Assessment of Falls in Older Patients Treated With Duloxetine: A Secondary Analysis of a 24-Week Randomized, Placebo-Controlled Trial
ABSTRACT
Objective: To assess fall events in older depressed patients during treatment with duloxetine.
Method: Post hoc analysis of solicited fall events collected at each study visit using a questionnaire during a 24-week, multicenter, randomized, placebo-controlled, double-blind, phase 4 trial (November 2006 to November 2009). Older outpatients (≥ 65 years) with major depressive disorder (DSM-IV criteria) were randomly assigned to duloxetine 60 mg/d (n = 249) or placebo (n = 121) for the 12-week acute phase, after which the duloxetine dose could be increased to 120 mg/d and nonresponding placebo patients could be switched to duloxetine 60 mg/d. Between-treatment differences in percentages of patients with fall events were compared by Fisher exact test. Exposure-adjusted incidence rates (EAIRs) of falls per patient-year were also estimated.
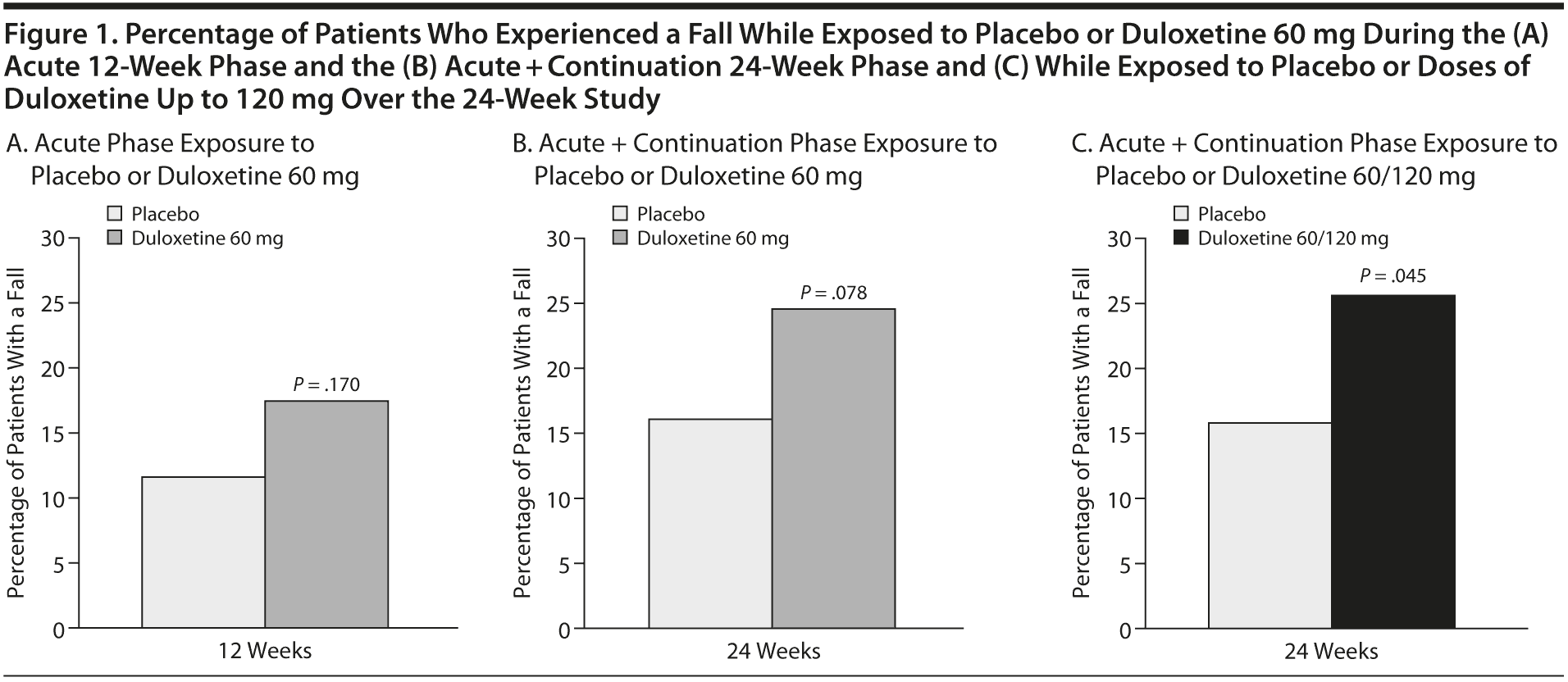
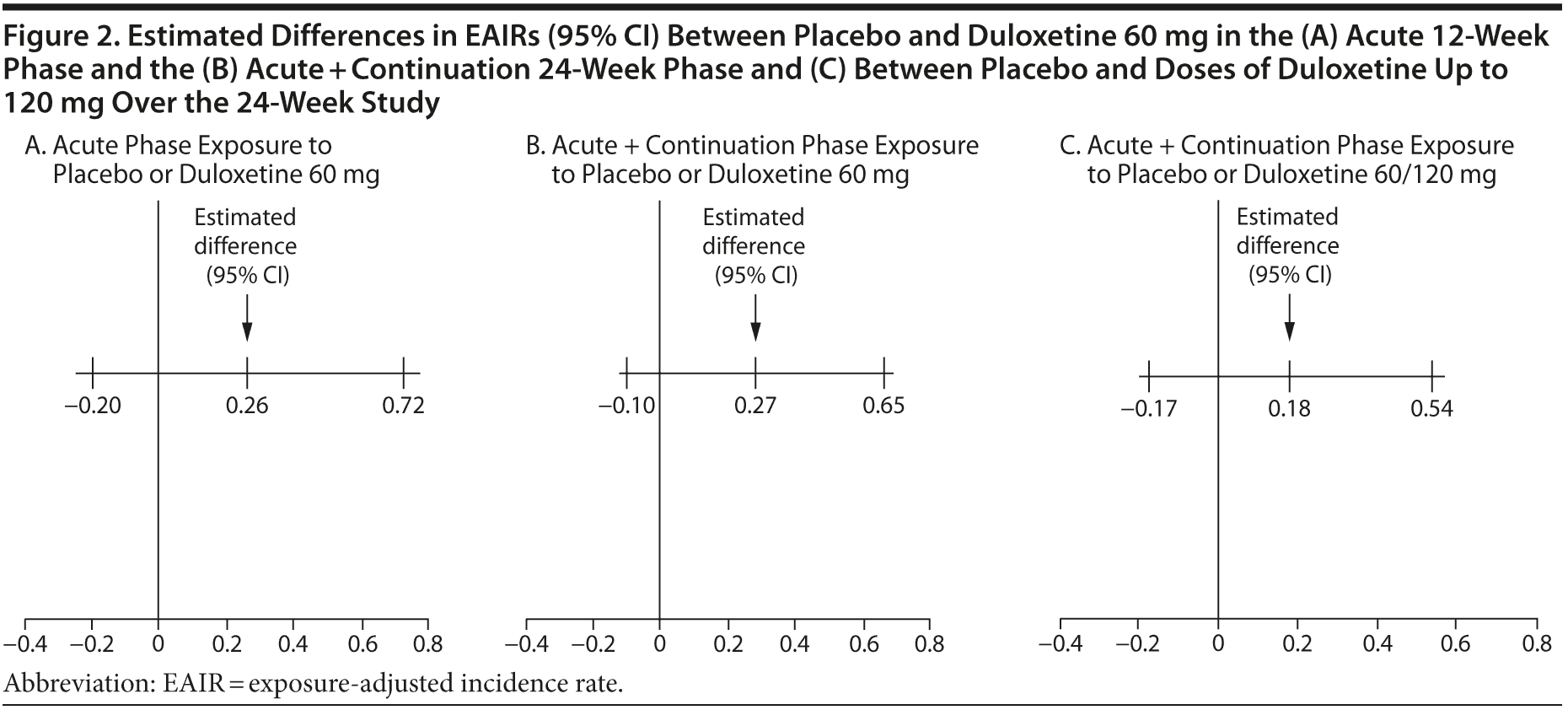
Results: In the acute phase, 17.3% of patients treated with duloxetine 60 mg versus 11.6% treated with placebo (P = .170) experienced a fall event. Over 24 weeks, the percentage of patients with a fall while taking duloxetine 60 mg versus placebo was 24.0% versus 15.7% (P = .078), and the percentage was significantly higher in patients taking duloxetine regardless of dose (25.3%) than with placebo (15.7%, P = .045). Between-treatment differences in EAIRs over 12 weeks (0.26; 95% CI, −0.20 to 0.72) and over 24 weeks (0.27; 95% CI, −0.10 to 0.65) were not significant.
Conclusions: Direct assessment of fall events greatly increases the number of falls reported by patients. Although the EAIR of falls per patient-year associated with duloxetine was not significant in this trial, clinicians should remain vigilant about the possibility of falls in older patients with duloxetine or any antidepressant treatment.
Trial Registration: ClinicalTrials.gov identifier NCT00406848
Prim Care Companion CNS Disord 2013;15(1):doi:10.4088/PCC.12m01419
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: May 30, 2012; accepted September 17, 2012.
Published online: January 3, 2013.
Corresponding author: Tina M. M. Oakes, PhD, Eli Lilly and Company, Drop Code 1542, Indianapolis, IN 46285 ([email protected]).
In the United States, approximately 22% to 40% of persons aged 65 years or older will experience a fall at least once each year.1,2 In this older population, falls are a significant cause of injuries, loss of confidence, increased morbidity, and, for some, loss of independence, institutionalization, and mortality.3,4 Accidental or environmental causes account for 30% to 50% of the falls, which are associated with gaits that are stiffer and less coordinated than in younger people.1 Age-related impairment in vision, hearing, and memory also contribute to tripping and stumbling.1 Other risk factors for falling include dizziness, drop attacks (defined as sudden falls without loss of consciousness or dizziness), syncope, postural hypotension, weak grip strength, low body weight, central nervous system disorders, cognitive deficits, drug side effects, depression, alcohol consumption, anemia, hypothyroidism, severe osteoporosis with spontaneous fracture, acute illness, fear of falling, and history of falling.1,5-7 In addition, psychoactive medications including antidepressants,6,8-12 anxiolytics, and sedatives9,10 are associated with an increased incidence of falls in older persons.
Duloxetine is a selective serotonin norepinephrine reuptake inhibitor that has been approved by the US Food and Drug Administration for the treatment of major depressive disorder (MDD) and generalized anxiety disorder (GAD) and for the management of diabetic peripheral neuropathic pain, fibromyalgia, and chronic musculoskeletal pain (as established in studies of chronic low back pain and chronic pain due to osteoarthritis). In addition to MDD, GAD, and diabetic peripheral neuropathic pain, duloxetine has also been approved by the European Medicines Agency for the treatment of lower urinary tract disorders.
In a recent 24-week study of duloxetine for treatment of MDD in older patients (F1J-US-HMFA),13 the incidence of experiencing a fall was assessed at each study visit in the following 2 ways: (1) as spontaneously reported treatment-emergent adverse events (TEAEs) and (2) as a solicited response to a specific fall assessment questionnaire. Over the course of the 24-week study using method 1, fall rates were significantly higher for duloxetine-treated patients compared to placebo-treated patients (23.7% vs 14.0%, P = .039),13 and these rates were much higher than what was reported in an 8-week trial of duloxetine in older patients with MDD14 in which only spontaneous TEAE reporting was utilized to assess falls. It was proposed that the solicitation of fall history (method 2) in this study may have influenced the rate of falls reported as spontaneous TEAEs.13
Here, we present a post hoc analysis of the solicited falls data from the 24-week study to further understand the incidence of falls associated with duloxetine or placebo treatment in elderly depressed patients. Specifically, in addition to the crude percentage of patients with a fall event, we examined whether variability in the duration of exposure to duloxetine and placebo influenced the solicited falls results. We also examined the potential influence of comorbid medical conditions and concomitant medications on the incidence of falls.
METHOD
For this post hoc analysis, data were derived from a 24-week, multicenter, randomized, placebo-controlled, double-blind phase 4 study (ClinicalTrials.gov identifier NCT00406848) conducted from November 2006 to November 2009 that compared duloxetine to placebo for treatment of MDD (DSM-IV criteria15) in older patients ≥ 65 years. Details of this study and the primary outcomes have been published,13 so only a brief overview is summarized here. The study included older patients who were randomly assigned to duloxetine 60 mg/d (n = 249) and placebo (n = 121) for the 12-week acute phase. During the following 12-week continuation phase, placebo rescue or duloxetine dose optimization were available if patients had < 50% reduction from baseline in the 17-item Hamilton Depression Rating Scale16 (HDRS-17) total score at week 12 or had a HDRS-17 total score > 10 at weeks 16 or 20 and therapy adjustment was deemed appropriate by the investigator. Placebo rescue and dose optimization were instituted in a double-blind fashion. Nonresponding duloxetine-treated patients had their dose increased to 120 mg/d, and patients taking placebo who had not responded could be switched to duloxetine 60 mg/d for the remainder of the trial. After dose escalation, 1 dose decrease due to safety or tolerability was allowed; if a second was requested, the patient was discontinued from the study. Duloxetine and placebo were administered once daily.

- The incidence of falling increases with age.
- Older patients may experience a fall when taking psychoactive medications.
- Patients may not spontaneously report falls; therefore, clinicians should ask their patients if they have fallen while taking antidepressants or other psychoactive medications.
At each visit, patients responded yes/no to a simple unpublished questionnaire that solicited fall events. If the patient responded yes, additional questions were asked regarding the status of the patient when the fall occurred, whether or not the patient used walking aids, what physical complaints the patient had at the time of the fall, and the outcome of the fall. For this article, we report the results of the analyses on the frequency and exposure-adjusted incidence rate (EAIR) of fall events based on the solicited fall assessment questionnaire.
The crude percentage of patients who experienced a fall was compared between duloxetine and placebo treatment for the acute phase and over the duration of the study. For those patients who remained on duloxetine 60 mg or placebo, the observation period was 12 weeks for the acute phase and 24 weeks for the entire study. However, for placebo-treated patients who were switched to duloxetine in the continuation phase, the observation period for fall events was determined by the length of time on placebo prior to rescue. Any fall events that occurred after placebo patients were switched to duloxetine were not included in the treatment comparison. Similarly, for patients randomly assigned to duloxetine, the observation period for fall events was determined by the length of time they were taking duloxetine 60 mg prior to dose escalation. In addition, the percentage of randomized duloxetine patients who experienced a fall while they were on the treatment regardless of dose was also determined for the entire 24 weeks of the study.
In clinical studies, especially those of longer duration similar to the current study, patients’ actual exposure to study drug or placebo could vary due to early discontinuation and study design features such as rescue and dose escalation. One measure that accounts for the potential exposure imbalance between treatment groups is the EAIR, which is defined as the number of patients with a particular event divided by the total exposure time among patients in the treatment group and at risk for an initial occurrence of the event.17 For the present study, the EAIR for number of falls per patient-year was estimated on the basis of the patient’s actual duration of exposure to duloxetine 60 mg or placebo in the acute phase and over the duration of the entire study. In addition, the EAIR of fall events per patient-year based on treatment with duloxetine regardless of dose was also estimated for the entire study.
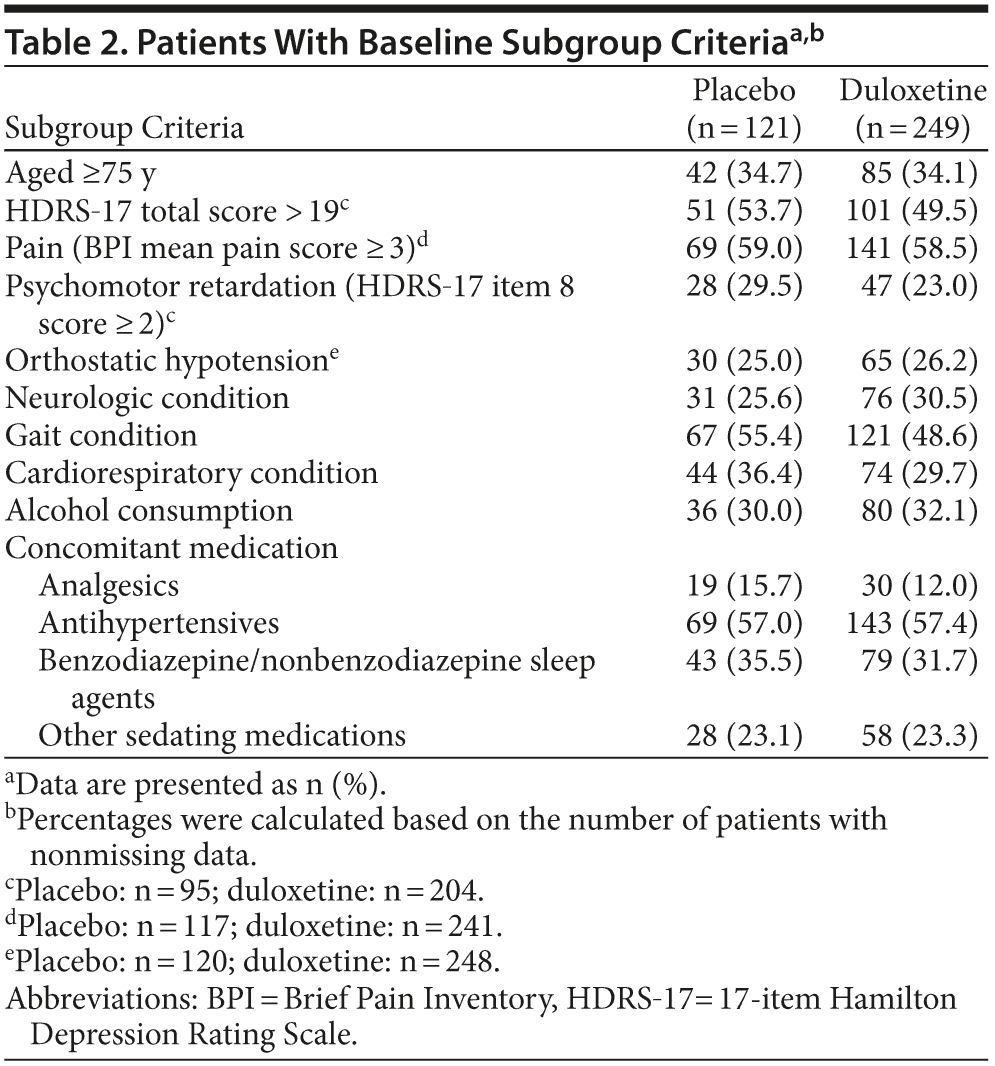
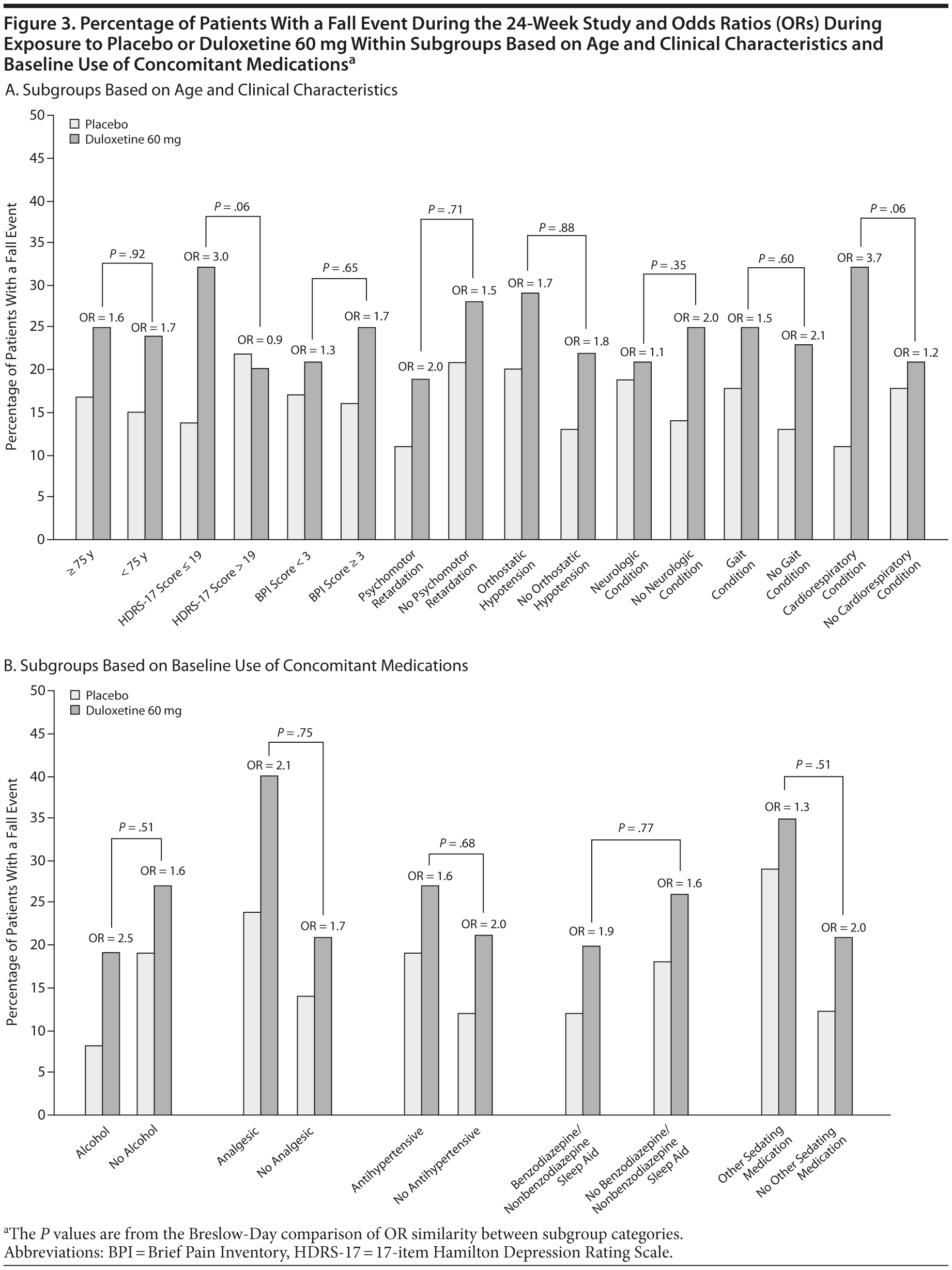
Subgroup analyses were performed to assess whether the effects of duloxetine relative to placebo on the frequency of falls were different between patients with and without specific characteristics. Subgroups were based on baseline characteristics of interest: high/low (> 19 vs ≤ 19) HDRS-17 total scores (HDRS-17 score ≤ 19 is mild severity; HDRS-17 score > 19 is moderate to more severe)17; Brief Pain Inventory 24-hour average pain severity (< 3 is mild; ≥3 is clinically significant pain)18; psychomotor retardation (item 8 of the HDRS ≥ 2); age (< 75 years of age vs older)19,20; baseline orthostatic hypotension; preexisting neurologic, cardiorespiratory, or gait-related conditions; and any alcohol consumption or concomitant medication use that included analgesics, antihypertensives, benzodiazepines and nonbenzodiazepine sleep agents, and other sedating medications. Baseline orthostatic hypotension was defined as standing diastolic blood pressure that was at least 10 mm Hg less than the supine diastolic blood pressure or standing systolic blood pressure that was at least 20 mm Hg less than the supine systolic blood pressure. Preexisting neurologic conditions included balance disorders and history of stroke; cognitive disorders/dementias; visual and hearing impairments; and migraine, psychomotor disorders, and somnolence. Preexisting conditions that might have an effect on gait included musculoskeletal disorders, history of falls, and peripheral neuropathies. Preexisting cardiorespiratory disorders included any cardiovascular and/or pulmonary disorder.
The difference between treatments in the crude percentage of patients who experienced a fall was compared by Fisher exact test, and statistical significance was noted at P ≤ .05. To compare the incidence of fall events per patient-year, the estimated difference between EAIRs for duloxetine and placebo was calculated along with corresponding 95% confidence intervals (CIs) using Wald’s method.21 EAIRs were considered significantly different if the 95% CI of the estimated treatment difference did not include zero.
The subgroup analyses estimated the percentage of patients with a fall by treatment in each subgroup category (ie, with vs without specified characteristic). Between-treatment odds ratios (ORs) of the percentages were estimated and compared between subgroup categories with the Breslow-Day test, and statistical significance was noted at P < .1. Statistical analyses were performed using SAS, version 9.1 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
As reported in the 24-week study,13 249 patients were randomly assigned to duloxetine and 121 to placebo. Most of the patients were women and white, and the mean age was 73 (65 to 90) years. Depression severity at baseline was moderate, with a mean total HDRS-17 score of 19; a few patients (n = 8) had mild dementia, but most had Mini-Mental State Examination22 total scores of approximately 28, indicating normal cognition. Study completion rates at 24 weeks were 55.4% for patients treated with placebo only (not rescued) and 62.7% for those treated with duloxetine.
The percentage of patients who experienced a fall during the study is shown in Figure 1A-C. Between-treatment differences in these percentages were not significant during the acute phase or over the duration of the study. During the acute phase, a fall was experienced by 17.3% of patients treated with duloxetine 60 mg and 11.6% of patients treated with placebo (P = .170). Over the 24 weeks of the study, falls were experienced by 24.0% of patients in the duloxetine group while they were treated with the 60-mg dose and by 15.7% of patients treated with placebo (P = .078). However, over the course of the entire 24-week study, 25.3% of patients randomly assigned to duloxetine experienced a fall while they were on treatment regardless of dose, and this was statistically greater than with placebo (15.7%, P = .045). Of those patients whose dose was increased to 120 mg (n = 66), only 3 experienced a fall.
In patients who were randomly assigned to duloxetine, the mean exposure duration to the drug regardless of dose was 133.2 days over the course of the 24-week study, which was longer than the mean exposure to placebo (103.7 days). Duloxetine patients’ mean exposure duration to the 60-mg dose (excluding time on 120 mg) was 113.9 days, which was still longer than, but more comparable to, exposure duration to placebo. This result is partly due to the placebo rescue feature of the study that led to shorter placebo exposure in the 12-week continuation phase.
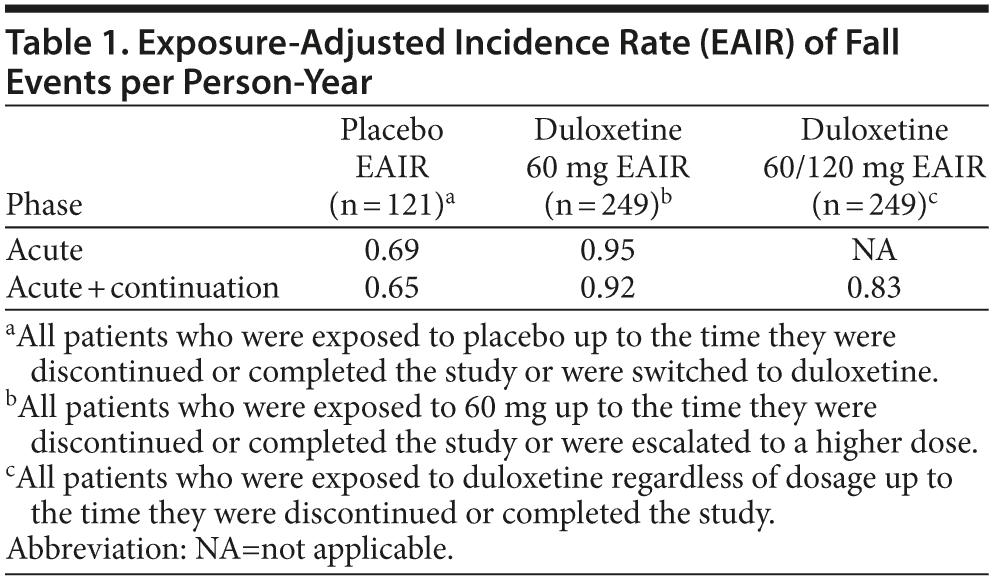
The EAIRs of fall events per patient-year for treatment with placebo, duloxetine 60 mg, and duloxetine regardless of dose are summarized in Table 1. The differences in EAIRs (and 95% CIs) between duloxetine and placebo were not significant during the acute phase or over the entire 24 weeks of the study, regardless of whether the EAIR was estimated based on patients’ exposure to the 60-mg dose only or titrated to 120 mg (Figure 2A-C).
The percentages of patients who met baseline criteria for subgroup inclusion are summarized in Table 2. The results of the subgroup analyses are shown in Figure 3. The analyses comparing subgroup categories based on age, illness severity, and comorbidity for the duration of the study are shown in Figure 3A; and the results based on concomitant medications and alcohol use are shown in Figure 3B. Most of the comparisons provided no substantial evidence for a differential effect of treatment with duloxetine as compared with placebo on the percentage of patients with a fall between subgroup categories, with the exception of baseline HDRS-17 total scores ≤ 19 and preexisting cardiorespiratory conditions. Over the course of the 24-week study, the OR of experiencing a fall with duloxetine 60 mg over placebo was significantly greater in patients with baseline HDRS-17 total scores ≤ 19 than it was for patients with higher scores. Also, over the course of the 24-week study, the OR of falling while taking duloxetine 60 mg versus placebo was significantly higher in patients with preexisting cardiorespiratory conditions as compared to patients without these conditions; a significant difference was, however, not seen during the acute phase (data not shown). Due to the small number of patients with preexisting cardiorespiratory conditions who had a fall (n = 29), it was not feasible to determine any particular condition that may have been associated with falls in patients who were exposed to duloxetine.
DISCUSSION
Solicitation of adverse events leads to higher reporting rates and is considered more sensitive and a potentially more accurate method of eliciting adverse events.23 Spontaneous adverse event reporting, which is the most common method of collecting adverse events in clinical trials, may only capture events that occurred most recently or that were particularly bothersome or that were considered serious in nature. In this 24-week study,13 a falls assessment questionnaire was utilized to specifically solicit fall events that occurred postbaseline. The percentage of patients with a fall while exposed to duloxetine 60 mg or placebo for 12 weeks in this study was about 7 times higher than the fall events reported spontaneously as TEAEs in a previous 8-week MDD study in older patients (duloxetine, 2.4%; placebo, 2.9%).24 When compared to spontaneously reported fall events in older patients (≥ 65 years) from an analysis of all placebo-controlled trials of duloxetine across all indications, solicited fall events associated with duloxetine reported in this 24-week study13 were over 15 times higher than falls reported as TEAEs (1.1%), and solicited events associated with placebo were nearly 30 times higher than their respective TEAE fall events (0.4%).
The duration of time that individuals are exposed to study drug or placebo in a clinical trial can vary due to early discontinuation, and this may impact the actual number of certain events observed, especially in longer trials and particularly for those events with an incidence rate that is relatively constant over time. In this study, the length of exposure to duloxetine or placebo could vary due to early discontinuation or to the design feature of dose escalation and placebo rescue that occurred during the 12-week continuation phase. To correct for exposure imbalance, we utilized EAIRs to compare fall events per person-year between duloxetine 60 mg/d and placebo during the acute phase and over the course of the entire study. The results suggest that the incidence of fall events was not significantly different between treatments when duration of exposure was taken into account. It is noteworthy that the EAIR of fall events based on exposure to either duloxetine 60 mg or 120 mg was no higher than that of duloxetine 60 mg only. Of 66 patients whose duloxetine dose was increased to 120 mg, only 3 more patients experienced a fall on the higher dose. In this study, it appeared that fall risk was not dose dependent; however, this study was not designed to determine the dose effect. Dose escalation to 120 mg occurred at different times, and patients were not randomly assigned to different doses. In addition, those patients who received the higher dose were more likely to have tolerated the lower dose well.
Significant differential effect of treatment with duloxetine over placebo on the OR of having a fall was not observed for most of the subgroups with the exception of patients with baseline HDRS-17 total scores ≤ 19 and those with preexisting cardiorespiratory conditions. The odds of falling while exposed to duloxetine versus placebo were greater in mild depression than in severe depression. These data do not indicate why this counterintuitive finding occurred. It is possible that in mild depression, adverse effects outweigh beneficial effects on depression, or that the effects of medication relative to placebo are more easily observed. In severe depression, the depressive symptoms may be a more important contributor to falls, and any increase in falls due to duloxetine side effects would be counterbalanced by decreases in falls due to improvements in underlying depression.
In patients with preexisting cardiorespiratory conditions, a different relationship was observed, particularly over the course of the 24 weeks of the study. In this subgroup, the significantly greater differential treatment effect of duloxetine over placebo on the percentage of patients with a fall, as compared to those patients without these conditions, may suggest a synergistic effect of duloxetine with this comorbidity. Even though there was no significant differential effect of duloxetine over placebo on fall events in patients with these conditions during the acute phase of the study, there is, nonetheless, a potential signal for the risk of falling in patients with these conditions who are treated with duloxetine.
There are limitations to this study to be considered. First, this was an exploratory post hoc analysis for which the primary study was not powered to compare between-treatment or between-subgroup differences in fall events. The lack of a randomized treatment arm for duloxetine 120 mg prevents any conclusions regarding a dose response on risk of falling. Further, the results of the subgroup analyses should be interpreted with caution because of the possibility of spurious results due to multiple testing and the possibility of false-negative results due to inadequate power.25
In conclusion, the direct assessment of fall history greatly increases the number of falls reported by patients. Although the EAIR of falls associated with duloxetine was not significantly different than that for placebo in this trial of older patients with MDD, clinicians should remain vigilant about the possibility of falls in older patients taking duloxetine or any antidepressant treatment.
Drug names: duloxetine (Cymbalta).
Author affiliations: Department of Psychiatry, University of California, San Francisco (Dr Nelson); Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, Indiana (Drs Oakes, Liu, Ahl, Bangs, and Robinson); Eli Lilly and Company, Toronto, Canada (Dr Raskin); and Lilly Research Center, Windlesham, Surrey, United Kingdom and the Gordon Hospital, London, United Kingdom (Dr Perahia).
Author contributions: All authors had full access to the data from this study, participated in the preparation of the manuscript, and approved it for publication.
Potential conflicts of interest: Dr Nelson has served as a consultant to Bristol-Myers Squibb, Cenestra Health, Corcept, Eli Lilly, Forest, Lundbeck, Medtronic, Merck, Otsuka, Pfizer, and Sunovion; has received grant/research support from Health Resources and Services Administration and National Institute of Mental Health; has received lecture honoraria from Eli Lilly Global, Lundbeck, Merck Asia, and Otsuka Asia; and is a stock shareholder in Atossa. Drs Oakes, Liu, Ahl, Bangs, Raskin, Perahia, and Robinson are employees of and stock shareholders in Eli Lilly or its subsidiaries.
Funding/support: This study was sponsored by Lilly USA, LLC, Indianapolis, Indiana.
Previous presentation: Presented in part at the Annual Scientific Meeting of the American Geriatrics Society; May 2-5, 2012; Seattle, Washington.
Acknowledgments: The authors wish to thank all the investigators and patients for their role in the conduct of this study.
REFERENCES
1. Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(suppl 2):ii37-ii41. PubMed
2. Shumway-Cook A, Ciol MA, Hoffman J, et al. Falls in the Medicare population: incidence, associated factors, and impact on health care. Phys Ther. 2009;89(4):324-332. PubMed doi:10.2522/ptj.20070107
3. Tinetti ME, Inouye SK, Gill TM, et al. Shared risk factors for falls, incontinence, and functional dependence: unifying the approach to geriatric syndromes. JAMA. 1995;273(17):1348-1353. PubMed doi:10.1001/jama.1995.03520410042024
4. Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337(18):1279-1284. PubMed doi:10.1056/NEJM199710303371806
5. Bloch F, Thibaud M, Dugué B, et al. Episodes of falling among elderly people: a systematic review and meta-analysis of social and demographic pre-disposing characteristics. Clinics (Sao Paulo). 2010;65(9):895-903. PubMed doi:10.1590/S1807-59322010000900013
6. Kerse N, Flicker L, Pfaff JJ, et al. Falls, depression and antidepressants in later life: a large primary care appraisal. PLoS ONE. 2008;3(6):e2423. PubMed doi:10.1371/journal.pone.0002423
7. Pluijm SM, Smit JH, Tromp EA, et al. A risk profile for identifying community-dwelling elderly with a high risk of recurrent falling: results of a 3-year prospective study. Osteoporos Int. 2006;17(3):417-425. PubMed doi:10.1007/s00198-005-0002-0
8. Coupland C, Dhiman P, Morriss R, et al. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. PubMed doi:10.1136/bmj.d4551
9. Sterke CS, van Beeck F, van der Velde N, et al. New insights: dose-response relationship between psychotropic drugs and falls: a study in nursing home residents with dementia. J Clin Pharmacol 2012;52(6):947-955. PubMed
10. Lavsa SM, Fabian TJ, Saul MI, et al. Influence of medications and diagnoses on fall risk in psychiatric inpatients. Am J Health Syst Pharm. 2010;67(15):1274-1280. PubMed doi:10.2146/ajhp090611
11. Darowski A, Chambers SA, Chambers DJ. Antidepressants and falls in the elderly. Drugs Aging. 2009;26(5):381-394. PubMed doi:10.2165/00002512-200926050-00002
12. Joo JH, Lenze EJ, Mulsant BH, et al. Risk factors for falls during treatment of late-life depression. J Clin Psychiatry. 2002;63(10):936-941. PubMed doi:10.4088/JCP.v63n1012
13. Robinson MJ, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine vs placebo [published online ahead of print July 21, 2012]. Am J Geriatr Psychiatry. PubMed
14. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900-909. PubMed doi:10.1176/appi.ajp.164.6.900
15. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994.
16. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
17. Shelton RC, Prakash A, Mallinckrodt CH, et al. Patterns of depressive symptom response in duloxetine-treated outpatients with mild, moderate or more severe depression. Int J Clin Pract. 2007;61(8):1337-1348. PubMed doi:10.1111/j.1742-1241.2007.01444.x
18. Ball SG, Desaiah D, Spann ME, et al. Efficacy of duloxetine on painful physical symptoms in major depressive disorder for patients with clinically significant painful physical symptoms at baseline: a meta-analysis of 11 double-blind, placebo-controlled clinical trials. Prim Care Companion CNS Disord. 2011;13(6): doi:10.4088/PCC.11r01181 PubMed
19. Roose SP, Sackeim HA, Krishnan KRR, et al; Old-Old Depression Study Group. Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo-controlled trial. Am J Psychiatry. 2004;161(11):2050-2059. PubMed doi:10.1176/appi.ajp.161.11.2050
20. Halpert BP, Zimmerman MK. The health status of the ‘ old-old’ : a reconsideration. Soc Sci Med. 1986;22(9):893-899. PubMed doi:10.1016/0277-9536(86)90162-0
21. Liu GF, Wang J, Liu K, et al. Confidence intervals for an exposure adjusted incidence rate difference with applications to clinical trials. Stat Med. 2006;25(8):1275-1286. PubMed doi:10.1002/sim.2335
22. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
23. Wernicke JF, Faries D, Milton D, et al. Detecting treatment emergent adverse events in clinical trials: a comparison of spontaneously reported and solicited collection methods. Drug Saf. 2005;28(11):1057-1063. PubMed doi:10.2165/00002018-200528110-00006
24. Raskin J, Wiltse CG, Dinkel JJ, et al. Safety and tolerability of duloxetine at 60 mg once daily in elderly patients with major depressive disorder. J Clin Psychopharmacol. 2008;28(1):32-38. PubMed doi:10.1097/jcp.0b013e318160738e
25. Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189-2194. PubMed doi:10.1056/NEJMsr077003