Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Memory Disorders Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows.
BANNER ALZHEIMER’ S INSTITUTE
The Banner Alzheimer’s Institute located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2014;16(1):doi:10.4088/PCC.13alz01625
© Copyright 2014 Physicians Postgraduate Press, Inc.
Received: December 19, 2013; accepted December 19, 2013.
Published online: February 27, 2014.
AUTHORS
Saurav Das, MBBS, is a visiting international physician from India, who currently rotates at the Stead Family Memory Clinic and the cognitive neuroimaging laboratory at Banner Alzheimer’s Institute.
Adam S. Fleisher, MD, MAS, is associate director of Brain Imaging at Banner Alzheimer’s Institute, a neurologist at the Institute’s Stead Family Memory Clinic, and an associate professor in the Department of Neurosciences at the University of California, San Diego.
Roy Yaari, MD, MAS, a neurologist, was director of the Stead Family Memory Clinic of Banner Alzheimer’s Institute and a clinical professor of neurology at the College of Medicine, University of Arizona, Phoenix, during development of this manuscript. Dr Yaari is currently an employee of Eli Lilly.
James D. Seward, PhD, ABPP, is a clinical neuropsychologist at Banner Alzheimer’s Institute.
Anna D. Burke, MD, is a geriatric psychiatrist and dementia specialist at the Stead Family Memory Clinic of Banner Alzheimer’s Institute.
Helle Brand, PA, is a physician assistant at the Stead Family Memory Clinic of Banner Alzheimer’s Institute.
Pierre N. Tariot, MD, a geriatric psychiatrist, is director of Banner Alzheimer’s Institute and a research professor of psychiatry at the College of Medicine, University of Arizona, Phoenix.
Corresponding author: Saurav Das, MBBS, 901 E Willetta St, Phoenix, AZ 85006 ([email protected]).
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute Staff. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer at least 70% of the questions in the Posttest, and complete the Evaluation. The Posttest and Evaluation are available at http://www.cmeinstitute.com/activities/journal.asp.
CME Objective
After studying this article, you should be able to:
- Conduct a differential diagnosis in a patient presenting with visual problems (despite normal ophthalmologic examinations) and cognitive decline
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Date of Original Release/Review
This educational activity is eligible for AMA PRA Category 1 Creditâ„¢ through February 28, 2017. The latest review of this material was February 2014.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for Forest, Lundbeck, Merck, Sunovion, and Takeda. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
HISTORY OF PRESENT ILLNESS
Mr A, a 62-year-old right-handed man, presented to the Banner Alzheimer’s Institute for an initial evaluation. He was accompanied by his wife. He reported approximately 3 years of insidious onset and gradually progressive difficulties with cognition.
First noted were challenges with completing sentences and staying on task. There was mild word-finding difficulty, and he had trouble reading and writing. Though Mr A could often recall dates and events correctly, he faced some challenges with calculations. His wife reported that he often misplaced items and sometimes was unable to locate objects, such as keys, even though they were directly placed in front of him, and he continued to search for the objects until she specifically showed him where they were placed. Mr A had some anxiety and mild related irritability regarding his current symptoms. He had 1 episode of visual hallucinations when he was increased from 10 mg to 40 mg of vilazodone, which had been prescribed for depression. The hallucinations resolved when the dose was returned to 10 mg. Mr A had periodic depressive features but no chronic anhedonia or suicidal ideation. His instrumental activities of daily living were generally intact, except that he could no longer function at his job. He found it was taking longer at work to complete tasks, including reading on the computer and typing reports. He had seen an ophthalmologist several times in the past few months, but no abnormality was reported.
He developed mild navigation problems while driving, though not getting lost. To cite an example, Mr A had almost crashed into the rear end of a car while navigating through the parking lot in a shopping mall the week before. He had experienced difficulty managing his phone but was able to shop independently with a list. He was independent with medications and other daily chores.
PAST MEDICAL HISTORY
Mr A had obstructive sleep apnea and refused to use his continuous positive airway pressure (CPAP) machine. Additionally, he had well-controlled hypertension, hyperlipidemia, chronic back pain, gastroesophageal reflux, and osteoarthritis.
ALLERGIES
Mr A had no known drug allergies.
MEDICATIONS
Mr A’s current medications included amlodipine, atorvastatin, dexlansoprazole, and cetirizine. He took vilazodone 10 mg once a day for depression and tramadol for management of chronic back pain. Additionally, he took flaxseed oil, glucosamine, chondroitin, a multivitamin, vitamin B complex, and garlic supplementation.
SUBSTANCE ABUSE HISTORY
Mr A had no significant history of alcohol consumption, cigarette smoking, or illicit drug use.
SOCIAL HISTORY
Mr A had 14 years of education and worked as a manager in an engineering firm. He currently lives with his wife.
FAMILY HISTORY
Mr A’s mother had vascular dementia.
LABORATORy findings
Mr A’s recent laboratory tests included a complete blood count and comprehensive metabolic profile; both were within normal limits.
Different dementias may be associated with different physical findings. For example, a vascular dementia may be associated with a residual focal neurologic deficit secondary to an old cerebrovascular insult. Dementia due to Alzheimer’s disease may be associated with subtle frontal release signs such as a positive snout, glabellar, or palmo-mental reflex (Links et al, 2010). Decline in cognitive test scores in general and a decline in visual acuity are strongly associated with increasing age. Poor vision may be a limiting factor for performance on cognitive tasks specifically requiring vision; however, it may also be related to neurodegenerative disorders like Alzheimer’s disease. Mr A’s pronounced visual complaints warrant a detailed ophthalmologic examination (Jefferis et al, 2012).
On the basis of the information so far, what would you expect to find on a neurologic examination of this patient?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Normal | 8% |
| B. A visual field defect | 25% |
| C. Diminished visual acuity | 42% |
| D. Visual agnosia | 83% |
| E. Abnormal retinal findings in the ophthalmoscopic examination | 17% |
| F. A focal sensory or motor deficit | 17% |
| G. Frontal release signs | 75% |
Case conference participants had the choice of selecting multiple options; thus, the percentages do not add up to 100. The majority of the case conference participants felt that Mr A should be screened for visual agnosia, and they also expected to see positive frontal release signs. About half of the participants felt that he might have a persistent problem with visual acuity in spite of the repeated ophthalmologist visits.
VITAL SIGNS
Mr A had a pulse of 72 bpm and blood pressure of 110/80 mm Hg. His weight was 205.6 lb.
NEUROLOGIC EXAMINATION
A full general neurologic examination was performed. Visual fields, visual acuity, and color vision were grossly intact, and there were no findings on cranial nerve examination. Mr A had slight decreased sensation to light touch and temperature in L5 distribution on the left leg. He had a positive palmomental reflex but no other significant or focal findings on neurologic examination.
A Mini-Mental State Examination score generally correlates with disease severity. Scores ≤ 9 points can indicate severe dementia, scores between 10-20 points can indicate moderate dementia, and scores > 20 points can indicate mild dementia (Mungas, 1991). Although MMSE scores must be interpreted in light of both the patient’s age and education, education is the primary demographic factor that affects scores. Therefore, whereas a cutoff of ≤ 23 is widely used in distinguishing between normal and abnormal performance, this cutoff may have less predictive ability in poorly educated individuals (Folstein et al, 1975). However, it is noteworthy that impairment in functionality is the principal determinant in the clinical diagnosis of dementia.
On the basis of the information so far, what would you predict Mr A’s score on the MMSE to be?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. 26-30 | 73% |
| B. 21-25 | 27% |
| C. 16-20 | 0% |
| D. 11-15 | 0% |
| E. ≤ 10 | 0% |
The majority of the participants (73%) thought the MMSE score for this patient would be in the range of 26-30. The rest of the participants thought he would score between 21 and 25.
The Montreal Cognitive Assessment (MoCA) is a 30-point test that assesses several cognitive domains. Because it is more challenging than the Mini-Mental State Examination, it has greater sensitivity for mild cognitive impairment and early stages of dementia. With a cutoff score < 26, the sensitivity for detecting mild cognitive impairment (N = 94) was 90% and the specificity was 87% (Nasreddine et al, 2005). Research has demonstrated that MoCA scores are highly correlated with education. It is recommended that education be taken into account when interpreting MoCA performance, but there are no formal specific cutoff scores for lower education at this time (Johns et al, 2008). This test is available online at http://mocatest.org/.
On the basis of the information so far, what would you expect Mr A’s MoCA score to be?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. 26-30 | 9% |
| B. 21-25 | 73% |
| C. 16-20 | 18% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
The majority of the participants (73%) thought that Mr A would score in the range of 21-25 on the MoCA. Two participants thought he would score between 16 and 20, and only 1 participant thought the score would be in the range of 26-30.
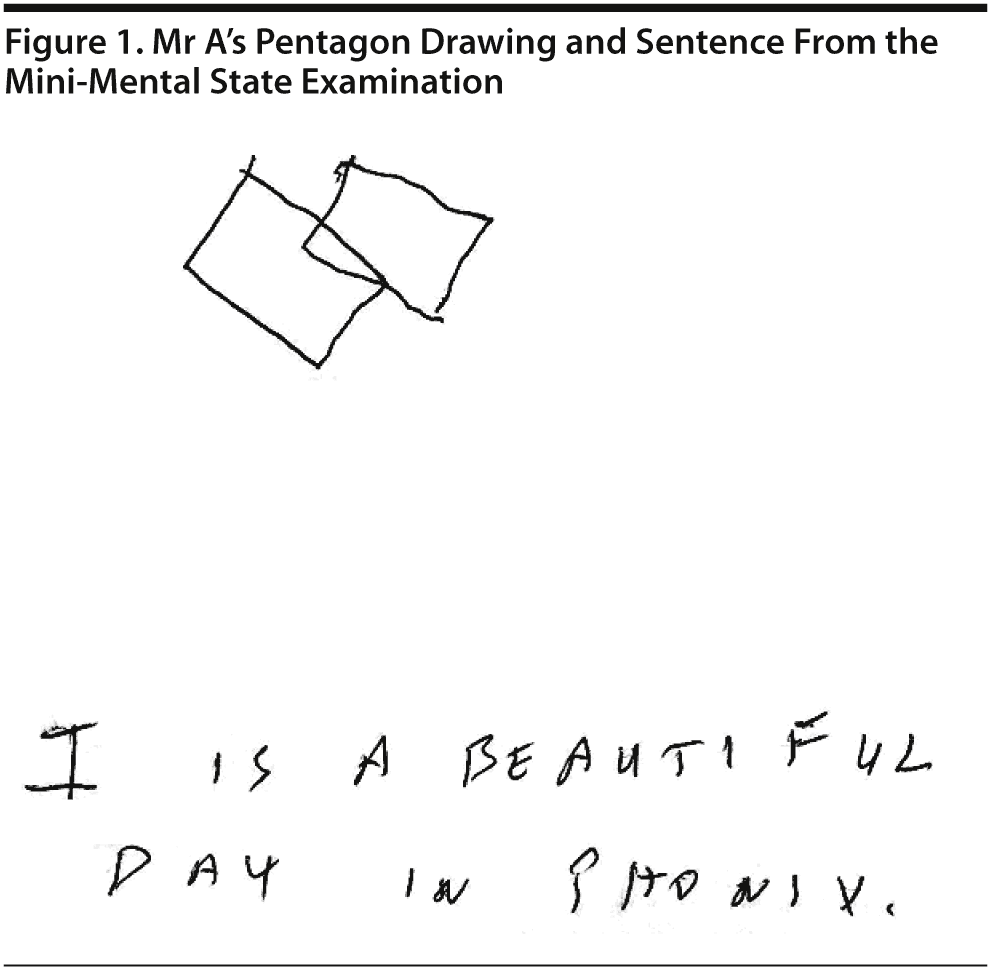
Mr A scored a 26/30 on the MMSE, missing 1 point for drawing intersecting pentagons, 1 point for memory, and 1 point in writing the sentence due to missing 1 letter in a word. He was able to copy 3 figures and to recall all 3 figures that he copied. He also perseverated on the drawing of pentagons, which he copied as intersecting squares (Figure 1). Calculations were intact except for complex ones. Picture interpretation was intact. Category retrieval showed 22 animals in a minute with 1 repetition. Fund of knowledge was intact, with a score of 5/7.
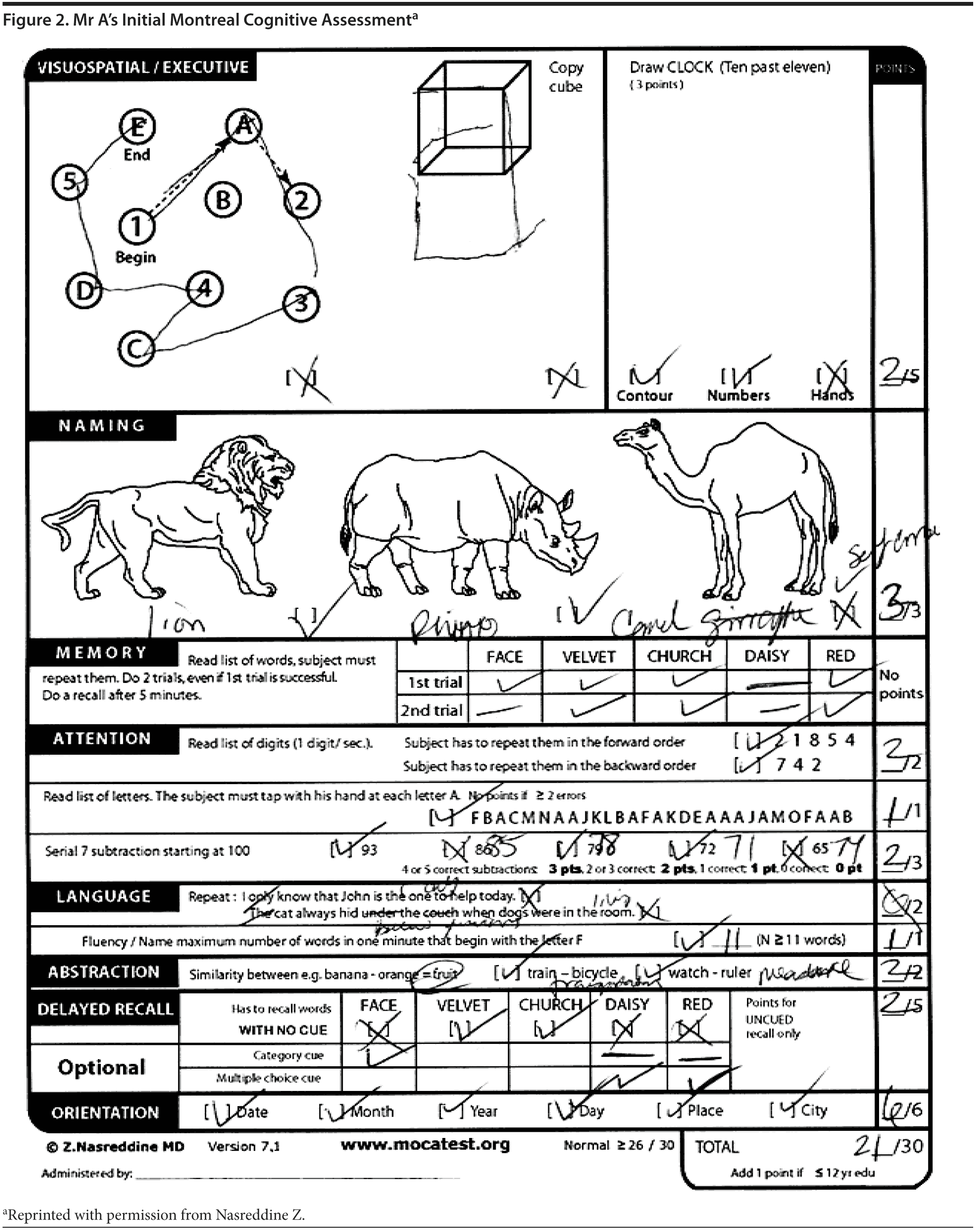
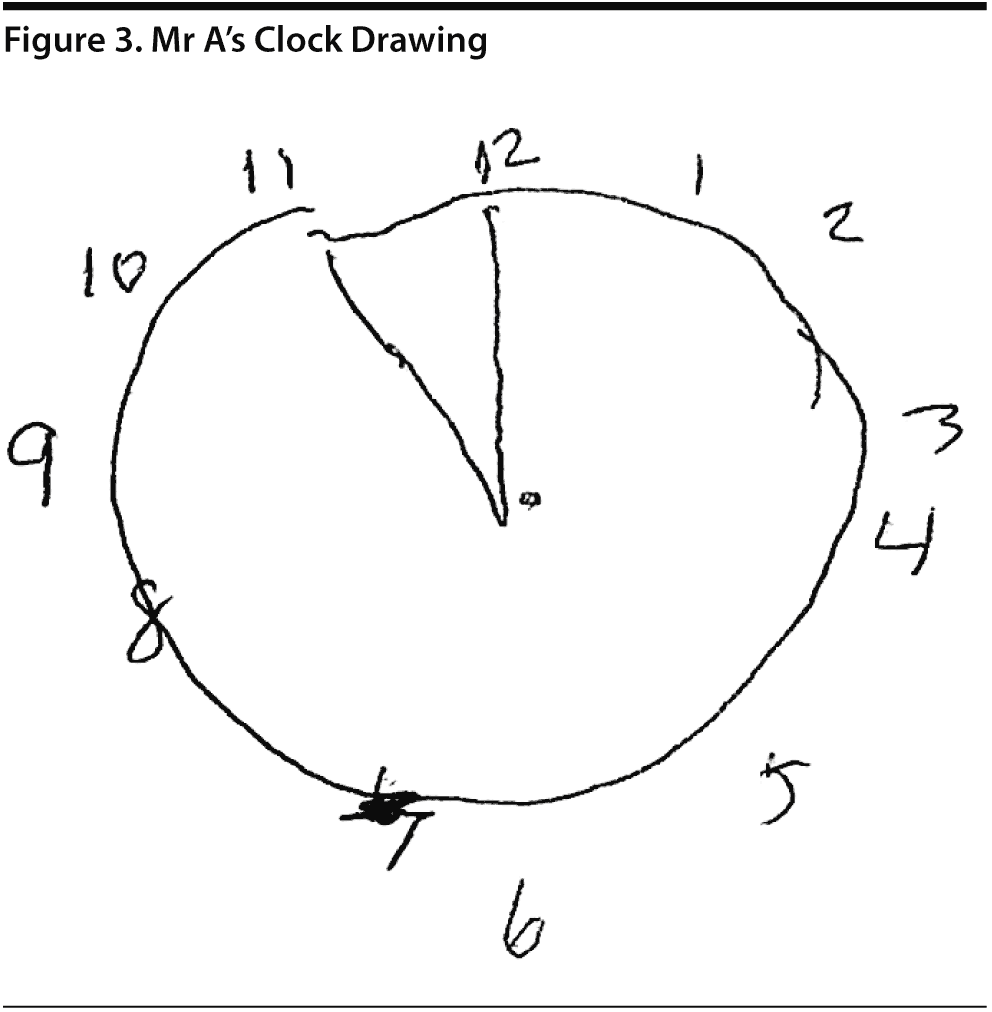
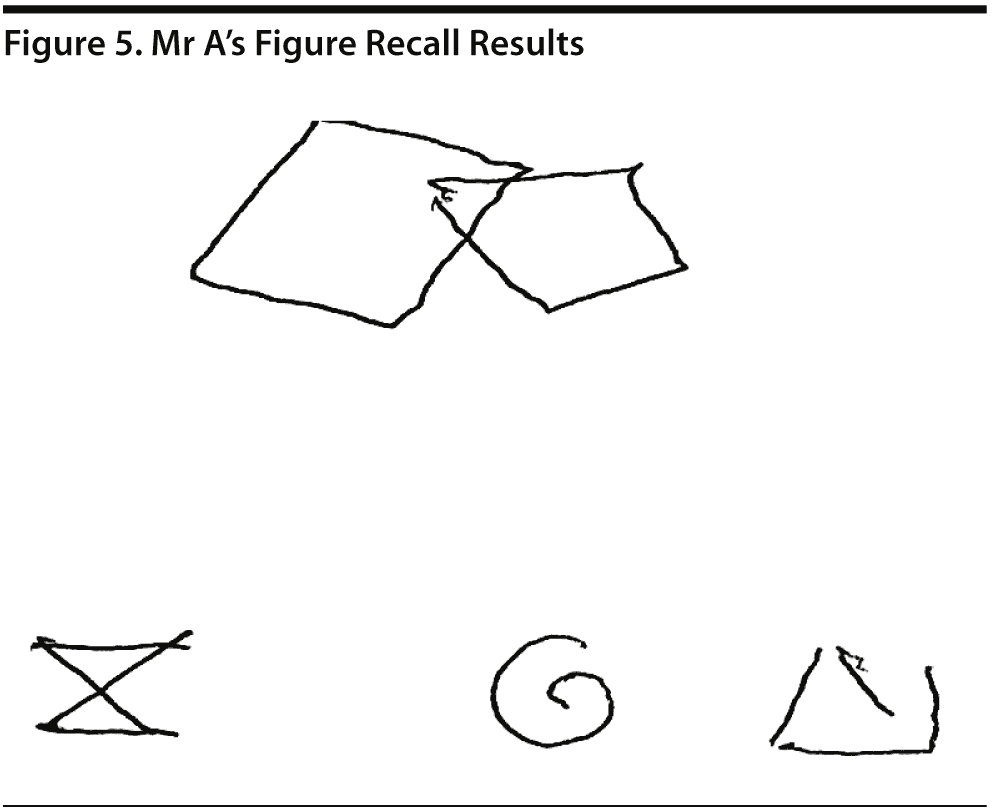
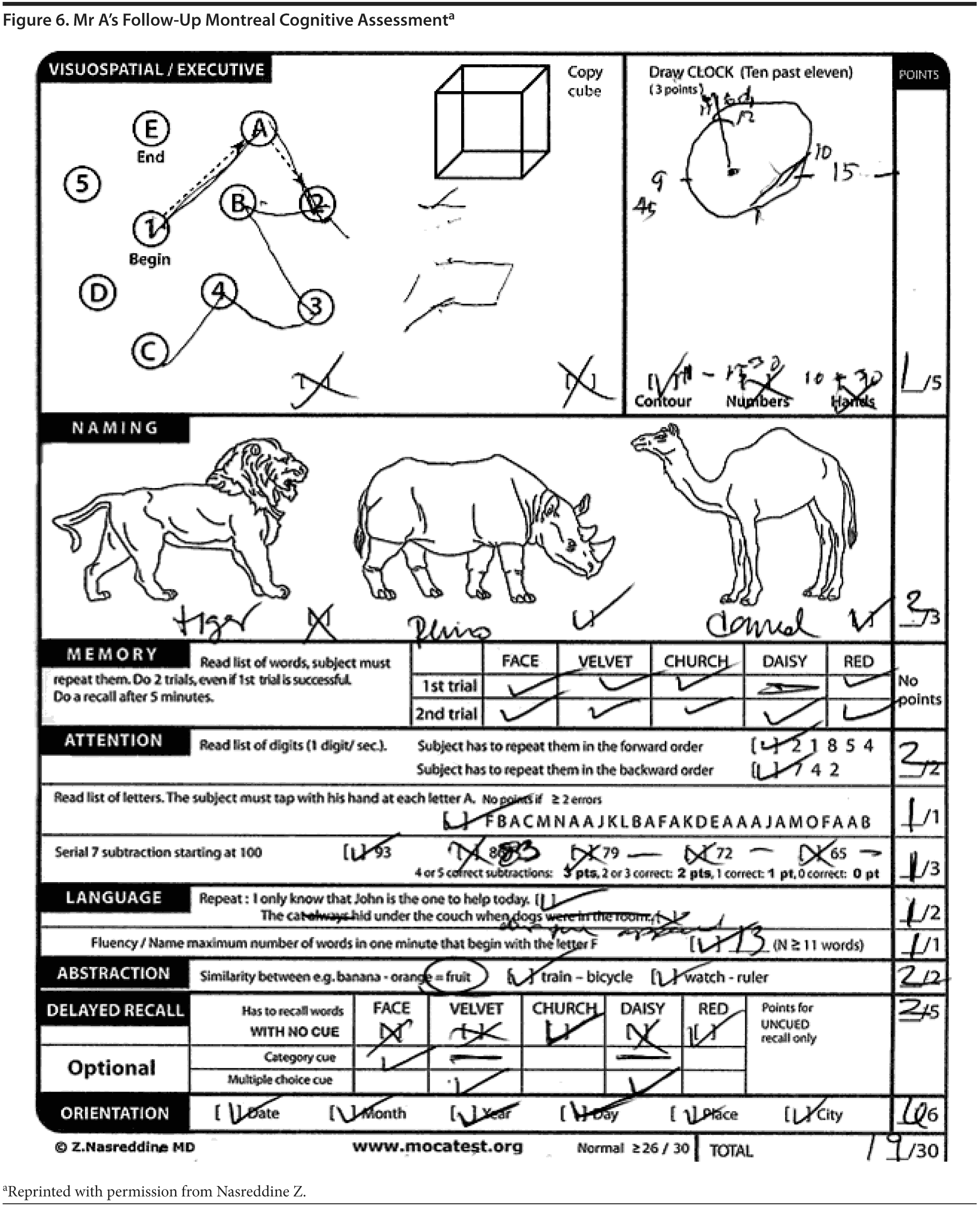
Mr A scored 21/30 on the MoCA. The results of Mr A’s MoCA test are provided in Figure 2. Mr A’s clock drawing showed minor errors with placement of hands (Figure 3). Figure 4 and Figure 5 show Mr A’s performance on figure copy and figure recall tests, respectively.
There was a discussion among the case conference participants regarding the scoring for the clock drawing. When asked whether they would give Mr A the 1 point for the placement of numbers on the clock, 81% said yes; however, 2 participants said no because the numbers were not equidistributed on each of the quadrants, though they would consider the placement outside the contour of the clock acceptable.
The DSM-5 defines dementia—or major neurocognitive disorder—as multiple cognitive deficits that include memory impairment and at least 1 of the following cognitive disturbances: aphasia, apraxia, agnosia, or a disturbance in executive functioning. The cognitive deficits must be at least 2 standard deviations from the normative mean by objective testing and sufficiently severe to cause impairment in social or occupational functioning and must represent a decline from a previously higher level of functioning. There was a discussion among the participants about the introduction of new terminology of major neurocognitive disorder. Some of the participants shared their experiences that the new nomenclature contributed to an additional stress to the patients bearing the one new diagnosis of a “major” neurocognitive disorder.
On the basis of the information so far, do you think Mr A falls under DSM-5 or National Institute on Aging-Alzheimer’s Association diagnostic criteria for dementia?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Yes | 82% |
| B. No | 9% |
| C. Not enough information | 9% |
Of the participants, 82% felt that Mr A would fit the diagnosis of dementia given his profound functional impairment. One participant thought he might not fit the criteria for dementia given that his scores on cognitive testing were not in the clear dementia range. One participant thought there was not enough information at this point, as the functional impairment could mostly be attributed to the problems in vision.
On further bedside testing, the attending neurologist asked Mr A to identify and describe a few complex pictures from a popular magazine in the office. Mr A found it quite challenging to interpret pictures out of a magazine. On several occasions, he was able to get the answer correct, but it took substantial prompting and time for him to be able to interpret the picture. For example, when showed a bowl of stir-fried noodles and vegetables, Mr A said, “This is either french fries or carrots.” He ultimately was able to individually identify the objects in the bowl after more prompting. He made other similar mistakes with similar pictures. When asked to bisect randomly placed horizontal lines on a sheet of paper, Mr A easily bisected the lines on the left side of the page, but had more difficulty and took more time to bisect lines on the right side of the page. Mr A was offered a page with numerous crosses and circles drawn. When asked to discriminate the crosses and circles on the page, he was able to perform with considerable ease on the left side of the page, but with more difficulty and time on the right side of the page. Mr A did not have difficulty in identifying people’s faces.
On the basis of the information presented thus far, which of the following provisional diagnoses would you rate most probable?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Mild cognitive impairment not due to Alzheimer’s disease | 27% |
| B. Mild cognitive impairment due to Alzheimer’s disease | 0% |
| C. Mixed dementia (vascular + Alzheimer’s disease) | 0% |
| D. Cognitive problems due to obstructive sleep apnea | 0% |
| E. Isolated visual agnosia | 64% |
| F. Visual variant of Alzheimer’s disease | 9% |
The majority of the participants (64%) felt that this was a case of isolated visual agnosia. Three participants felt it was a case of mild cognitive impairment due to a cause other than Alzheimer’s disease. One participant felt it could be a case of visual variant of Alzheimer’s disease.
Which of the following would you order for this patient as the immediate next workup?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. A detailed neuropsychological examination | 0% |
| B. Vitamin B12, TSH, brain MRI | 0% |
| C. FDG-PET | 0% |
| D. Amyloid PET | 0% |
| E. A and B | 100% |
| F. B and C | 0% |
All of the participants unanimously agreed that Mr A needed to be tested for vitamin B12 and TSH levels and to have an MRI scan and a detailed neuropsychological examination.
The American Academy of Neurology practice guidelines include a metabolic panel, vitamin B12, TSH, and structural neuroimaging comprising a computed tomography or MRI brain scan as routine workup in all suspected cases of dementia (Knopman et al, 2001).
Treating Physician’s Impression
Mr A, a 62-year-old right-handed man, presented with 3 years of insidious onset and gradually progressive cognitive complaints. His presentation was somewhat atypical in that he had a remarkably sound episodic memory, but prominent cortical visual impairment, including visual agnosias. He had difficulty with visual recall, but visual recognition was mostly intact. Auditory memory was better than visual memory. He also had difficulty with executive function and attention. There was no substantial component of anxiety or depression during test taking.
This presentation, according to the treating physician, at that point had a somewhat broad differential diagnosis. The atypical posterior cerebral atrophy variants of Alzheimer’s disease could present with prominent visuospatial and executive function difficulties. This was certainly on the differential diagnosis. Other etiologies, particularly cerebrovascular disease and space-occupying lesions, must be ruled out first. Once those etiologies are ruled out, the plan would be to consider functional imaging and testing specific to Alzheimer’s pathology to better elucidate the cause of Mr A’s symptoms. Even though he scored in the “mild cognitive impairment” range on all of the testing, Mr A had a disproportionate dysfunction in instrumental activities of daily living, particularly regarding his inability to continue functioning at his job. Therefore, the treating physician thought that Mr A met clinical diagnostic criteria for mild dementia of unspecified etiology at this time.
Would you consider starting Mr A on an acetylcholinesterase inhibitor at this time?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Yes | 19% |
| B. No | 81% |
The majority (81%) of the participants thought that it was inappropriate to start Mr A on an acetylcholinesterase inhibitor until non-Alzheimer’s disease pathologies were ruled out and given the fact that Mr A did not have prominent memory impairment. Two participants felt that, given Mr A’s level of functional impairment, it was a reasonable option to consider starting him on a potentially off-label acetylcholinesterase inhibitor.
TREATING PHYSICIAN’ S PLAN
The attending neurologist ordered vitamin B12 and TSH tests, which were normal. Detailed neuropsychological testing and MRI scan (with and without contrast) were ordered. Mr A was also advised to be reevaluated for sleep apnea and fitting for CPAP, which may be influencing his day time cognitive function. Mr A was asked to follow up after completing these additional tests.
FOLLOW-UP
Mr A followed up in the clinic after completing the workup. He had self-discontinued all of his antidepressant medication. Overall, he felt well and did not report any substantial anhedonia or suicidal ideation. He continued to have mild anxiety and stress. He had repeat sleep studies performed and was found to no longer have any significant sleep apnea. He had another visual optometrist examination and was given new prescription lenses for his glasses. However, the visual symptoms persisted.
An MRI of the brain showed moderate atrophy of the hippocampus as well as global atrophy with a posterior predominance. There was no significant cerebrovascular disease.
NEUROPSYCHOLOGICAL TESTING
Detailed neuropsychological testing showed significant impairment in concentration and attention as well as slowing of cognitive processing. Figure copy was significantly impaired. Calculations showed impairment. Visual memory was impaired. There was severe slowing across visual motor speed tasks. He was severely slowed on a fine dexterity speed task and on a task requiring detection of a target in an array and symbol-digit copy. He became confused on some visuospatial tasks such as copying a complex figure. He could not complete the visual copy task due to confusion regarding the complex lines in the figure; he had errors with elementary calculations and did not appear to understand mathematics beyond an elementary school level. The neuropsychologist suggested that it would be important to rule out any eye problems that may be contributing to his extremely poor functioning on the above tests. The tests that required visual scanning and tracking and interpretation of symbols and numbers were also severely impaired. He was mildly impaired on a test of semantic language and had occasional mild anomia. On memory testing, recall performance was ultimately low average on a measure of rote verbal learning, although his performance was impaired during the learning trials. Memory for logical information was average after a 30-minute delay. Mild-to-moderate impairment in learning and recall of visual information was reported.
TREATING PHYSICIAN’ S DIAGNOSIS AND PLAN
The diagnostic workup at this point had ruled out other significant treatable dementias or structural abnormalities. In summary, Mr A was a 62-year-old right-handed man with several years of worsening visual perceptual problems, mild executive dysfunction, and memory problems. His depression and sleep problems were unlikely to be contributing to the cognitive complaints but required further monitoring and management. Given the progressive history of the cognitive symptoms, this mostly represented a neurodegenerative process. It was felt that the most likely etiologies were posterior cortical atrophy variant of Alzheimer’s disease, dementia with Lewy bodies, or an atypical tauopathy. The follow-up MMSE and MoCA (Figure 6) scores were relatively stable at 26 and 19, respectively.
Mr A was started on citalopram 10 mg once a day for the mild anxiety and depression. After detailed discussion about the relationship of his cognitive symptoms to possible Alzheimer’s disease dementia, advanced biomarker testing was suggested to improve diagnostic certainty in the underlying disease process. This testing would help inform management given the differential diagnosis. The potential risks, costs, and benefits of spinal fluid analysis for amyloid and tau proteins, FDG-PET for patterns of brain metabolism, and amyloid PET for evidence of Alzheimer’s disease pathology were discussed. Information about ongoing clinical treatment trials in Alzheimer’s disease was provided. The option of acetylcholinesterase inhibitors was deferred until after further testing, considering mild memory and functional impairment in this patient with an uncertain diagnosis of possible Alzheimer’s disease.
On the basis of the information so far, what do you think would be the result of an amyloid PET scan in this patient?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Positive | 63% |
| B. Negative | 9% |
Of the conference participants, 63% thought that amyloid PET testing would be positive and 1 participant thought it would be negative. Others thought it might not be appropriate to have an amyloid PET scan for this patient. The participants in support of the scan thought that the amyloid PET would help to establish a more definitive diagnosis given the atypical presentation of this patient and would help outline the prognosis and treatment. The scan results might also contribute to the patient’s eligibility to participate in different clinical research trials. The one disadvantage of the scan that came out of the discussion was its cost, which is around $3,500, and the fact that it is not covered under most of the insurance plans.
UPDATE
Mr A subsequently decided to have an amyloid PET scan to rule out Alzheimer’s disease. The scan showed multiple areas of β-amyloid aggregates in bilateral parietal and frontal cortices. The most intense tracer uptake was noticed in bilateral temporal lobes.
CONCLUSION AND PLAN
Mr A was formally diagnosed with visual variant Alzheimer’s disease. After 4-month repeat testing showed a mild decline in memory, it was recommended that he start an acetylcholinesterase inhibitor as indicated for Alzheimer’s disease dementia. He also opted to enroll in a clinical treatment trial to potentially obtain newer and possibly beneficial medications in development for Alzheimer’s disease. Mr A will be followed in the clinic with repeat testing every 4 to 6 months for further management decisions. He was introduced to local resources related to Alzheimer’s disease and enrolled in the Family and Community Services program for social, emotional, and behavioral support for him and his family.
DISCUSSION
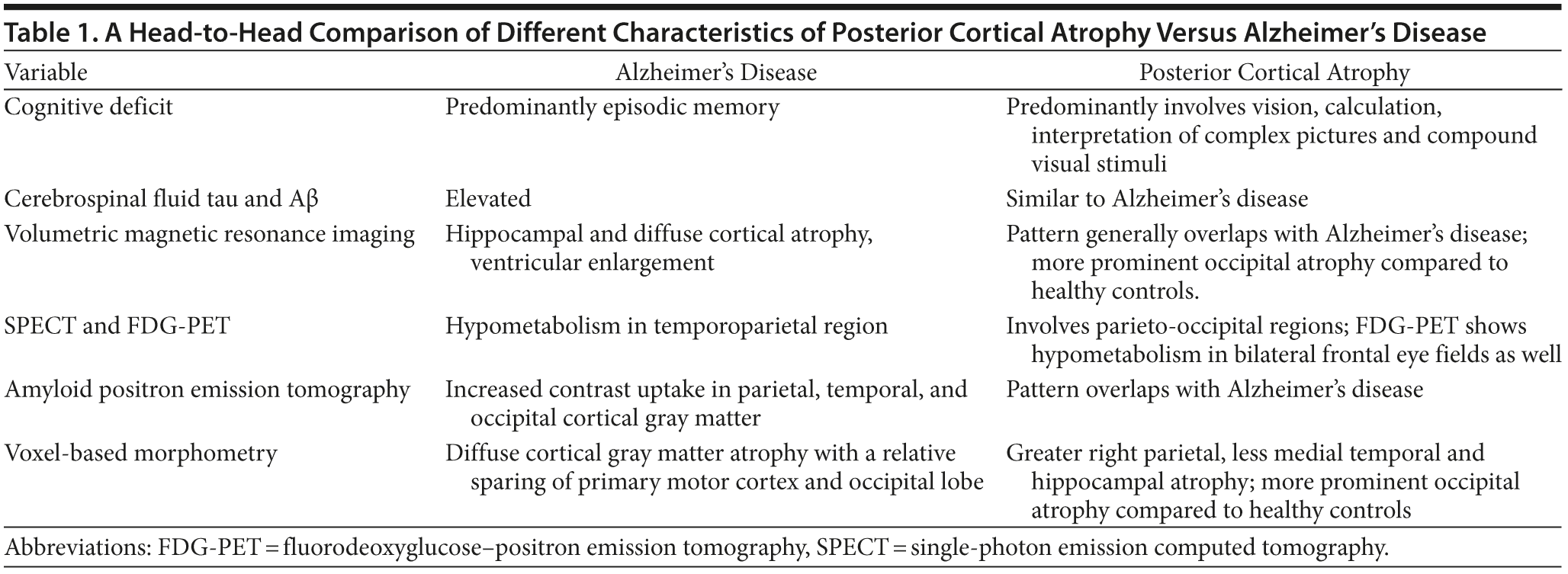
Magnetic resonance imaging of the brain in Alzheimer’s disease often shows global cortical atrophy, most prominent in the medial temporal lobe. However, in posterior cortical atrophy, the MRI shows a posterior predominance, which is consistent with our findings. Objective scoring systems have been developed to help in the visual analysis of these scans (Koedam et al, 2011). Studies have differed as to how neuroimaging may be used to support the diagnosis of posterior cortical atrophy. FDG-PET may demonstrate a posterior prominent hypometabolism but often is not sensitive to changes in the early stages of Alzheimer’s disease (Ishii, 2013). Moreover, posterior cortical atrophy and Alzheimer’s disease have been shown to have similar topography in β-amyloid deposition with amyloid PET (de Souza et al, 2011).
POSTERIOR CORTICAL ATROPHY
Posterior cortical atrophy is a clinical radiologic syndrome characterized by progressive decline in visual processing skills, with relatively intact episodic memory and language in the early stages, and atrophy of posterior brain regions (Crutch et al, 2012). Posterior cortical atrophy typically presents in the mid-50s and early 60s with a variety of symptoms, such as difficulty with visual interpretation, object locating, or reaching for objects under visual guidance, as well as difficulty with navigation. Misdiagnosis of posterior cortical atrophy is common because of its relative rarity and unusual and variable presentation, but also because patients frequently seek the opinion of an ophthalmologist who may note normal eye examinations, not always appreciating the cortical brain dysfunction. Moreover, neurologists do not often test for simultanagnosia (the inability to perceive more than 1 object at the same time) and other visuospatial or visuoperceptual disturbances (Crutch et al, 2013b). Studies have shown that in addition to the well-reported degradation of vision, literacy, and numeracy, posterior cortical atrophy is also characterized by progressive oral language dysfunction with prominent word retrieval difficulties (Crutch et al, 2013a). This is in agreement with the findings in our case. Multiple groups have previously proposed diagnostic criteria for this syndrome (Kas et al, 2011; McMonagle et al, 2006; Mendez et al, 2002; Tang-Wai et al, 2004). In July 2012, a multidisciplinary group of posterior cortical atrophy research clinicians formed an International Working Party to lay out consensus criteria for the diagnosis of posterior cortical atrophy (Crutch et al, 2013b). According to these criteria, the core features of posterior cortical atrophy comprise the following: (a) insidious onset and gradual progression, (b) prominent visuoperceptual and visuospatial impairments but no significant impairment of vision itself, (c) relative preservation of memory and insight, (d) evidence of complex visual disorders (eg, elements of Balint syndrome, Gerstman syndrome, visual field defects, visual agnosia, environmental disorientation), and (e) absence of stroke or tumor. The other features supportive to the diagnosis include (a) presenile onset, (b) alexia, (c) ideomotor or dressing apraxia, (d) prosopagnosia, and (e) prolonged color afterimages (Crutch et al, 2013b).
Ideomotor apraxia refers to the difficulty to plan or complete complex motor tasks that rely on memory. Interestingly, these patients may be able to perform an act automatically when cued; also, they may be able to explain how to perform an action (eg, Patients with ideomotor apraxia may not be able to carry out the following instruction: “Pretend to brush your teeth.” They may not be able to pick up a phone when asked to do so, but may be able to do the same when the phone bell rings).
Prosopagnosia refers to the inability to identify faces while other aspects of visual processing remain intact.
Posterior Cortical Atrophy Versus Alzheimer’s Disease
Table 1 shows a head-to-head comparison of posterior cortical atrophy and Alzheimer’s disease. The visual deficits in posterior cortical atrophy can be evaluated using tests of basic visual processing and higher visual processing. While basic visual processing includes the ability of form detection, form coherence, form discrimination, color discrimination, motion coherence, and point localization, higher visual processing includes object perception and space perception. Basic visual processing dysfunction is shown to have significant impact on higher-order visual dysfunctions. Form detection, form coherence, and color performance correlate with object and space perception. Form detection could predict only object but not space perception. Point localization predicted only space perception but not object perception (Lehmann et al, 2011).
There are several proposed anatomic and functional classifications of posterior cortical atrophy. For example, posterior cortical atrophy with posterior parietal cortical thinning is associated with space perception problems, while inferior temporal thinning is associated with object perception problems. Posterior cortical atrophy can also be classified on the basis of several other parameters. For example, those primarily affecting dominant versus those affecting nondominant hemispheres and those that are unilateral versus those that are bilateral. It is also classified on the basis of the patient’s visual deficits, which may affect dorsal visual stream, ventral visual stream, or primary visual processing. Others consider posterior cortical atrophy as a phenotypic continuum (Lehmann et al, 2011; Ross et al, 1996).
In summary, the fact that posterior cortical atrophy is much rarer in the clinical spectrum of dementias warrants the clinician to be more vigilant in administering screening tests of basic cortical visual functions and higher visual processing when suspicion arises. It would not be inappropriate to describe posterior cortical atrophy as part of a continuum of cognitive dysfunction that manifests as visual symptoms, yet arises from the β-amyloid pathology associated with Alzheimer’s disease.
Disclosure of off-label usage
The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
FACULTY FINANCIAL DISCLOSURE
Dr Yaari is currently an employee of Eli Lilly. Dr Tariot has served as a consultant for Abbott, AbbVie, AC Immune, Adamas, Boehringer-Ingelheim, California Pacific Medical Center, Chase, Chiesi, CME Inc, Elan, MedAvante, Merz, Otsuka, and Sanofi-Aventis; has received consulting fees and research support from Avanir, Avid, Bristol-Myers Squibb, Cognoptix, Eli Lilly, GlaxoSmithKline, Janssen, Medivation, Merck, and Roche; has received research support only from AstraZeneca, Baxter, Functional Neuromodulation, GE, Genentech, Pfizer, Targacept, and Toyama; has received other research support from Arizona Department of Health Services and National Institute on Aging; is a stock shareholder in Adamas; and is listed as a contributor to a patent owned by the University of Rochester (Rochester, New York) for “Biomarkers of Alzheimer’s Disease.” Drs Das, Fleisher, Seward, and Burke and Ms Brand have no personal affiliations or financial relationships with any commercial interest to disclose relative to the activity.
Clinical Points
- Posterior cortical atrophy is a clinical radiologic syndrome characterized by progressive decline in visual processing skills, with relatively intact episodic memory and language in the early stages, and atrophy of posterior brain regions.
- Patients with posterior cortical atrophy present at a relatively early age compared to Alzheimer’s disease and predominantly evidence visual symptoms.
- A careful history taking and a detailed bedside examination, including testing for complex figure copy and simultanagnosia, are important to make the diagnosis.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
Crutch SJ, Lehmann M, Schott JM, et al. Posterior cortical atrophy. Lancet Neurol. 2012;11(2):170-178. PubMed doi:10.1016/S1474-4422(11)70289-7
Crutch SJ, Lehmann M, Warren JD, et al. The language profile of posterior cortical atrophy. J Neurol Neurosurg Psychiatry. 2013a;84(4):460-466. PubMed doi:10.1136/jnnp-2012-303309
Crutch SJ, Schott JM, Rabinovici GD, et al. Shining a light on posterior cortical atrophy. Alzheimers Dement. 2013b;9(4):463-465. PubMed doi:10.1016/j.jalz.2012.11.004
de Souza LC, Corlier F, Habert MO, et al. Similar amyloid-β burden in posterior cortical atrophy and Alzheimer’s disease. Brain. 2011;134(Pt 7):2036-2043. PubMed doi:10.1093/brain/awr130
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
Ishii K. PET Approaches for diagnosis of dementia [published online ahead of print August 14, 2013]. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A3695
Jefferis JM, Collerton J, Taylor JP, et al. The impact of visual impairment on Mini-Mental State Examination Scores in the Newcastle 85+ study. Age Ageing. 2012;41(4):565-568. PubMed doi:10.1093/ageing/afs042
Johns EK, Phillips NA, Chertkow H, et al. The Montreal Cognitive Assessment: normative data in the community. In: Final Program of the 36th Annual Meeting of the International Neuropsychological Society; February 6-9, 2008; Waikoloa, Hawaii. J Int Neuropsychol Soc. 2008;41(suppl 1):58.
Johns EK, Phillips NA, Chertkow H, et al. The effect of education on performance on the Montreal Cognitive Assessment (MoCA): normative data from the community [poster]. Canadian J Geriatrics. 2008:11(1):62. Presented at the 28th Annual Meeting of the Canadian Geriatrics Society; April 2008; Montreal, Quebec, Canada
Kas A, de Souza LC, Samri D, et al. Neural correlates of cognitive impairment in posterior cortical atrophy. Brain. 2011;134(Pt 5):1464-1478. PubMed doi:10.1093/brain/awr055
Knopman DS, DeKosky ST, Cummings JL, et al. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153. PubMed doi:10.1212/WNL.56.9.1143
Koedam EL, Lehmann M, van der Flier WM, et al. Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol. 2011;21(12):2618-2625. PubMed doi:10.1007/s00330-011-2205-4
Lehmann M, Barnes J, Ridgway GR, et al. Basic visual function and cortical thickness patterns in posterior cortical atrophy. Cereb Cortex. 2011;21(9):2122-2132. PubMed doi:10.1093/cercor/bhq287
Links KA, Merims D, Binns MA, et al. Prevalence of primitive reflexes and Parkinsonian signs in dementia. Can J Neurol Sci. 2010;37(5):601-607. PubMed
McMonagle P, Deering F, Berliner Y, et al. The cognitive profile of posterior cortical atrophy. Neurology. 2006;66(3):331-338. PubMed doi:10.1212/01.wnl.0000196477.78548.db
Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14(1):33-40. PubMed doi:10.1159/000058331
Mungas D. In-office mental status testing: a practical guide. Geriatrics. 1991;46(7):54-58, 63, 66. PubMed
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. PubMed doi:10.1111/j.1532-5415.2005.53221.x
Ross SJ, Graham N, Stuart-Green L, et al. Progressive biparietal atrophy: an atypical presentation of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;61(4):388-395. PubMed doi:10.1136/jnnp.61.4.388
Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168-1174. PubMed doi:10.1212/01.WNL.0000140289.18472.15
Enjoy this premium PDF as part of your membership benefits!