Selegiline Transdermal System: Use Pattern and Adherence in Patients With Major Depressive Disorder
ABSTRACT
Objective: To discern the pattern of use of selegiline transdermal system as well as the level of adherence relative to other pharmacotherapies for treatment of major depressive disorder.
Method: Deidentified patient-level data (2010-2011; N = 2,985) were abstracted from US longitudinal archives (Medicaid, Medicare, managed care) in this retrospective exploratory claims-based analysis. Major depressive disorder was defined as ICD-9-CM codes 292.2, 296.3, 300.4, or 311. Antidepressant treatment failure was defined as receipt of < 90 days of initial antidepressant.
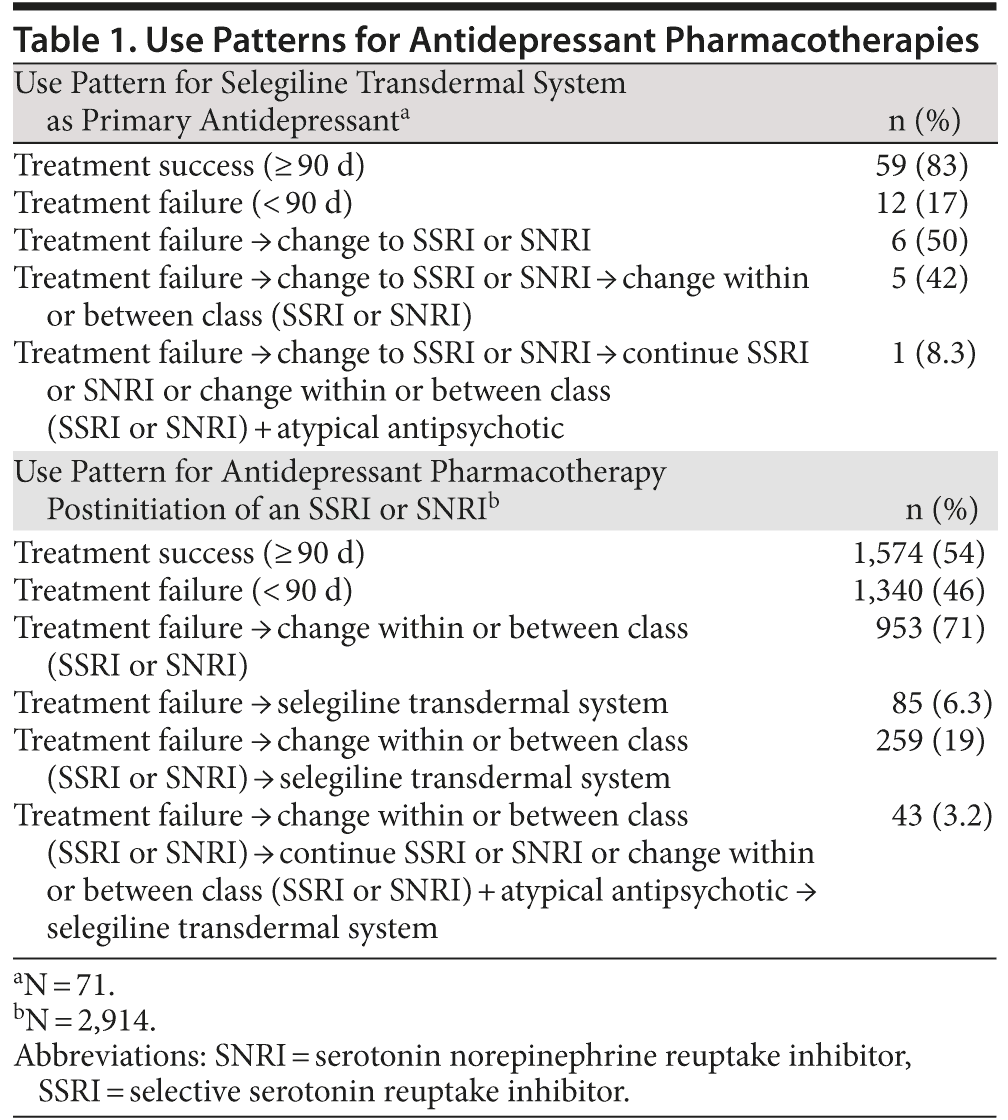
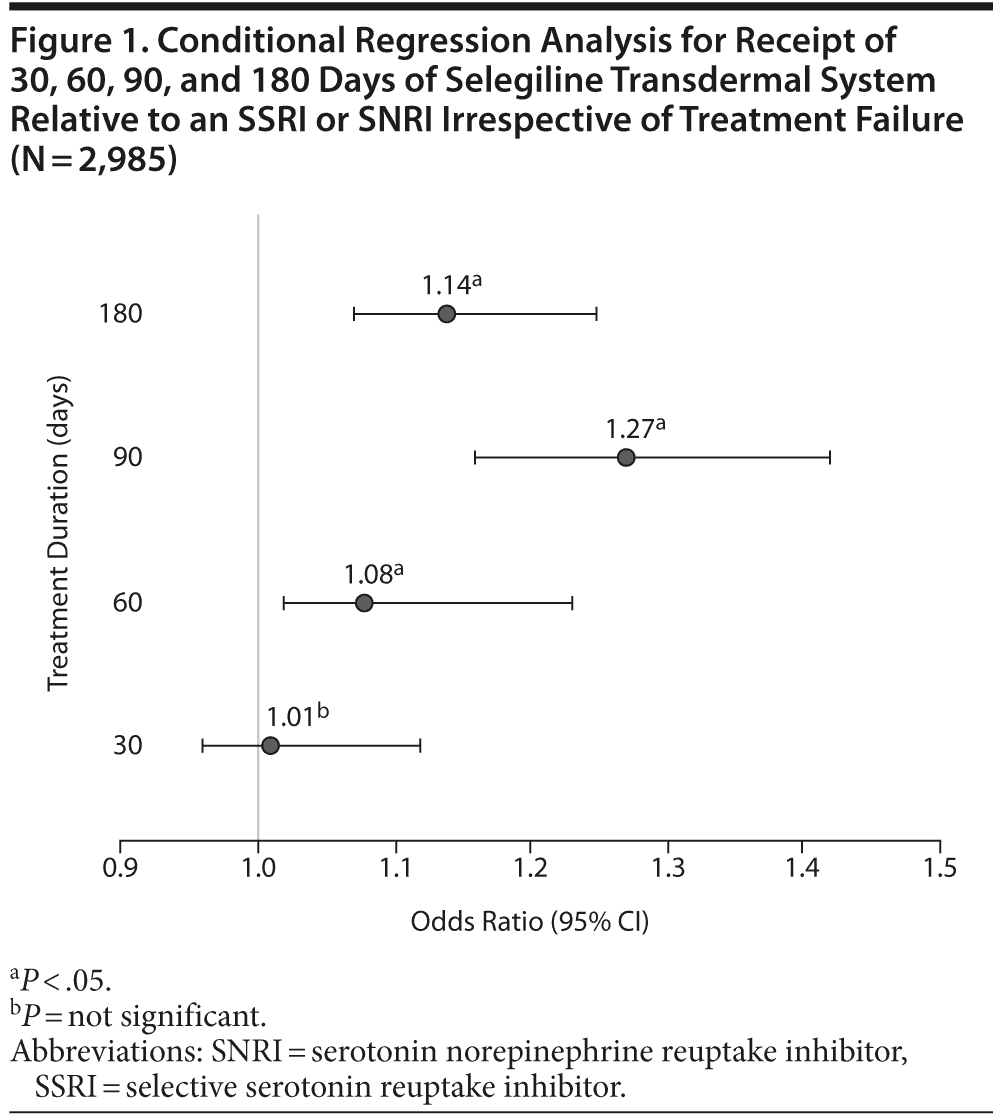
Results: Most patients received selegiline transdermal system as a second or third treatment option following treatment failure, and only 71 patients received it as first-line therapy. Patients were more likely to receive selegiline transdermal system for 60, 90, or 180 days compared to other therapies irrespective of treatment failure (P < .05). Among patients who did not fail treatment in the first 90 days, selegiline transdermal system was associated with a greater probability of receipt compared to selective serotonin reuptake inhibitors or serotonin norepinephrine reuptake inhibitors at 120 days (odds ratio [OR] = 1.21; 95% CI, 1.14-1.47) and 180 days (OR = 1.09; 95% CI, 1.01-1.28).
Conclusion: Although limited by the small sample size of patients receiving selegiline transdermal system versus other pharmacotherapies, the results suggest that after antidepressant treatment failure, earlier use of selegiline transdermal system may be warranted.
Prim Care Companion CNS Disord 2013;15(1):doi:10.4088/PCC.12br01466
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: September 14, 2012; accepted December 7, 2012.
Published online: February 21, 2013.
Corresponding author: David A. Sclar, BPharm, PhD, Midwestern University College of Pharmacy, 19555 N 59th Ave, Glendale, AZ 85308 ([email protected]).
Monoamine oxidase inhibitors (MAOIs) have played an important role in psychiatry since the initial introduction of iproniazid into clinical practice as an antidepressant in the 1950s. While MAOIs are still considered to be highly effective antidepressants, the use of MAOIs for the treatment of depression has declined significantly, perhaps due to the risk of potentially serious side effects stemming from food and drug interactions (eg, a vasopressor effect as a consequence of inhibiting MAO in the gut, thereby leading to decreased clearance of dietary tyramine and elevated risk of serotonin syndrome when concomitantly administered with other serotonergic agents).
Selegiline is an irreversible inhibitor of MAO enzymes. Selegiline transdermal system provides a novel mechanism to overcome some of the safety concerns associated with oral administration.1,2 The short-term and long-term safety and efficacy of selegiline transdermal system 6 mg/24 h (20 mg/20 cm2), 9 mg/24 h (30 mg/30 cm2), and 12 mg/24 h (40 mg/40 cm2) have been previously studied in the treatment of major depressive disorder (MDD) in randomized, double-blind, placebo-controlled trials of 6, 8, and 52 weeks’ duration. Selegiline transdermal system3 is available in the 3 doses listed above.
In 1 selegiline transdermal system clinical trial,1 more than 40% of the patients with MDD had failed at least 1 prior antidepressant treatment. Prior treatment failure with first-line therapies (eg, selective serotonin reuptake inhibitors [SSRIs], selective norepinephrine reuptake inhibitors [SNRIs]) may be due to treatment resistance and/or nonadherence to treatment instructions. In a large retrospective study of SSRIs, approximately 57% of patients were nonadherent to their prescribed antidepressant therapy within 6 months.4 Almost one-third of patients treated for depression discontinue their antidepressant therapy in the first month of treatment.5 The majority of patients discontinuing antidepressant therapy do not inform their physician of this change.
METHOD
Since adherence and health outcomes are strongly associated, we conducted a retrospective exploratory claims-based analysis to discern the following: the pattern (sequence) of use of selegiline transdermal system relative to other pharmacotherapies for treatment of MDD and the level of adherence to selegiline transdermal system relative to other antidepressant pharmacotherapies. Deidentified patient-level data (2010-2011) were abstracted from US longitudinal archives (Medicaid, Medicare, managed care). Major depressive disorder was defined as ICD-9-CM codes 292.2, 296.3, 300.4, or 311. Antidepressant treatment failure was defined as receipt of < 90 days of initial antidepressant. Criteria for inclusion were ambulatory patients aged 18 to 75 years with continuous enrollment ≥ 18 months (beginning 6 months prior to an ICD-9-CM code for MDD [index date]), enrollment ≥ 12 months postindex date, no ICD-9-CM code for a comorbid mental illness, and prescribed SSRI, SNRI, or selegiline transdermal system. Using an intent-to-treat approach, multivariate logistic regression was used to assess sequential use of antidepressant pharmacotherapy and adherence. Models were adjusted for age, gender, race, insurance coverage (Medicaid, Medicare, managed care), and Deyo/Charlson Comorbidity Index6 and health service utilization costs for nonpsychiatric illness.
- Treatment adherence to antidepressant pharmacotherapy can have a significant effect on health outcomes.
- Use of selegiline transdermal system was associated with a greater probability of receipt compared to selective serotonin reuptake inhibitors or serotonin norepinephrine reuptake inhibitors at 120 days and 180 days.
- Results suggest that after antidepressant treatment failure, earlier use of selegiline transdermal system may be warranted.
RESULTS
Of the patient records identified (N = 2,985), the majority of patients received selegiline transdermal system as a second or third treatment option following treatment failure (Table 1). Only 71 patients received selegiline transdermal system as first-line therapy. Patients were more likely to receive selegiline transdermal system for 60, 90, or 180 days compared to other therapies irrespective of treatment failure (P < .05; Figure 1). Among patients who did not fail treatment in the first 90 days, selegiline transdermal system was associated with a greater probability of receipt compared to SSRIs or SNRIs at 120 days (odds ratio [OR] = 1.21; 95% CI, 1.14-1.47) and 180 days (OR = 1.09; 95% CI, 1.01-1.28).
CONCLUSION
Treatment adherence to antidepressant pharmacotherapy can have a significant effect on health outcomes. Although limited by the small sample size of patients receiving selegiline transdermal system versus other pharmacotherapies, our results suggest that after antidepressant treatment failure, earlier use of selegiline transdermal system may be warranted.
Drug names: selegiline transdermal system (EMSAM).
Author affiliations: Midwestern University College of Pharmacy, Glendale, Arizona (Dr Sclar); University of North Texas System College of Pharmacy, Fort Worth (Dr Cohen); and Medical Affairs, Mylan Specialty L.P., Basking Ridge, New Jersey (Dr Portland).
Potential conflicts of interest: Drs Sclar and Cohen have received grant/research support from and served as consultants to Mylan Specialty L.P. Dr Portland is an employee of Mylan Specialty L.P., and may hold stock and/or stock options in Mylan.
Funding/support: Support for this research was provided by Mylan Specialty L.P., Basking Ridge, New Jersey.
Previous presentation: Presented at the 52nd Annual Meeting of the New Clinical Drug Evaluation Unit; May 29-June 1, 2012; Phoenix, Arizona.
Acknowledgment: The authors thank Paul F. Cavanaugh, Jr, PhD, Mylan Specialty L.P., Basking Ridge, New Jersey, for editorial assistance. Dr Cavanaugh may hold stock and/or stock options in Mylan.
REFERENCES
1. Patkar AA, Portland KB, Pae CU. Selegiline transdermal system in major depressive disorder. Neuropsychiatry. 2012;2(2):125-134. doi:10.2217/npy.12.9
2. Pae CU, Bodkin JA, Portland KB, et al. Safety of selegiline transdermal system in clinical practice: analysis of adverse events from postmarketing exposures. J Clin Psychiatry. 2012;73(5):661-668. PubMed doi:10.4088/JCP.12m07648
3. EMSAM [package insert]. Napa, CA: Dey, LP; 2009.
4. Cantrell CR, Eaddy MT, Shah MB, et al. Methods for evaluating patient adherence to antidepressant therapy: a real-world comparison of adherence and economic outcomes. Med Care. 2006;44(4):300-303. PubMed doi:10.1097/01.mlr.0000204287.82701.9b
5. Sawada N, Uchida H, Suzuki T, et al. Persistence and compliance to antidepressant treatment in patients with depression: a chart review. BMC Psychiatry. 2009;9(1):38. PubMed doi:10.1186/1471-244X-9-38
6. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. PubMed doi:10.1016/0895-4356(92)90133-8