CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Memory Disorders Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows.
BANNER ALZHEIMER’ S INSTITUTE
The Banner Alzheimer’s Institute located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2012;14(5):doi:10.4088/PCC.12alz01463
© Copyright 2012 Physicians Postgraduate Press, Inc.
Received: September 6, 2012; accepted September 6, 2012.
Published online: October 25, 2012.
AUTHORS
Anna D. Burke, MD, is a geriatric psychiatrist and dementia specialist at the Memory Disorders Clinic of Banner Alzheimer’s Institute.
Roy Yaari, MD, MAS, a neurologist, is associate director of the Memory Disorders Clinic of Banner Alzheimer’s Institute and a clinical professor of neurology at the College of Medicine, University of Arizona, Tucson.
Geri R. Hall, PhD, ARNP, GCNS, FAAN, is a gerontology clinical nurse specialist at Banner Alzheimer’s Institute and an adjunct clinical professor at the College of Nursing, University of Arizona, Tucson.
Helle Brand, PA, is a physician assistant at the Memory Disorders Clinic of Banner Alzheimer’s Institute.
Pierre N. Tariot, MD, a geriatric psychiatrist, is director of Banner Alzheimer’s Institute and a research professor of psychiatry at the College of Medicine, University of Arizona, Tucson.
Adam S. Fleisher, MD, MAS, is associate director of Brain Imaging at the Banner Alzheimer’s Institute, a neurologist at the Institute’s Memory Disorders Clinic, and an associate professor in the Department of Neurosciences at the University of California, San Diego.
Jan Dougherty, RN, MS, is director of Family and Community Services at Banner Alzheimer’s Institute.
Corresponding author: Anna D. Burke, MD, Banner Alzheimer’s Institute, 901 E. Willetta St, Phoenix, AZ 85006 ([email protected]).
CME Background
Original material is selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME activities on a variety of topics from volume to volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute Staff. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the material and go to PrimaryCareCompanion.com to complete the Posttest and Evaluation online.
CME Objective
After studying this case, you should be able to:
- Assess middle-aged patients who present with behavioral changes and memory impairment, provide appropriate diagnosis and available treatment, and support families in their caregiving efforts
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Date of Original Release/Review
This educational activity is eligible for AMA PRA Category 1 Creditâ„¢ through October 31, 2015. The latest review of this material was September 2012.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for AstraZeneca, Pfizer, Takeda, and Trovis and has been a member of the speakers/advisory boards for Forest and Merck. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
HISTORY OF PRESENT ILLNESS
Ms A was a very pleasant, 61-year-old, divorced, white woman who presented to the Memory Disorders Clinic at Banner Alzheimer’s Institute with her mother for evaluation of cognitive impairment. As per family report, symptoms were initially noted approximately 1 year prior to the evaluation. The onset of symptoms was insidious, with increasing repetition during conversations and questioning. Ms A stated that this repetition was frequently associated with her simply not listening to her mother and just nodding and saying “yes.” However, Ms A admitted that her short-term memory deficits were somewhat excessive and she was concerned due to a strong family history of dementia. She reported difficulty remembering dates and times as well as recent events. Occasional geographic disorientation was also noted. Ms A intermittently required reminders in order to be able to remember appointments. She occasionally misplaced items around the house. She reported some frustration with forgetting phone numbers that she frequently used such as that of her son. She continued to write and read without difficulty and continued to enjoy doing crossword puzzles. Ms A also endorsed anomia with difficulty finding words to express herself, as well as sporadic use of vague words. The progression of symptoms was gradual over the course of the past year, with subtle worsening. In addition, she described worsening insomnia over the past several months related to excessive worrying. Ms A began using over-the-counter sleep aids as a result.
No apathy or dysphoria was reported. No personality changes, inappropriate behaviors, or aggression was noted. Ms A’s mother expressed concern about her daughter’s increasing anxiety and somatic preoccupation related to vague gastrointestinal symptoms. Ms A frequently ruminated about a sensation of her stomach being “stuck.” She also ruminated about fears of developing an eating disorder. She had difficulty eating and drinking due to her somatic preoccupation. Over the course of the past several years, Ms A underwent numerous gastrointestinal workups, all of which were negative. Many of her symptoms were believed to be related to an anxiety disorder, although no formal diagnosis was ever made and no psychiatric workup had been completed.
Functionally, Ms A remained independent in all personal hygiene and grooming activities. She continued to drive and manage her finances. She continued to work in retail and had no difficulty performing her duties at work. She managed her own medications with no known errors. Ms A continued to use all household appliances appropriately. There was no difficulty using cash, calculating tips, or shopping.
Rapid eye movement sleep behavior disorder is a sleep disorder that involves abnormal behavior during the sleep phase with rapid eye movement. The dreamer acts out his or her dreams, which often involve kicking, screaming, punching, grabbing, and even jumping out of bed.
No rapid eye movement sleep disturbances were present. Ms A did report that she recently developed some difficulty swallowing and a sensation of her stomach “getting stuck” and was in the emergency room the night prior to the evaluation. She was diagnosed with gastroesophageal reflux disease and was started on omeprazole. Recent onset of urinary frequency was reported. Ms A denied any urinary accidents or fecal incontinence. She endorsed no other symptoms consistent with a urinary tract infection such as dysuria or change in the character of urine.
PAST MEDICAL HISTORY
Ms A reported a history of gastroesophageal reflux disease as well as a history of partial resection of the lung due to an unclear bacterial infection in the mid 1990s. She had a negative tuberculosis test per primary care records.
ALLERGIES
Ms A had no known drug allergies.
MEDICATIONS
Ms A took the over-the-counter generic sleep aid omeprazole (she was unable to recall the name), iron, calcium and vitamin D, a multivitamin, vitamin D3, glucosamine, and chondroitin.
SOCIAL HISTORY
Ms A had a high school education plus 1½ years of college. She was studying to become a teacher. She currently worked in retail with her 84-year-old mother, with whom she also lived. She was divorced and not in a long-term relationship. Ms A had 2 children who lived out of state from whom she was estranged due to their substance abuse issues.
SUBSTANCE ABUSE HISTORY
Ms A had no history of alcohol or illicit drug abuse. She reported smoking cigarettes (approximately 1½ packs per day) on and off since her 20s, but had quit in 2010.
FAMILY HISTORY
Ms A reported a significant family history of dementia. Most of the family members on her father’s side developed dementia associated with significant psychotic and behavioral disturbances in their mid-50s; the family described it as “they became nutty.” Increasing impulsive behaviors during this period were also noted. For example, when questioned in further detail, Ms A and her mother described her father as becoming more lax in his personal hygiene and grooming habits, having a greater tendency to make inappropriate comments, and becoming increasingly obsessive during this period. He would also drink alcohol excessively during his later life. Ms A’s paternal aunt developed psychosis during the course of her dementia. The family reported that she would be talking to them on the phone and would have to leave suddenly because Jesus was knocking on her door. There was also a family history of depression, with 1 of Ms A’s uncles committing suicide by hanging. There was no known family history of stroke or Parkinson’s disease.
The DSM-IV defines dementia as multiple cognitive deficits that include memory impairment and at least 1 of the following cognitive disturbances: aphasia, apraxia, agnosia, or a disturbance in executive functioning. The cognitive deficits must be sufficiently severe to cause impairment in social or occupational functioning and must represent a decline from a previously higher level of functioning. A diagnosis of dementia should not be made if the cognitive deficits occur exclusively during the course of a delirium (American Psychiatric Association, 2000).
On the basis of the clinical history alone, do you think?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. She meets criteria for dementia | 0% |
| B. She is most likely cognitively normal | 0% |
| C. She possibly has MCI | 40% |
| D. Her cognitive issues are most likely due to an underlying psychiatric disorder | 60% |
On the basis of the clinical history alone, which do to you believe is most accurate?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. She most likely does not have a progressive neurodegenerative condition | 0% |
| B. Her symptoms are most likely due to depression and anxiety | 50% |
| C. Her symptoms are more consistent with FTD | 5% |
| D. Her symptoms are more consistent with Alzheimer’s disease | 15% |
| E. She most likely has dementia, not otherwise specified | 30% |
Of those present, 50% felt that all of Ms A’s current cognitive symptoms could be accounted for by anxiety, which could affect her ability to sustain attention and in turn impair her ability to recall recent events. Fifteen percent felt that Ms A might be presenting early symptoms of Alzheimer’s disease, since initial symptoms may include limited cognitive deficits and more prominent affective and anxiety symptoms. Another 5% believed that there was compelling evidence of possible frontotemporal pathology, including a strong family history of early onset of dementia with prominent behavioral symptoms, and 30% felt that although a neurodegenerative process was present, additional workup was necessary to clarify the exact etiology.
Different dementias may be associated with various physical examination findings. However, most often the physical examination is normal in the early stages. Some subtle general findings can include frontal release signs such as a positive snout, glabellar, or palmomental reflex (Links et al, 2010).
On the basis of the information so far, what would you expect to see on the neurologic examination?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Normal | 95% |
| B. Objective nonfocal neurologic findings (including frontal release signs) | 5% |
| C. Focal neurologic findings | 0% |
PHYSICAL EXAMINATION
The physical examination was unremarkable.
NEUROLOGIC EXAMINATION
The neurologic examination was unremarkable. No frontal release signs were present. There were no abnormalities in deep tendon reflexes, muscle strength, or muscle tone. No changes in gait or station were noted. Cranial nerve examination was completely intact.
MENTAL STATUS EXAMINATION
Ms A was a very well groomed, white woman who appeared to be in no acute distress. She was notably anxious but cooperative with the examination. Her eye contact was appropriate, as was her affect, which was noted to be full. Her mood was “good,” and her thought process was linear, with no evidence of any paranoid, suicidal, or homicidal ideations. No auditory or visual hallucinations were noted; however, somatic delusions of her stomach “getting stuck” were present. Speech was of normal volume, rate, and amount. Judgment and insight were good. Fund of knowledge was slightly decreased for age and educational level. Orientation to time, place, and person was maintained, with grossly intact recent and remote memory, as well as attention and concentration.
LABORATORY STUDIES/RADIOLOGY
Laboratory studies available at the time of the visit, which included a complete blood count, comprehensive metabolic panel, and thyroid-stimulating hormone and vitamin B12 levels, revealed no clinically significant abnormalities. No neuroimaging was available at the time of the visit.
A Mini-Mental State Examination (MMSE) score generally correlates with disease severity. Scores ≤ 9 points can indicate severe dementia, scores between 10-20 points can indicate moderate dementia, and a score > 20 can indicate mild dementia (Mungas, 1991). Although MMSE scores must be interpreted in light of both the patient’s age and education, education is the primary demographic factor that affects scores. Therefore, whereas a cutoff of ≤ 23 is widely used in distinguishing between normal and abnormal performance, this cutoff may have less predictive ability in poorly educated individuals (Folstein et al, 1975).
On the basis of the information so far, what would you expect the MMSE score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 26-30 | 75% |
| B. 21-25 | 25% |
| C. 16-20 | 0% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
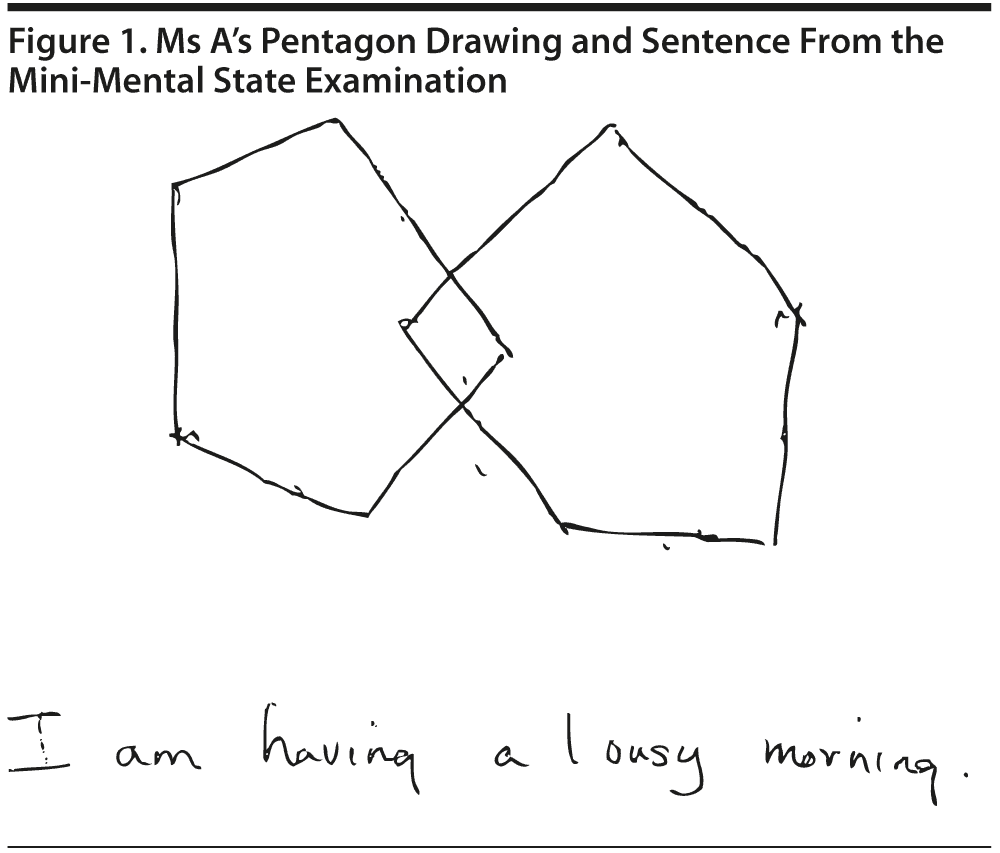
The MMSE revealed a total score of 29/30, with Ms A losing 1 point on delayed recall. Figure 1 shows Ms A’s pentagon drawing and sentence from the MMSE.
The Montreal Cognitive Assessment (MoCA) is a 30-point test that assesses several cognitive domains. Because it is more challenging than the Mini-Mental State Examination, the MoCA has greater sensitivity for mild cognitive impairment and early stages of dementia. With a cutoff score < 26, the sensitivity for detecting mild cognitive impairment (N = 94) was 90% and the specificity was 87% (Nasreddine et al, 2005). Research has demonstrated that MoCA scores are highly correlated with education. It is recommended that education be taken into account when interpreting MoCA performance, but there are no formal specific cutoff scores for lower education at this time (Johns et al, 2008). The MoCA is available online at http://mocatest.org/.
On the basis of the information so far, what would you expect the MoCA score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 26-30 | 20% |
| B. 21-25 | 75% |
| B. 16-20 | 5% |
| B. 11-15 | 0% |
| B. < 11 | 0% |
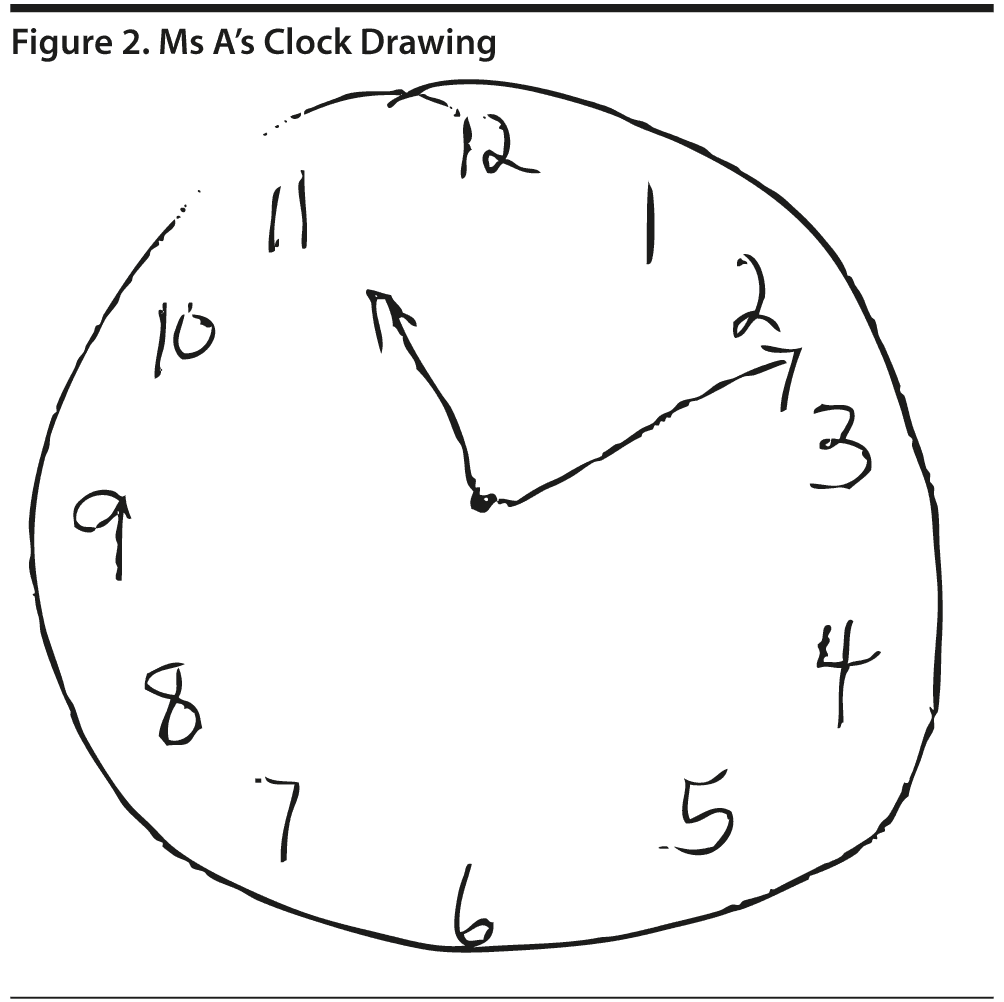
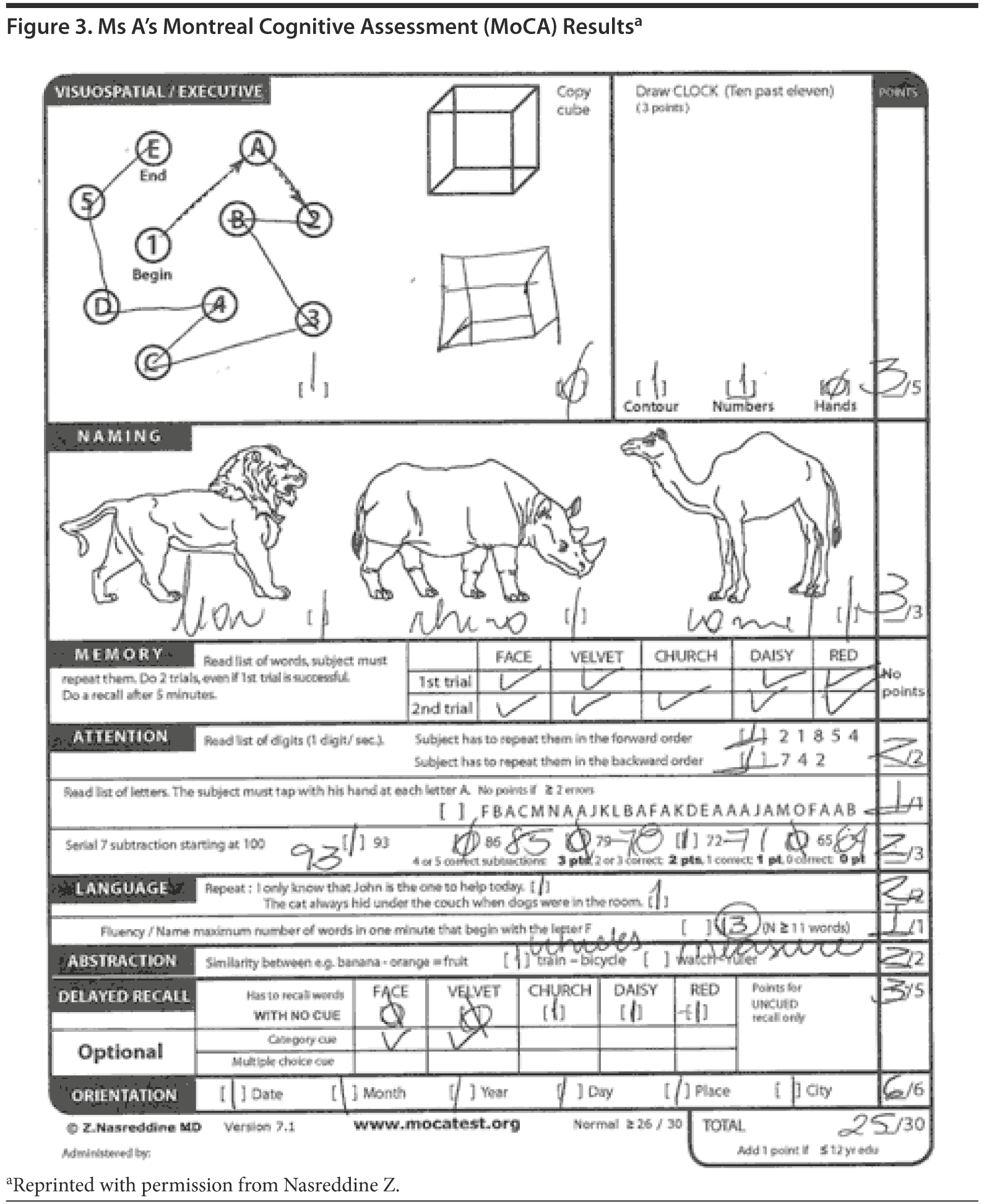
All of the clinicians present believed that the MoCA would be more sensitive to detect subtle cognitive deficits, particularly in areas of visuospatial abilities and executive function, regardless of the patient’s cognitive reserve. The MoCA revealed a total score of 25/30, with Ms A losing 1 point on visuospatial abilities, 2 points on delayed recall, 1 point on serial subtractions, and 1 point on clock drawing (Figures 2 and 3).
On the basis of the information so far, do you think this is a progressive neurodegenerative condition?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Yes | 30% |
| B. No | 10% |
| B. Cannot determine at this time | 60% |
Most of the conference attendees felt that further workup was indicated before a diagnosis could be established.
What should the next step be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Neuropsychological testing | 0% |
| B. Structural brain scan | 0% |
| C. FDG-PET scan | 0% |
| D. Laboratory studies | 0% |
| E. Refer the patient to a psychiatrist | 0% |
| F. A and B | 40% |
| G. B and C | 10% |
| H. A, B, C | 50% |
IMPRESSION
The clinician did not believe that Ms A met criteria for a dementia at the time of her initial visit. However, her symptoms were concerning for the presence of a possible underlying neurodegenerative process that could meet criteria for MCI.
Standard structural neuroimaging used in diagnosis of dementia includes magnetic resonance imaging or computed tomography scans. These tests are helpful in excluding intracranial pathology such as infarction, mass, hemorrhage, or trauma, which may be causing cognitive impairment. Although cortical atrophy or hippocampal atrophy are frequently visible on imaging, the diagnosis of dementia remains a clinical one. A dementia diagnosis should not be based solely on structural neuroimaging. Functional neuroimaging such as fluorodeoxyglucose-positron emission tomography allows a clinician to assess not only for physical changes in the brain but also for changes in metabolic activity. Due to its prohibitive cost, this modality is less frequently used. However, it remains an important tool in differentiating between Alzheimer’s dementia and frontotemporal dementia.
PLAN OF CARE
- Neuropsychological testing was ordered to help clarify the pattern of cognitive strengths and weaknesses, to assist with differential diagnosis, to serve as a basis for rehabilitative suggestions, and to provide a basis against which to measure future changes.
- A magnetic resonance image of the brain was ordered to assess for an intracranial pathology contributing to Ms A’s current clinical presentation.
- A urinalysis and urine culture were ordered to assess for the possibility of any urinary tract infection due to recently noted urinary frequency.
- The clinician recommended that Ms A stop the over-the-counter sleep aid, because it could further impair her cognitive abilities, as most of these medications contain diphenhydramine, which has anticholinergic properties. Instead, mirtazapine 7.5 mg per night was prescribed. Mirtazapine was chosen due to its antidepressant, antianxiety, antiemetic, and sedating properties.
Mirtazapine is a noradrenergic and specific serotonergic antidepressant. The medication is highly sedating at lower doses (eg, 7.5-30 mg per night). Increases in the dose above 30 mg result in decreased sedation. Mirtazapine also significantly stimulates appetite and provides potent antidepressive, antianxiety, and antiemetic effects.
FOLLOW-UP
Ms A returned to the Memory Disorders Clinic 3 months later. Since that time, she was briefly hospitalized due to exacerbation of anxiety symptoms and development of suicidal ideations related to her gastrointestinal complaints, which were felt by the emergency room physician to be somatic delusions. She became increasingly somatically focused and so distressed that she was experiencing difficulty sleeping or functioning. She was hospitalized for approximately 1 week on an inpatient psychiatric unit, during which time her medications were adjusted and quetiapine was added. Her anxiety symptoms improved, but did not resolve. Her somatic preoccupation also did not fully resolve. Ms A now focused on her new medications as the cause of excessive constipation. As a result, she was noncompliant with her quetiapine and mirtazapine. She reported not having taken quetiapine, which had been increased to 100 mg twice daily, for the past 3 days prior to her follow-up visit at Banner Alzheimer’s Institute and had only taken a single dose the morning of her appointment. She also took her mirtazapine inconsistently, as she believed that this was a “sleeping pill.”
Ms A continued to experience cognitive symptoms. She easily forgot recent events and peoples’ names and faces. No changes in functional abilities were noted by Ms A’s mother, who was felt to be a good historian. No significant decline in cognitive abilities had occurred since her initial visit.
On the basis of the clinical history alone, which do you believe is most accurate?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Her symptoms are most likely due to depression and anxiety | 35% |
| B. Her symptoms are more consistent with FTD | 0% |
| B. Her symptoms are more consistent with Alzheimer’s disease | 0% |
| B. She most likely has dementia, not otherwise specified | 65% |
Most of the conference attendees felt that the symptoms could be related to a neurodegenerative disorder; however, additional workup was necessary to establish the exact etiology. Although Ms A’s symptoms might be psychiatric in nature, the attendees felt that her family history of early onset of progressively worsening psychotic symptoms and cognitive impairment was concerning for a rare neurodegenerative disorder.
FOLLOW-UP
Ms A underwent neuropsychological testing. Ms A’s full-scale IQ was in the average range, with a marked pattern of strengths and weaknesses. She received relatively low scores on subtests assessing her verbal abilities and very high scores on subtests that measured her ability to quickly and correctly scan sequence or discriminate simple visual information. Between these 2 extremes, she received scores in the low average to average range on measures of nonverbal problem solving and attention and concentration. Ms A’s scores were generally within normal limits on neuropsychological tests assessing various cognitive domains. Her relative strengths included measures that required rapid information processing. She also did well on measures that required attention and concentration. Her language abilities were roughly intact. Ms A gave mixed results on measures of executive functioning. Her scores were average on a test that required her to keep track of 2 sequences simultaneously and shift back and forth between them, although borderline impairment was found on a different task that required her to focus her concentration and resist distracting stimuli.
Although her scores were not markedly impaired, Ms A displayed a relative weakness on measures of memory. Her ability to learn and immediately recall a word list was mildly impaired and included intrusions; that is, she would recall words that were not on the list. After a delay, her ability to recall the words from the list was average-borderline. Although Ms A’s performance on the tests of recognition memory for this list were average, in contrast, her ability to recall a passage was average, both immediate and after a delay. Her delayed reproduction of geometric designs was mildly impaired. The overall pattern of neuropsychological test findings was more cortical than subcortical in nature, primarily as a consequence of her relative weakness in memory.
Taking into account the results of the testing, the neuropsychologist felt that Ms A’s presentation was most consistent with a possible amnestic MCI. However, given the early onset and behavioral disturbances, a frontotemporal process could not fully be ruled out at this time. The neuropsychologist questioned whether a mild mania may have been present at the time of the evaluation. Increased impulsivity was noted during the course of the neuropsychological testing, as well as during the course of Ms A’s clinic visit. As the clinician entered the room, Ms A could not wait to discuss the neuropsychological testing and was unable to answer any other questions regarding what occurred over the course of the past several weeks. Ms A continued to experience panic attacks according to family history, but she had not followed through on outpatient treatment with a psychiatrist.
What should the next step be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. FDG-PET scan | 100% |
| B. Her symptoms are most likely due to depression and anxiety | 0% |
| B. Initiate a cholinesterase inhibitor | 0% |
| B. Initiate memantine | 0% |
| B. Observe, repeating in-office cognitive testing biannually | 0% |
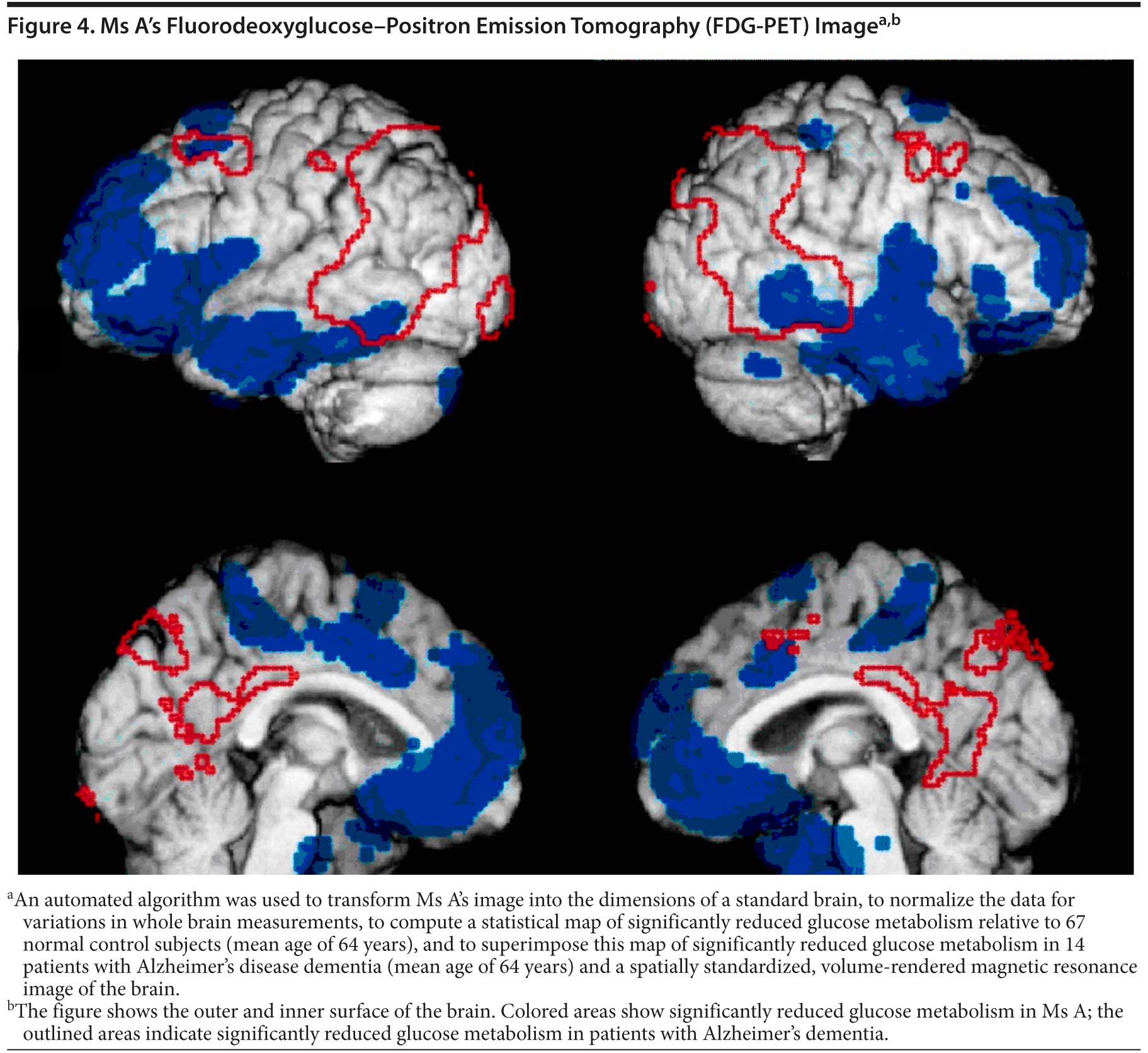
The clinician felt that sufficient evidence was present as a result of behavioral symptoms to warrant an FDG-PET scan. The scan revealed hypometabolism in the frontal and temporal lobes (Figure 4). The pattern was consistent with FTD. Ms A was diagnosed with FTD, behavioral variant.
DISCUSSION
Frontotemporal dementia is a relatively rare progressive neurodegenerative disorder accounting for approximately 5% of all dementia diagnoses. Most patients tend to begin showing symptoms in their 50s, and due to the young age at onset, these symptoms are frequently thought to be related to psychiatric disorders. In contrast to Alzheimer’s disease, FTD typically affects memory to a lesser extent in the early stages. The initial symptoms usually include dramatic alterations in behaviors and language abilities. On average, onset of FTD occurs approximately a decade earlier than Alzheimer’s disease, with some cases being reported as early as 21 years of age and as late as 80 years of age. It has been established that FTD is a diagnosis of exclusion, used only after immunohistochemical methods fail to identify other distinctive pathological conditions. The broad diagnosis of FTD comprises a heterogeneous cluster of syndromes including the behavioral variant and dysexecutive syndrome, primary progressive aphasia, and a semantic variant. These syndromes are briefly defined below.
- Behavioral variant/dysexecutive syndrome: Characterized by prominent changes in personality and impulse control, patients display cognitive rigidity, inflexibility, distractibility, and irritability. Patients tend to be more disinhibited and impulsive, frequently displaying socially inappropriate behaviors or lack of empathy. Features of Kl×¼ver-Bucy syndrome emerge in some of these patients, including hyperorality, hypersexuality, and hypervisual behavior. Atypical forms of depression, psychosis, or mania have also been reported.
- Primary progressive aphasia: Characterized by anomic aphasia that insidiously worsens over time, as well as gradual progressive loss of speech output and impaired repetition that occurs over time, despite relatively preserved aural comprehension of single words. Ultimately, patients become completely mute. Comprehension of spoken language also deteriorates throughout the disease process.
- Semantic variant: Characterized by empty, circumlocutious spontaneous speech with frequent paraphasic errors, patients display difficulty on language expression tasks dependent on semantic memory such as defining words and confrontation naming. Comprehension of single words is frequently impaired. Patients may also display difficulty recognizing visually presented objects despite no apparent visual-perceptual deficits. Dyslexia and dysgraphia are also common. Syntax and repetition are relatively preserved.
There is currently no US Food and Drug Administration-approved therapy for FTD; hence, all of the pharmacotherapies discussed here are currently being used off-label on the basis of evidence of efficacy in other disorders with similar symptom profiles. No controlled trials have been conducted to guide practice.
Due to the prevalence of apathy, depressive symptoms, anxiety, loss of impulse control, and agitation, selective serotonin reuptake inhibitors (SSRIs) are frequently used as a first-line therapy to help control symptoms. If behavioral control cannot be attained using an SSRI, mood stabilizers and atypical antipsychotics may offer benefits (Lenz et al, 2009; Mendez et al, 2009; Rabinovici and Miller, 2010).
There is currently no compelling evidence that medications used for Alzheimer’s disease are of benefit for patients suffering from FTD. In fact, there are reports of worsening of behavioral disturbances in patients suffering from FTD after a cholinesterase inhibitor, such as donepezil, is initiated (Kertesz et al, 2008; Mendez et al, 2007; Moretti et al, 2004). Unlike cholinesterase inhibitors, N-methyl-d-aspartate receptor antagonists, such as memantine, are hypothesized to be of potential benefit since they decrease aberrant glutamatergic activity thought to also be impacted in FTD. Treatment of FTD focuses on symptom management that is aimed at maximizing the patient’s quality of life and minimizing the caregiver’s burden and may include medications to manage particular symptoms, regular supervision, and assistance.
NONpHARMACOLOGIC MANAGEMENT FOR PEOPLE WITH BEHAVIORAL VARIANT Frontotemporal Dementia
By the time a patient is diagnosed with behavioral variant FTD, the family has been living with behavioral unpredictability, narcissism, impulsivity, and lack of insight for several years. In pursuit of a diagnosis, patients are often diagnosed as having a psychiatric disorder including depression, bipolar disorder, and/or personality disorder. While the diagnosis of behavioral variant FTD is terrible, families are often relieved to know the diagnosis. Care of people with behavioral variant FTD is vastly different from that of other dementias because of the loss of insight, inability to inhibit, and narcissism. Common symptoms of behavioral variant FTD that affect care are listed below.
- Loss of insight
- Reduced capacity for empathy
- Narcissism
- Emotional lability
- Semantic aphasias
- Reduced ability to initiate activity, apathy
- Decreased interpersonal skills
- Obsessions
- Self-absorption
- Consistently poor judgment
- Severe disinhibition
- Able to understand cause and effect but cannot take action
- Anger with aggression
The first task for families is typically to develop mechanisms to stem damage from disinhibited behavior, usually overspending (shopping, making poor investments, enrolling in scams, gambling, etc). If possible, durable powers of attorney should be obtained; however, because of the lack of insight, patients often refuse. Families may need to pursue guardianship, although it is often difficult to obtain, as few attorneys understand behavioral variant FTD and the patient often scores quite well on mental status screens. Thus, it is important to provide the family support and encourage contact with an attorney who specializes in elder law while they navigate the legal and financial options.
The second task is to provide for safety. A driving assessment is essential when available, and removal of the car due to tendencies toward impulsive driving, speeding, and vengeful driving patterns may be necessary. Many patients experience a stage of severe anger, heightened by the inability to inhibit responses. Guns, ammunition, power tools, garden machines, and other materials that could be used as weapons must be removed from the house, and care must be taken to ensure that they are not replaced. Some patients may exhibit sexually inappropriate behaviors toward family, strangers, or even children. These behaviors should be monitored and treated with psychiatric follow-up before the patient harms someone or is arrested.
A third task is to help the family develop activities that support the patient’s obsessions and accommodate shortened attention spans. For example, if the patient obsesses about a particular television show, have the family record it to play repeatedly, as trying to remove the object of an obsession increases patient anger.
Caregivers at Banner Alzheimer’s Institute are still in the process of helping Ms A’s family accept her behavioral variant FTD diagnosis and establish appropriate care (eg, legal issues, powers of attorneys, living environment). Supervision and assistance will be vital in appropriate treatment. Ms A will now be seen every 2 months at the Memory Disorders Clinic, and additional support will be provided to Ms A’s mother via telephone and through the FTD support group.
Fortunately, the symptoms of behavioral variant FTD are worse early in the disease and become less oppressive with disease progression and helping families understand this may lighten their load. Support groups for families of people with behavioral variant FTD are being developed across the country. To locate these groups and information on behavioral variant FTD management, contact the following: Association for Frontotemporal Degeneration (www.theaftd.org) and Alzheimer’s Association (http://www.alz.org/dementia/fronto-temporal-dementia-ftd-symptoms.asp). There are also 2 free resource pamphlets available for families:
- Frontotemporal Disorders: Information for Patient, Families, and Caregivers. From the National Institute on Aging, the National Institutes of Health (http://www.nia.nih.gov/alzheimers/publication/frontotemporal-disorders-information-patients-families-and-caregivers-4)
- What About the Kids: Frontotemporal Degeneration, Information for Parents with Young Children and Teens (www.theaftd.org).
Disclosure of off-label usage
The authors have determined that, to the best of their knowledge, memantine is not approved by the US Food and Drug Administration for the treatment of frontotemporal dementia and quetiapine is not approved for the treatment of behavioral disturbance in dementia.
FINANCIAL DISCLOSURE
Dr Yaari is a consultant for Amedisys Home Health. Dr Tariot has served as a consultant for Acadia, AC Immune, Allergan, AstraZeneca, Eisai, Eli Lilly, Epix, Forest, Genentech, MedAvante, Memory Pharmaceuticals, Myriad, Novartis, Sanofi-Aventis, Schering-Plough, and Worldwide Clinical Trials; has received consulting fees and grant/research support from Abbott, AstraZeneca, Avid, Baxter, Bristol-Myers Squibb, Elan, Eli Lilly, GlaxoSmithKline, Medivation, Merck, Pfizer, Toyama, and Wyeth; has received educational fees from Alzheimer’s Foundation of America; has received other research support from Alzheimer’s Association, Arizona Department of Health Services, GE, Institute for Mental Health Research, Janssen, National Institute of Mental Health, and National Institute on Aging; has received honoraria from AstraZeneca, Eisai, Eli Lilly, and Pfizer; is a stock shareholder in Adamas and MedAvante; and holds a patent for “Biomarkers of Alzheimer’s Disease.” Drs Burke, Hall, and Fleisher and Mss Brand and Dougherty have no personal affiliations or financial relationships with any commercial interest to disclose relative to the activity.
FUNDING/SUPPORT
None reported.
DISCLAIMER
The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press, Inc.
Clinical Points
- Frontotemporal dementia (FTD) frequently presents with severe personality changes, affective symptoms, psychosis, and/or anxiety and may mimic a psychiatric disorder.
- On average, onset of FTD occurs approximately a decade earlier than Alzheimer’s disease, with some cases reported as early as 21 years of age and as late as 80 years of age.
- There are currently no FDA-approved pharmacologic treatments for FTD. Treatment is symptomatic and should always include behavioral and environmental modifications as well as caregiver education and support.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Arlington, VA: American Psychiatric Association; 2000.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6PubMed
Johns EK, Phillips NA, Chertkow H, et al. The effect of education on performance on the Montreal Cognitive Assessment (MoCA): normative data from the community. Canadian J Geriatrics. 2008;11(1):62. Poster presented at the 28th Annual Meeting of the Canadian Geriatrics Society; April 2008; Montreal, Quebec, Canada.
Johns EK, Phillips NA, Chertkow H, et al. The Montreal Cognitive Assessment: normative data in the community. In: Final Program of the 36th Annual Meeting of the International Neuropsychological Society; February 6-9, 2008; Waikoloa, Hawaii. J International Neuropsychological Society. 2008;41(suppl 1):58. doi: http://dx.doi.org/10.1017/S1355617708080429 >
Kertesz A, Morlog D, Light M, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord. 2008;25(2):178-185. doi:10.1159/000113034 PubMed
Lenz B, Sidiropoulos C, Bleich S, et al. Frontotemporal dementia: neurotransmitter and clinical symptoms with focus on therapeutic targets. Neurol Psychiatr. 2009;77(5):289-294.PubMed
Links KA, Merims D, Binns MA, et al. Prevalence of primitive reflexes and parkinsonian signs in dementia. Can J Neurol Sci. 2010;37(5):601-607. PubMed
Mendez MF, Shapira JS, McMurtray A, et al. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15(1):84-87. doi:10.1097/01.JGP.0000231744.69631.33 PubMed
Mendez MF. Frontotemporal dementia: therapeutic interventions. Front Neurol Neurosci. 2009;24:168-178. doi:10.1159/000197896 PubMed
Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003 PubMed
Mungas D. In-office mental status testing: a practical guide. Geriatrics. 1991;46(7):54-58, 63, 66. PubMed
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x PubMed
Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24(5):375-398. doi: 10.2165/11533100-000000000-00000 PubMed
Enjoy this premium PDF as part of your membership benefits!