Psychiatric Features in High-Functioning Adult Brothers With Fragile X Spectrum Disorders
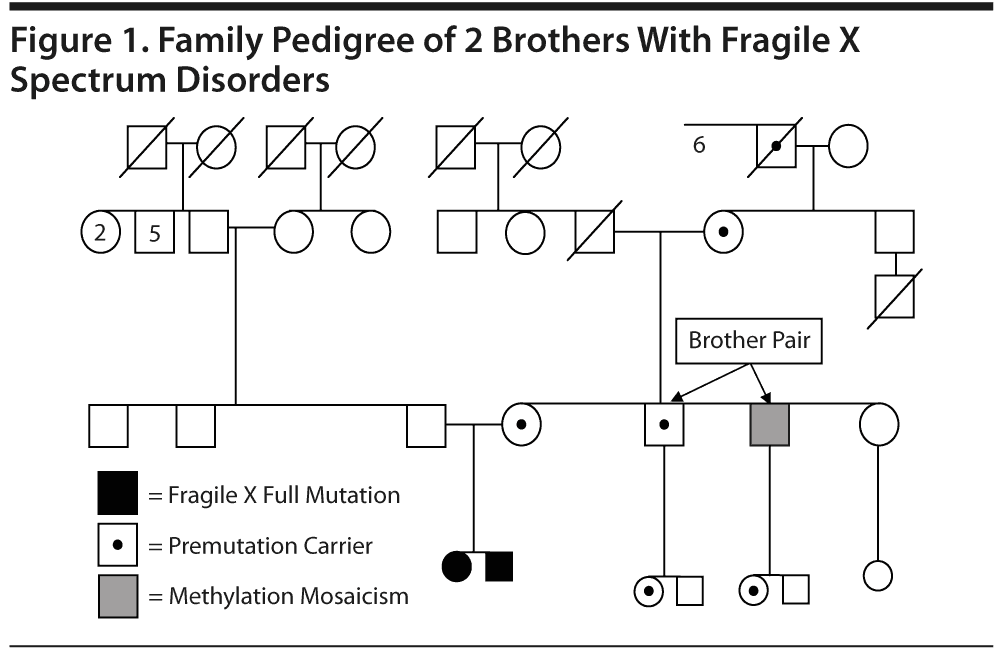
To the Editor: The awareness of psychiatric disability in the fragile X premutation (55-200 CGG repeats on Xq27.3) is low because these problems are not as obvious as in the full-mutation fragile X syndrome.1-5 Symptoms of hyperactivity, social deficits, and autism spectrum disorders as well as anxiety disorders and mood disorders are present in premutation carriers of both genders.1,2,6,7 Some individuals with the premutation have a mild-to-moderate deficit of fragile X-related mental retardation protein (FMRP).8 The level of FMRP decreases with increased CGG repeat number more evident in the upper end of the premutation range, leading to physical and behavioral features similar to fragile X syndrome.9,10 We present 2 cases of brothers with average and above intellectual abilities but emotional/neurocognitive deficits associated with the presence of expanded alleles from a larger fragile X family pedigree (see Figure 1) with significant psychopathology.
The brothers’ 62-year-old mother was identified as a premutation carrier with an unknown CGG repeat size. She is affected by hypertension and underwent menopause at age 49 years. One of the brothers’ sisters, who has 2 children with fragile X syndrome, was diagnosed with bipolar II disorder with intermittent hypomanic episodes and panic disorder without agoraphobia (predominantly, but not only, in social situations; diagnosis made using the Structured Clinical Interview for DSM-IV Axis I Disorders [SCID-I]11). The maternal grandfather died in his early 50s after a sudden stroke. The maternal grandmother is 86 years old and healthy. The brothers’ father suffered from alcoholism and died recently at age 67 years. A paternal uncle committed suicide in his 30s, while both paternal grandparents were "odd" and either depressed or irritable and had been hospitalized in psychiatric institutions. The paternal grandfather had received electroconvulsive treatment and had a history of alcoholism.
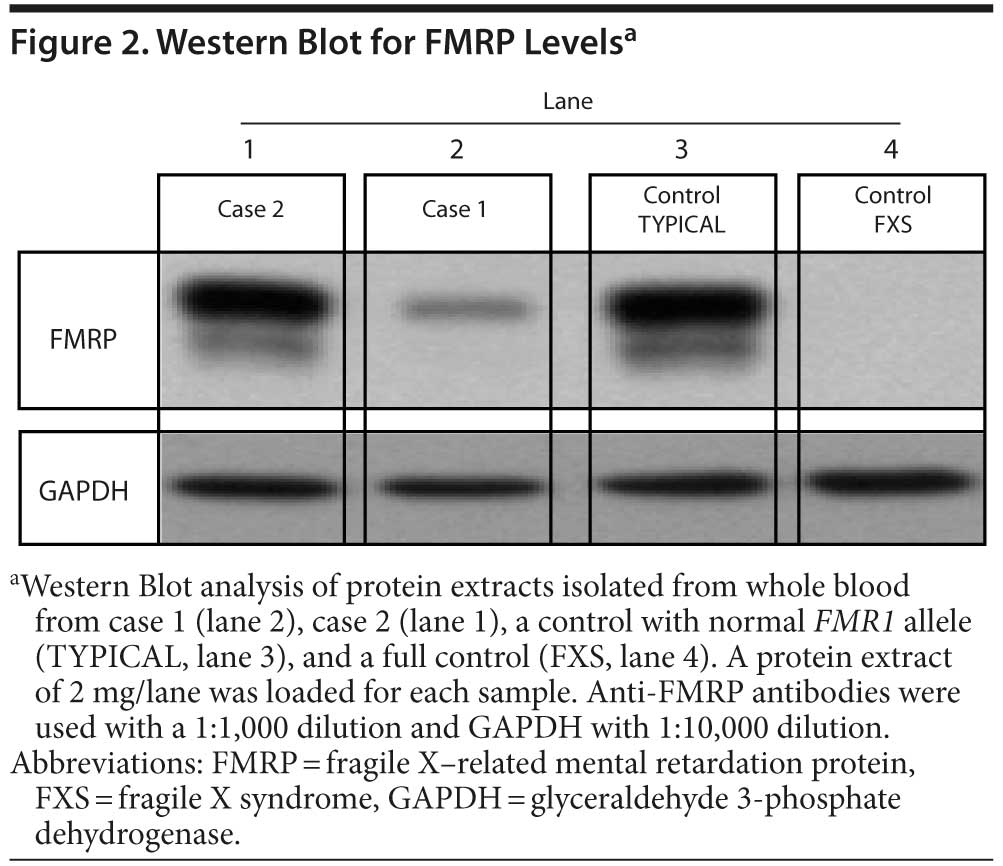
Case 1. Mr A is a 40-year-old man with full mutation and methylation mosaicism, including a methylated allele (190 CGG repeats) and unmethylated alleles in both the premutation and the full-mutation range (98 and 225-547 CGG repeats, respectively; full mutation detected in approximately 30% of the cells). His FMR1 messenger RNA (mRNA) level was 5.8-fold above normal, and he showed decreased FMRP expression level of approximately 20% of normal (see Figure 2).
His developmental history included a premature birth, delayed motor development, and delayed onset of language. He showed poor eye contact, signs of attention deficit and hyperactivity, and language and reading problems in childhood. His medical history included leg cramps, recurrent sinusitis, and varicose veins. He complained about poor balance and visual-motor coordination deficits since childhood. The medical examination demonstrated a prominent jaw, flat feet, increased deep tendon reflexes of 3+, macroorchidism, a mild hearing loss, and motor coordination problems including inability to tandem walk. His cognitive testing12 at age 40 years demonstrated a full scale intelligence quotient (FSIQ) of 107 (68th percentile, in the average range), and his general memory score13 was 98 (45th percentile). On psychiatric interview, Mr A reported several manic episodes with psychotic features during his teenage years, meeting SCID-I criteria for bipolar disorder type I, most recent episode manic with psychotic features in full remission at the time of the evaluation. He had had 3 psychiatric hospitalizations during his teenage years. As an inpatient, he was started on lithium treatment, which he did not tolerate and discontinued, followed by haloperidol and benztropine. After 3 years, he discontinued medications and psychiatric treatment and remained relatively symptom-free. Currently, he meets SCID-I criteria for alcoholism.
Although Mr A presented with an IQ considered to be average, which is remarkable for an adult man with FMR1 mosaicism, he showed features of premutation and full-mutation involvement related to high mRNA levels and low FMRP levels. Overall, we hypothesize that Mr A demonstrates a "double hit" phenomenon—he is affected by both lowered FMRP and elevated mRNA levels.
Case 2. Mr B is a 41-year-old male premutation carrier with 118 CGG repeats and the brother of Mr A. Elevated FMR1 mRNA levels (3.92-fold elevation) and a moderate decrease in FMRP expression were observed in blood (approximately 65% of normal levels; see Figure 2). Mr B had normal development. His medical history included migraines, a blocked nasal passage secondary to a deviated septum, asthma, and eczema. On examination, he presented with a long, narrow face, long ears (> 7 cm), and a prominent jaw. His neurologic examination showed slightly increased reflexes and a loss of vibratory sensation in the lower extremities. On psychiatric interview, he reported an untreated major depressive episode lasting 6 months at age 20 years; therefore, he met SCID-I criteria for major depressive disorder, single episode in full remission, at the time of the evaluation. His cognitive testing11 at age 41 years demonstrated a superior FSIQ of 122 (93rd percentile), and his general memory score13 was 103 (58th percentile).
We present 2 brothers with expanded FMR1 alleles, elevated FMR1 mRNA levels, and a distinct psychopathological profile, including mood disorders and substance abuse. The high prevalence of premutation alleles in the general population (approximately 1:250-810 in males and 1:130-250 in females) warrants increased awareness of the possible connection to medical and psychopathological features.14,15
Individuals who have both lowered FMRP and elevated FMR1 mRNA (ie, a "double hit"), carriers with a CGG repeat number in the upper premutation range and some mosaic full mutations, may be more common than previously thought, and they often present with psychiatric features.16 The phenomenon of double involvement of toxic elevation of FMR1 mRNA and reduced FMRP is worthy of further study, and it may represent a new phenotype in between the premutation and the full mutation with a more severe psychopathology that combines features of both types of mutations.
References
1. Cornish KM, Kogan C, Turk J, et al. The emerging fragile X premutation phenotype: evidence from the domain of social cognition. Brain Cogn. 2005;57(1):53-60. PubMed doi:10.1016/j.bandc.2004.08.020
2. Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27(suppl):S137-S144. PubMed doi:10.1097/00004703-200604002-00012
3. Hessl D, Tassone F, Loesch DZ, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139B(1):115-121. PubMed doi:10.1002/ajmg.b.30241
4. Johnston C, Eliez S, Dyer-Friedman J, et al. Neurobehavioral phenotype in carriers of the fragile X premutation. Am J Med Genet. 2001;103(4):314-319. PubMed doi:10.1002/ajmg.1561
5. Roberts JE, Bailey DB Jr, Mankowski J, et al. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(1):130-139. PubMed
6. Aziz M, Stathopulu E, Callias M, et al. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet. 2003;121B(1):119-127. PubMed doi:10.1002/ajmg.b.20030
7. Goodlin-Jones BL, Tassone F, Gane LW, et al. Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr. 2004;25(6):392-398. PubMed doi:10.1097/00004703-200412000-00002
8. Tassone F, Hagerman RJ, Taylor AK, et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66(1):6-15. PubMed doi:10.1086/302720
9. Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome—an older face of the fragile X gene. Nat Clin Pract Neurol. 2007;3(2):107-112. PubMed doi:10.1038/ncpneuro0373
10. Tassone F, Hagerman RJ, Iklé DN, et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84(3):250-261. PubMed doi:10.1002/(SICI)1096-8628(19990528)84:3<250::AID-AJMG17>3.0.CO;2-4
11. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). Washington, DC: American Psychiatric Press; 1996.
12. Wechsler D. Wechsler Adult Intelligence Scale: Administration and Scoring Manual, Third Edition. San Antonio, TX: Harcourt Assessment, Inc; 1997.
13. Wechsler D. Wechsler Memory Scale-Third Edition). San Antonio, TX: Harcourt Assessment, Inc; 1997.
14. Bourgeois JA, Coffey SM, Rivera SM, et al. A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry. 2009;70(6):852-862. PubMed doi:10.4088/JCP.08r04476
15. Bourgeois JA, Seritan AL, Casillas EM, et al. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2011;72(2):175-182. PubMed
16. Hessl D, Wang JM, Schneider A, et al. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiatry. 2011;70(9):859-865. PubMed
Author affiliations: MIND Medical Investigation of Neurodevelopmental Disorders Institute (Drs Schneider, Tassone, Hagerman, and Hessl) and Departments of Psychiatry and Behavioral Sciences (Drs Seritan and Hessl), Biochemistry (Dr Tassone), Psychology (Dr Rivera), and Pediatrics (Drs Schneider and Hagerman), University of California-Davis Medical Center, Sacramento.
Potential conflicts of interest: Dr Hagerman has been a consultant for Novartis and has received grant/research support from Seaside Therapeutics, Novartis, Roche, Forest, and Curemark. Dr Hessl has been a consultant for Roche, Seaside Therapeutics, and Novartis; has received grant/research support from the National Institute of Mental Health; and has received honoraria from Adelphi. Drs Schneider, Seritan, Tassone, and Rivera report no potential conflicts of interest relevant to the subject of this letter.
Funding/support: This work was supported by National Institutes of Health (NIH) grant MH078041 to Dr Hessl, grants HD036071 and AG032115 to Dr Hagerman, and grant HD02274 to Dr Tassone; NIH/National Institute on Aging grant RL1AG032115 (overall PI: Dr P. J. Hagerman; component PI: Dr R. J. Hagerman); NIH/National Institute of Mental Health grant R01MH078041 (PIs: Drs Hessl and Rivera); and Health and Human Services Administration of Developmental Disabilities grant 90DD0596.
Acknowledgments: The authors thank the participants and the MIND Institute.
Published online: April 11, 2013.
Prim Care Companion CNS Disord 2013;15(2):doi:10.4088/PCC.12l01492
© Copyright 2013 Physicians Postgraduate Press, Inc.