels. Mr A’s visual perception was intact.
Diagnostically, the results of this evaluation are consistent with MCI with subtle memory and language problems. However, the pattern of test findings is more typical of a subcortical than a cortical etiology.
Discussion
On neuropsychological testing, patients with subcortical dementia tend to have greater difficulties with tasks assessing attentional, visuospatial, and constructional abilities, with relatively well-preserved memory and language skills; the reverse pattern is seen in cortical dementias. However, patterns of neuropsychological test findings are not robust enough to make this distinction in isolation, with additional clinical input needed (Randolph et al, 1998 and Beatty et al, 2003).
Although the distinction is not clear-cut, cortical dementias are disease processes that primarily affect the cerebral cortex, the outer covering of the brain. Alzheimer’s disease is the best known example of a cortical dementia. Subcortical dementias primarily affect underlying, deeper brain structures. An example is seen in dementias associated with Parkinson’s disease and related conditions, wherein the pathology affects the basal ganglia and nigrostriatal system, which are deeper in the brain (Attix and Welsh-Bohmer, 2006). Lezak et al (2004) described the following cognitive profile as being typical of subcortical dementias: “. . . cognitive slowing . . . with disturbances of attention and concentration, executive disabilities including impaired concept manipulation and use of strategies, visuospatial abnormalities, and a memory disorder that affects retrieval more than learning. . . .”(p224) In contrast, in addition to difficulty learning new information, the classic symptoms of cortical involvement include aphasia, agnosia, and apraxia. In the case of Mr A, his relative strength on measures of verbal fluency and delayed recall and relative weakness on a measure of construction were suggestive of a subcortical process.
FOLLOW-UP VISIT With THE Treating Physician
Impression
Mr A is a 70-year-old man with a significant family history of Alzheimer’s disease who has been diagnosed with MCI. Neuropsychological testing corroborates the presence of MCI mainly affecting memory and language. Further workup will be initiated to help determine the etiology.
Plan
- Order an MRI of the brain to exclude intracranial pathology, as well as a vitamin B12 and a TSH to exclude low vitamin B12 levels or thyroid disease as an etiology of his cognitive changes.
- Continue to monitor his mood. At this time, pharmaceuticals are not indicated.
- Discussed the possibility of participating in an amyloid imaging research study. The patient is very interested and met with the study coordinator today.
- Discussed the possibility of initiating a cholinesterase inhibitor and decided to hold off at this time and to have this discussion after the cognitive workup has been completed.
- Follow up in the clinic after the amyloid scan. The MRI, vitamin B12, and TSH will be deferred until after the amyloid scan results have been reviewed with the patient.
In April 2012, the FDA approved florbetapir F-18 amyloid positron emission tomography (PET) imaging in patients with cognitive impairment who are being evaluated for Alzheimer’s disease and other causes of cognitive decline. Florbetapir F-18 is a radioactive diagnostic agent that binds to amyloid plaques, a hallmark characteristic of Alzheimer’s disease, and is detected using PET scan images of the brain. Although FDA approved, florbetapir F-18 PET was not yet readily available for clinical use at the time of this case. A florbetapir F-18 PET scan was obtained for Mr A through a research study protocol.
Based on the information attained thus far, what would you expect the result of the amyloid brain scan to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Amyloid positive | 77% |
| B. Amyloid negative | 23% |
The majority of conference attendees predicted that the amyloid imaging study would be positive for amyloid, indicating a higher likelihood of Alzheimer’s disease pathology. A negative florbetapir F-18 PET scan indicates little to no fibrillar amyloid deposition, which is not consistent with a neuropathological diagnosis of Alzheimer’s disease (Hyman et al, 2012 and McKhann et al, 2011). A positive florbetapir F-18 PET scan indicates the presence of moderate to frequent fibrillar amyloid. This amount of fibrillar amyloid is seen in patients with Alzheimer’s disease, but should be interpreted carefully, as amyloid plaque may also be present in patients with other types of neurologic conditions and in older people with normal cognition (Thies and Bleiler, 2012 and Sperling et al, 2011). Thus, the florbetapir F-18 PET scan is intended to be used as an adjunct to other diagnostic evaluations to rule out amyloid pathology associated with Alzheimer’s disease.
FOLLOW-UP VISIT
Mr A’s florbetapir F-18 PET scan was negative, which is not consistent with Alzheimer’s disease as the underlying pathology of his MCI. Mr A’s TSH and vitamin B12 levels were normal. The MRI results were unremarkable except for enlarged ventricles that could be consistent with normal pressure hydrocephalus (NPH). The MRI was re-reviewed, and multiple physicians agreed that the MRI result looks to be consistent with NPH.
Normal pressure hydrocephalus is a condition of communicating hydrocephalus (as opposed to an obstructive or noncommunicating hydrocephalus) of pathologically enlarged brain ventricles that leads to a clinical triad of dementia, gait disturbance, and urinary incontinence. This condition is potentially reversible by placement of a ventriculoperitoneal shunt, although there is very little consensus regarding the diagnosis of NPH and the selection of patients for shunt placement (Krauss and Halve, 2004).
Although the clinical history of Mr A is not consistent with NPH (no gait disturbance or urinary incontinence), he was referred to a NPH specialist for further evaluation. Mr A’s physician does not believe that he has NPH, and the etiology of his MCI remains uncertain. Mr A will be followed clinically every 4 to 6 months. No further testing is planned at this time. A sleep study was recommended, but since neither Mr A nor his wife report sleep disturbances, it was declined. A repeat MRI will be performed if there are clinical changes in the future.
CASE 2
History of Presenting Illness
Ms B is a 75-year-old woman who presented to the Memory Disorders Clinic at the Banner Alzheimer’s Institute with her husband and daughter, both of whom supplemented the clinical history. She was first noted to have cognitive changes about 2 years prior. She was “a little bit” repetitive in telephone conversations with her children. About 1 year ago, Ms B’s husband had complicated medical issues, creating a great deal of stress on the patient. Although his medical issues resolved, at that time, Ms B was more repetitive and depressed. Family had attributed her changes to the stressful situation. About 3 months ago, the daughter found her mother to be increasingly repetitive. She was beginning to have difficulty recalling names of grandchildren and other relatives. She had a tendency to write things down more than she used to and would ask her husband what day of the week it was. Ms B is able to track appointments on the calendar. She does misplace items and does have some word-finding difficulties. She continues to read in a book club and is going to lead the next book discussion. Ms B has been taking more notes. She reads slower than she used to. Her activities of daily life are intact. Ms B’s husband takes care of the finances, but she is able to handle her own cash, track her own medications, shop, and adapt to a new computer. She continues to drive without any difficulty. Ms B’s family has no concerns regarding her driving at the current time. The patient concurs with the observations reported by her husband and daughter; she is aware of and concerned by her short-term memory changes.
Past Medical History
Ms B has a history of glaucoma of the left eye with some loss of vision. She broke her right radius after a mechanical fall in 2008. Ms B is treated for leg cramps, osteopenia, and migraine. She has had a remote appendectomy, tonsillectomy, and hysterectomy.
Medications
Ms B’s current medications include raloxifene, travoprost eye drops, aspirin, and several as-needed medications including naproxen, cetirizine, and sumatriptan. She also takes calcium, vitamin D, omega-3, vitamin B12, magnesium, and potassium gluconates.
Allergies
Ms B has had adverse reactions to alendronate, risedronate, and diphenhydramine.
Social History
Ms B has 17 years of education and worked as a nurse and administrator. She lives with her husband in Arizona. There is no significant history of alcohol or tobacco use.
Family History
Ms B’s father had “dementia.”
Review of Systems
Review of systems was positive for muscle cramps and infrequent constipation. There was no report of gait/balance changes or urinary incontinence.
Physical Examination
Ms B’s general physical examination was unremarkable.
Neurologic Examination
Ms B’s neurologic examination was unremarkable except for a very mild bilateral upper extremity essential tremor, broken smooth pursuits, glabellar, snout, and right palmomental reflex.
Smooth pursuit can be tested by asking the patient to track a small moving target at a distance of about 1 m, while keeping his/her head stationary. Both horizontal and vertical smooth pursuit should be assessed. The target should be moved at a slow uniform speed, and the pursuit eye movements are observed to determine whether they are smooth or broken up by catch-up saccades or a fast movement of the eye. Because smooth pursuit requires the coordination of many brain regions, this is a nonspecific finding, but could be indicative of cerebral degeneration. Sudo et al (2010) reported that impaired smooth pursuit can be indicative of impaired intellectual and frontal lobe function and can be regarded as a primitive reflex (frontal release sign).
Different dementias may be associated with various physical examination findings. However, most often the physical examination is normal in the early stages. Some subtle general findings can include frontal release signs such as a positive snout, glabellar, or palmomental reflex (Links et al, 2010).
The DSM-IV defines dementia as multiple cognitive deficits that include memory impairment and at least 1 of the following cognitive disturbances: aphasia, apraxia, agnosia, or a disturbance in executive functioning. The cognitive deficits must be sufficiently severe to cause impairment in social or occupational functioning and must represent a decline from a previously higher level of functioning. A diagnosis of dementia should not be made if the cognitive deficits occur exclusively during the course of a delirium (American Psychiatric Association, 2000).
Mild cognitive impairment (MCI) refers to cognitive impairment that does not meet the criteria for normal aging or dementia because the cognitive impairment does not impair activities of daily living. Several criteria for, and subtypes of, MCI have been proposed (Voisin et al, 2003). Originally, MCI emphasized memory impairment as a precursor state for Alzheimer’s disease (Petersen et al, 1999). It then became apparent that MCI is a heterogeneous entity that affects other cognitive domains and includes the prodromal stages of other dementias. The diagnostic criteria for MCI are not exact and require subjectivity in determining whether a cognitive impairment is present or what constitutes impairment in activities of daily living.
Based on the clinical history alone do you think?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. She meets criteria for dementia | 0% |
| B. She is most likely to be cognitively normal | 0% |
| C. She possibly has MCI | 100% |
| D. Her cognitive issues are most likely due to an underlying psychiatric disorder | 0% |
Case conference attendees were unanimous in selecting MCI as the most likely diagnosis. All attendees believed that cognitive impairment was present but had not affected activities of daily living.
A MMSE score generally correlates with disease severity. Scores ≤ 9 points can indicate severe dementia, scores between 10-20 points can indicate moderate dementia, and a score > 20 can indicate mild dementia (Mungas, 1991). Although MMSE scores must be interpreted in light of both the patient’s age and education, education is the primary demographic factor that affects scores. Therefore, whereas a cutoff ≤ 23 is widely used in distinguishing between normal and abnormal performance, this cutoff may have less predictive ability in poorly educated individuals (Folstein et al, 1975).
Based on the clinical history alone, what would you expect Ms B’s MMSE score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 28-30 | 6% |
| B. 25-27 | 50% |
| C. 22-24 | 38% |
| D. 19-21 | 6% |
| E. 15-18 | 0% |
Most attendees predicted that Ms B would have a relatively high MMSE score, given that she most likely has MCI. However, many attendees voted for scores < 24, which would not be common in MCI. Generally, a MMSE score < 24 points suggests that dementia may be present. With a cutoff score of 24, the MMSE has a sensitivity of 87% and a specificity of 82% for determining dementia (Crum et al, 1993).
Ms B scored 27 points on the MMSE. She missed 1 point on comprehension and 2 points on delayed recall. Figure 5 shows Ms B’s pentagon drawing and sentence from the MMSE.
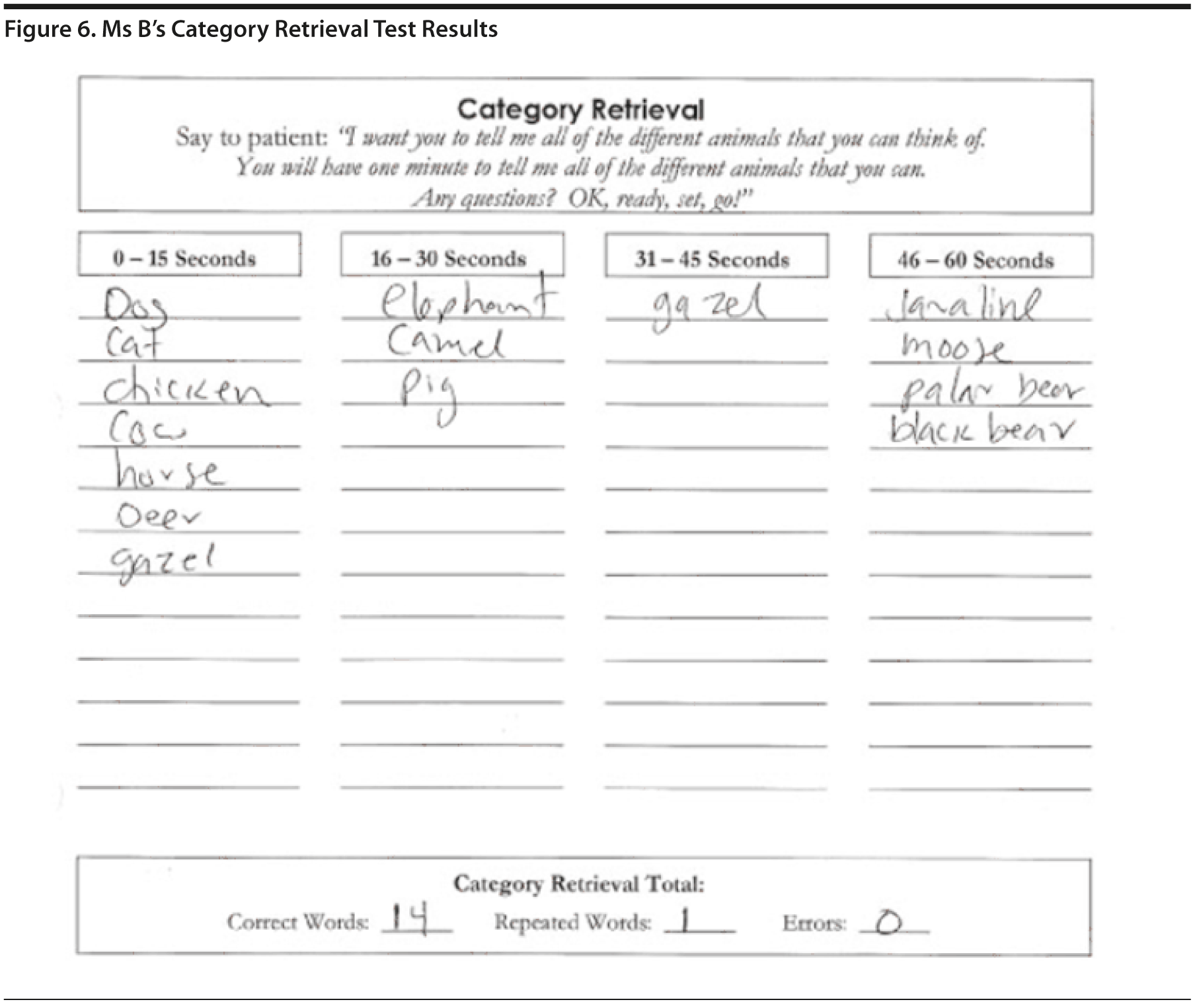
In the Category Retrieval Test, the examiner asks the patient to name as many animals as possible in 1 minute. The examiner records the responses. Performance on this measure is influenced by age; unimpaired people in their 60s should name about 18 animals, whereas people in their 80s should name about 15 (Mitrushina et al, 2005). There is no hard-and-fast cutoff for impairment. However, patients who name 4 or more animals less than expected raise concerns. Note that bilingual individuals are at a disadvantage on this and other measures of verbal fluency (Gollan et al, 2002).
Based on the information known so far, what would you expect Ms B’s Category Retrieval Test score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 0-5 | 0% |
| B. 6-10 | 44% |
| C. 11-15 | 56% |
| D. 16-20 | 0% |
| E. 21-25 | 0% |
Ms B listed 14 animals, with 1 repetition. This result could indicate a mild impairment with language (Figure 6).
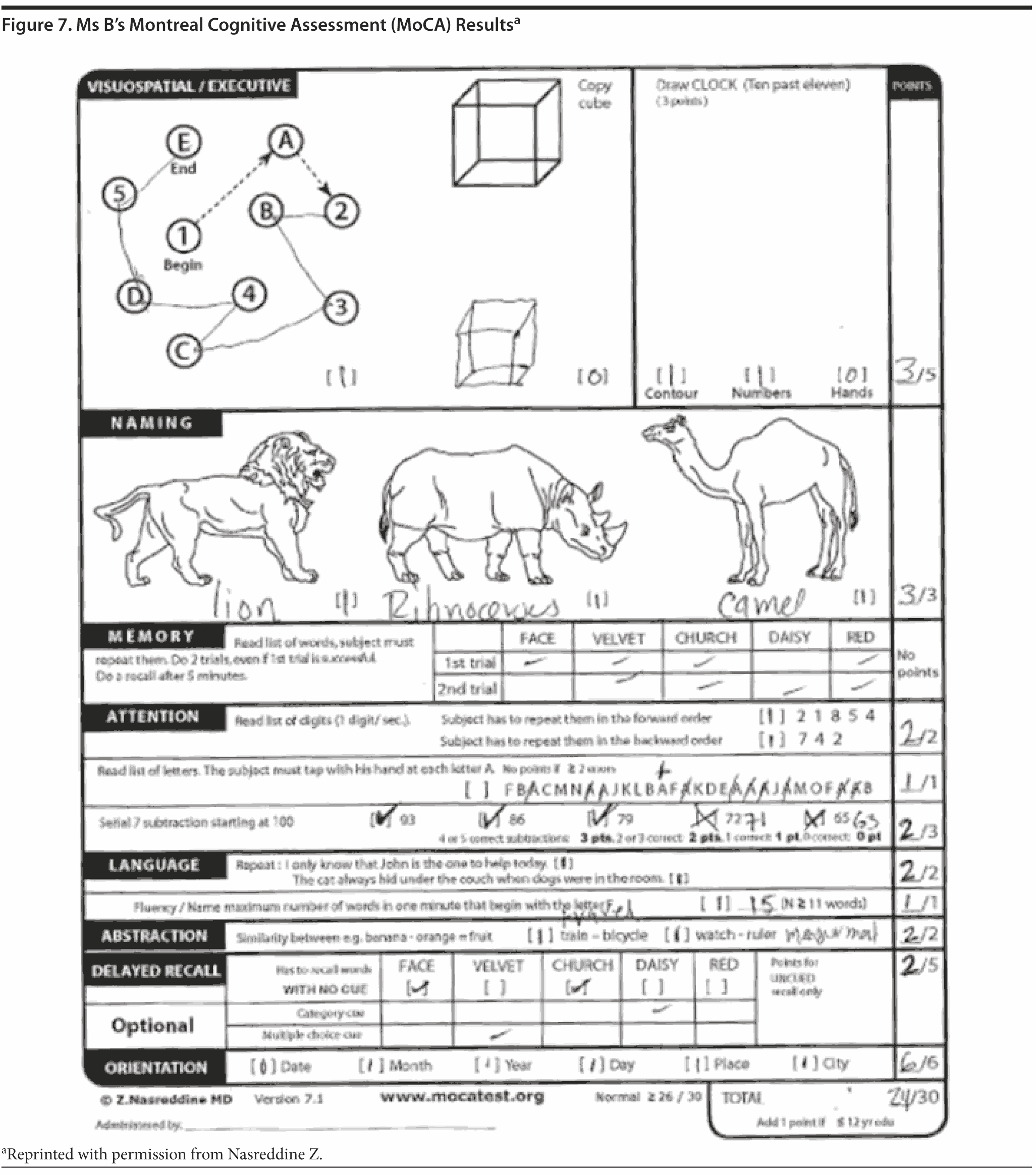
The MoCA is a 30-point test that assesses several cognitive domains. Because it is more challenging than the MMSE, the MoCA has greater sensitivity for MCI and early stages of dementia. With a cutoff score < 26, the sensitivity for detecting MCI (N = 94) was 90% and the specificity was 87% (Nasreddine et al, 2005). Research has demonstrated that MoCA scores are highly correlated with education. It is recommended that education be taken into account when interpreting MoCA performance, but there are no formal specific cutoff scores for lower education at this time (Johns et al, 2008). This test is available online at http://mocatest.org/.
Based on the information known so far, what would you expect Ms B’s MoCA score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 28-30 | 0% |
| B. 25-27 | 31% |
| C. 22-24 | 69% |
| D. 19-21 | 0% |
| E. 15-18 | 0% |
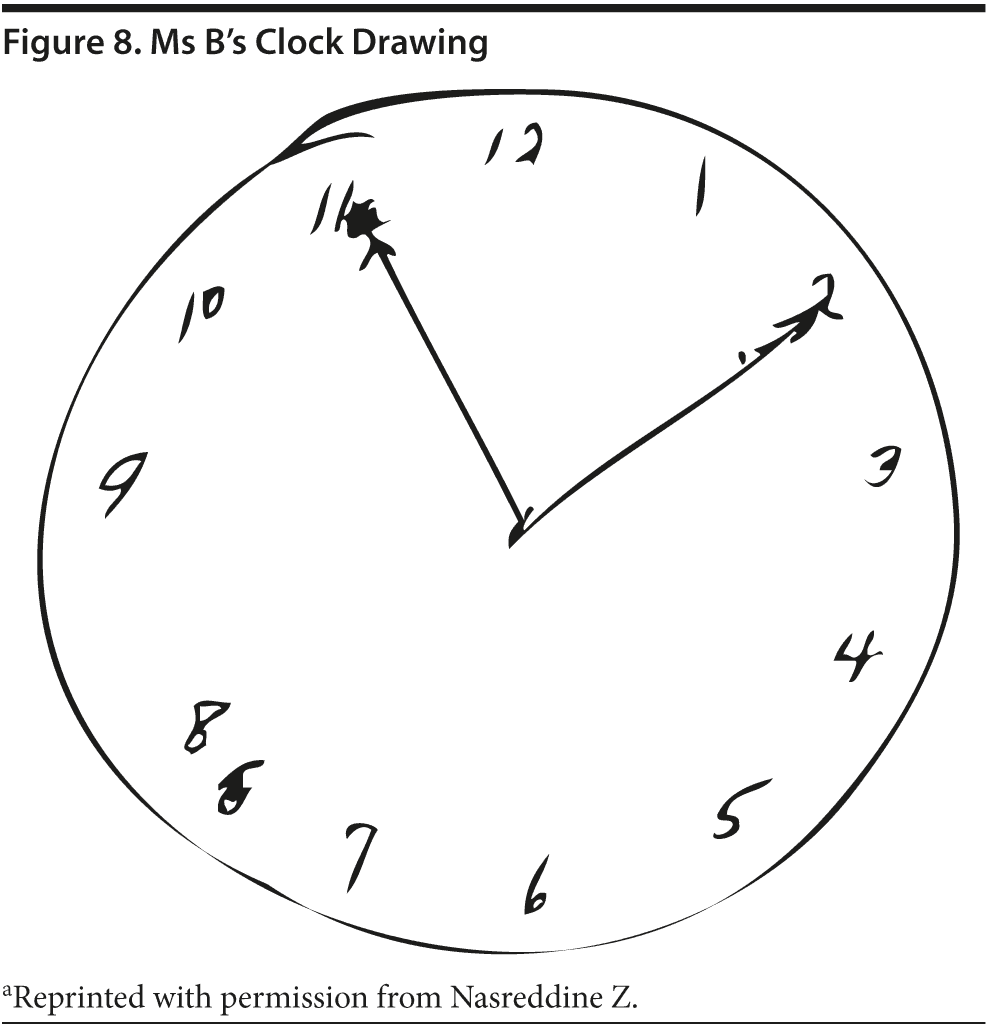
Given Ms B’s relatively high MMSE score, most conference attendees predicted a high MoCA score. Ms B scored 24 points, which is in the abnormal range. She missed 1 point on the cube copy (although it was very close to normal), 1 point on the clock drawing (the hands are equal length), 1 point on serial 7’s , and 3 points on delayed recall. Figures 7 and 8 show Ms B’s MoCA results and clock drawing, respectively.
LABORATORIES/RADIOLOGY
No laboratory or radiologic tests had been performed.
What further laboratory, radiology, or other tests would you order at this time?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Structural brain scan, TSH, vitamin B12, CBC, and CMP | 6% |
| B. Structural brain scan, TSH, vitamin B12, CBC, CMP, and neuropsychological testing | 88% |
| C. Neuropsychological testing | 6% |
| D. TSH and vitamin B12 | 0% |
| E. Structural brain scan | 0% |
| F. Structural brain scan and neuropsychological testing | 0% |
As in the prior case, most conference attendees believed that Ms B did have MCI and thus opted to order a brain MRI, laboratory studies, and neuropsychological testing. In this case, since no recent laboratory studies have been completed, a CBC, CMP, TSH, and vitamin B12 should be ordered. Guidelines for a routine dementia workup include a CBC, CMP, vitamin B12, TSH, and structural brain imaging with either an MRI or computed tomography (Knopman et al, 2001).
In contrast to the prior case, in which the treating physician was unclear whether Mr A met criteria for normal aging versus MCI, the treating physician was more confident of the MCI diagnosis in the case of Ms B and ordered an MRI, CMP, CBC, TSH, and vitamin B12. Additionally, neuropsychological testing was ordered.
Should a cholinesterase inhibitor be started at this time?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Yes | 6% |
| B. No | 94% |
Most attendees at the case conference did not think a cholinesterase inhibitor was indicated at this time. As in the prior case, attendees wished to wait for the neuropsychological test results to help confirm whether Ms B has MCI and then discuss whether to initiate a cholinesterase inhibitor.
As mentioned above, cholinesterase inhibitors are not FDA approved for treatment of MCI, and several studies have investigated cholinesterase inhibitors in the treatment of MCI, but these studies did not show significant evidence to support their use (Doody et al, 2009; Feldman et al, 2007; Mayor, 2005; Petersen et al, 2005; Salloway et al, 2004). The treating physician and Ms B opted to discuss use of a cholinesterase inhibitor after the neuropsychological testing.
THE TREATING PHYSICIAN’ S IMPRESSION AND PLAN
Impression
The patient is a 75-year-old woman who presents for a cognitive evaluation. The clinical history and the cognitive screening tests are consistent with MCI. Further testing will be initiated to help confirm this diagnosis.
Plan
- Order an MRI of the brain to rule out structural brain lesions; order a CBC, CMP, vitamin B12, and TSH to rule out potential reversible causes of dementia.
- Order formal neuropsychological testing to help confirm suspicion of MCI.
- Discussed the possibility of initiating a cholinesterase inhibitor at this time but agreed to pursue with the tests; once testing is complete, discuss again the issue of initiating a cholinesterase inhibitor.
- Discuss potential research options if the suspicion of MCI is confirmed, as the patient is very interested in clinical research.
- Follow up with the patient after the neuropsychological testing has been completed.
NEUROPSYCHOLOGICAL TESTING
Results
Results of this evaluation reveal that Ms B’s overall level of intellectual and cognitive functioning is generally within normal limits. However, she gave subtle indications of difficulty with auditory verbal memory and also received relatively low scores on measures of verbal fluency. These findings are in addition to low scores received on measures that had a visual component. The results of this evaluation, when considered in the context of the information contained in her chart, support the presence of a very mild amnestic MCI.
Discussion
Specifically, Ms B’s poor delayed free recall of a word list was concerning for the presence of a dementia prodrome; tests requiring the delayed free recall of new material appear to be the most sensitive to detect the early dementia processes (Smith and Rush, 2006).
3-MONTH FOLLOW-UP
Ms B and her husband report continued short-term memory issues, although not worse. Ms B has had an MRI of the brain, CBC, CMP, vitamin B12, and TSH, all of which were unremarkable.
Impression
Ms B is a 75-year-old woman with cognitive concerns corroborated by her husband. Formal neuropsychological testing was consistent with “very mild amnestic MCI.”
Plan
- Discussed in detail whether to initiate a cholinesterase inhibitor and agreed to hold off at this time.
- Discussed the possibility of participating in an amyloid imaging study; Ms B is very interested in this, and she met with the study coordinator.
Based on the information attained thus far, what would you expect the result of the amyloid brain scan to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Amyloid positive | 100% |
| B. Amyloid negative | 0% |
All conference attendees predicted that the amyloid imaging study would be positive for amyloid, indicating a higher likelihood of Alzheimer’s disease pathology. Ms B was positive for amyloid, which is consistent with a neuropathological diagnosis of Alzheimer’s disease (Hyman et al, 2012 and McKhann et al, 2011). As mentioned previously, this result should be interpreted carefully, as amyloid plaque may also be present in patients with other types of neurologic conditions and in older people with normal cognition (Thies and Bleiler, 2012 and Sperling et al, 2011).
The results of the amyloid imaging led the treating physician to conclude that Alzheimer’s disease pathology is the most likely etiology of this patient’s MCI. After discussion with Ms B, a cholinesterase inhibitor was initiated, although it is off-label for treatment of MCI. She and her family were also referred to the appropriate educational and family support programs, as well as to other clinical studies.
DISCUSSION
Until recently, amyloid plaques, one of the characteristic pathological features of Alzheimer’s disease, could not be visualized until autopsy (or biopsy). With the advent of amyloid PET imaging in 2002 using Pittsburgh compound B (PiB), researchers have been able to quantify brain amyloid through a minimally invasive technique. PiB, and similar compounds, are radioisotopes that are injected into a vein and bind to amyloid plaques in the brain. The radioisotope produces a positron signal that is detected using PET scan technology.
Up to this point, use of amyloid imaging has been restricted to research purposes to evaluate its usefulness as an Alzheimer’s disease biomarker, as well as to track efficacy of antiamyloid agents in clinical trials. Since PiB was developed, several other amyloid imaging agents have been developed and studied, and in April 2012, the FDA approved florbetapir as a diagnostic aid in patients with cognitive impairment who are being evaluated for Alzheimer’s disease or other causes of cognitive decline.
A negative scan indicates low levels or no fibrillar amyloid plaques are present, which is not consistent with Alzheimer’s disease. This result significantly reduces the likelihood that a patient’s cognitive impairment is due to Alzheimer’s disease. A positive scan indicates moderate to frequent amyloid plaques are present, which is consistent with a diagnosis of Alzheimer’s disease; however, high levels of amyloid may also be present in patients with other types of brain conditions as well as in cognitively normal elderly adults. Therefore, a positive scan does not necessarily establish a diagnosis of Alzheimer’s disease, but a negative scan strongly suggests that Alzheimer’s disease pathology is not present. In the case of Ms B, the amyloid scan suggests that the underlying pathology of this patient is due to Alzheimer’s disease. She was initiated on antidementia therapy, and the family was referred to appropriate education and counseling for adjustment to a chronic illness.
As illustrated in the 2 cases, amyloid brain imaging can be used in conjunction with other tests to provide additional information to assist with diagnosis. In the case of Mr A, the amyloid scan helped determine that the etiology of his MCI was not due to Alzheimer’s disease, and further workup was initiated. At the time of publication, the etiology of Mr A’s MCI remains unknown. It was a significant relief for Mr A to learn that amyloid is not present at this time, given his strong family history of Alzheimer’s disease.
As mentioned previously, florbetapir F-18 PET imaging was FDA approved in April 2012 and became clinically available in June of 2012. Given the paucity of experience of use of amyloid imaging in the clinical setting, it is not yet known how this tool will be utilized. As more experience is gained with this new technology, more will be learned about whether it is an appropriate tool to be used in the primary care setting to aid with diagnosis in people with cognitive concerns or if it will be used by specialists in the memory care setting to help distinguish Alzheimer’s disease from other types of dementia.
Disclosure of off-label usage
The authors have determined that, to the best of their knowledge, donepezil is not approved by the US Food and Drug Administration for the treatment of mild cognitive impairment.
FINANCIAL DISCLOSURE
Dr Yaari is a consultant for Amedisys Home Health. Dr Tariot has served as a consultant for Acadia, AC Immune, Allergan, Eisai, Epix, Forest, Genentech, MedAvante, Memory Pharmaceuticals, Myriad, Novartis, Sanofi-Aventis, Schering-Plough, and Worldwide Clinical Trials; has received consulting fees and grant/research support from Abbott, AstraZeneca, Avid, Baxter, Bristol-Myers Squibb, GlaxoSmithKline, Elan, Eli Lilly, Medivation, Merck, Pfizer, Toyama, and Wyeth; has received educational fees from Alzheimer’s Foundation of America; has received other research support from Alzheimer’s Association, Arizona Department of Health Services, GE, Institute for Mental Health Research, Janssen, National Institute of Mental Health, and National Institute on Aging; has received honoraria from AstraZeneca, Eisai, Eli Lilly, and Pfizer; is a stock shareholder in Adamas and MedAvante; and holds a patent for “Biomarkers of Alzheimer’s Disease.” Drs Fleisher, Seward, and Burke and Ms Brand have no personal affiliations or financial relationships with any commercial interest to disclose relative to the activity.
FUNDING/SUPPORT
None reported.
DISCLAIMER
The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press.
Clinical Points
- Amyloid positron emission tomography (PET) imaging can be used in the workup of patients with mild cognitive impairment (MCI).
- Negative results of amyloid PET imaging (showing little to no amyloid deposition) can help rule out an Alzheimer’s etiology of MCI.
- A positive amyloid PET scan (showing moderate to frequent amyloid deposition) does not conclusively confirm an Alzheimer’s etiology of MCI.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Arlington, VA: American Psychiatric Association; 2000.
Attix DK, Welsh-Bohmer KA, eds. Geriatric Neuropsychiatry: Assessment and Intervention. New York, NY: Guilford; 2006.
Beatty WW, Ryder KA, Gontkovsky ST, et al. Analyzing the subcortical dementia syndrome of Parkinson’s disease using the RBANS. Arch Clin Neuropsychol. 2003;18(5):509-520. PubMed
Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386-2391. doi:10.1001/jama.1993.03500180078038 PubMed
Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555-1561. doi:10.1212/01.wnl.0000344650.95823.03 PubMed
Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6(6):501-512. doi:10.1016/S1474-4422(07)70109-6 PubMed
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6 PubMed
Gollan TH, Montoya RI, Werner GA. Semantic and letter fluency in Spanish-English bilinguals. Neuropsychology. 2002;16(4):562-576. doi:10.1037/0894-4105.16.4.562 PubMed
Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1-13. doi:10.1016/j.jalz.2011.10.007 PubMed
Johns EK, Phillips NA, Chertkow H, et al. The effect of education on performance on the Montreal Cognitive Assessment (MoCA): normative data from the community. Canadian J Geriatrics. 2008:11(1):62. Poster presented at the 28th Annual Meeting of the Canadian Geriatrics Society; April 2008; Montreal, Quebec, Canada.
Johns EK, Phillips NA, Chertkow H, et al. The Montreal Cognitive Assessment: normative data in the community. In: Final Program of the 36th Annual Meeting of the International Neuropsychological Society; February 6-9, 2008; Waikoloa, Hawaii. J International Neuropsychological Society. 2008;41(suppl 1):58. doi: http://dx.doi.org/10.1017/S1355617708080429
Knopman DS, DeKosky ST, Cummings JL, et al. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153. doi:10.1212/WNL.56.9.1143 PubMed
Knopman DS, DeKosky ST, Cummings JL, et al. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153. doi:10.1212/WNL.56.9.1143 PubMed
Krauss JK, Halve B. Normal pressure hydrocephalus: survey on contemporary diagnostic algorithms and therapeutic decision-making in clinical practice. Acta Neurochir (Wien). 2004;146(4):379-388. doi:10.1007/s00701-004-0234-3 PubMed
Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment, 4th ed. New York, NY: Oxford; 2004.
Links KA, Merims D, Binns MA, et al. Prevalence of primitive reflexes and Parkinsonian signs in dementia. Can J Neurol Sci. 2010;37(5):601-607. PubMed
Links KA, Merims D, Binns MA, et al. Prevalence of primitive reflexes and Parkinsonian signs in dementia. Can J NSudo K, Mito Y, Tajima Y, et al. Smooth-pursuit eye movement: a convenient bedside indicator for evaluating frontal lobe and intellectual function. In Vivo. 2010;24(5):795-797. PubMedeurol Sci. 2010;37(5):601-607. PubMed
Mayor S. Regulatory authorities review use of galantamine in mild cognitive impairment. BMJ. 2005;330(7486):276. doi:10.1136/bmj.330.7486.276-b PubMed
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi:10.1016/j.jalz.2011.03.005 PubMed
Mitrushina M, Boone KB, Razani J, et al. Handbook of Normative Data for Neuropsychological Assessment. Second edition. New York, NY: Oxford University Press; 2005.
Mungas D. In-office mental status testing: a practical guide. Geriatrics. 1991;46(7):54-58, 63, 66. PubMed
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x PubMed
Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303-308. doi:10.1001/archneur.56.3.303 PubMed
Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379-2388. PubMed
Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. doi:10.1076/jcen.20.3.310.823 PubMed
Salloway S, Ferris S, Kluger A, et a. Donepezil 401 Study Group. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63(4):651-657. doi:10.1212/01.WNL.0000134664.80320.92 PubMed
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. doi:10.1016/j.jalz.2011.03.003 PubMed
Smith G, Rush BK. Normal aging and mild cognitive impairment. In: Attix DK, Welsh-Bohmer KA, eds. Geriatric Neuropsychiatry: Assessment and Intervention. New York, NY: Guilford; 2006.
Sudo K, Mito Y, Tajima Y, et al. Smooth-pursuit eye movement: a convenient bedside indicator for evaluating frontal lobe and intellectual function. In Vivo. 2010;24(5):795-797. PubMed
Thies W, Bleiler L; Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131-168. doi:10.1016/j.jalz.2012.02.001 PubMed
Voisin T, Touchon J, Vellas B. Mild cognitive impairment: a nosological entity? Curr Opin Neurol. 2003;16(suppl 2):S43-S45. doi:10.1097/00019052-200312002-00008 PubMed
Enjoy this premium PDF as part of your membership benefits!