Objective: To identify clinical characteristics common among epileptic patients prescribed levetiracetam who report suicidal ideation or who exhibit suicidal behavior. A case is also provided that highlights the need for increased vigilance for neuropsychiatric sequelae in fragile epileptic patients prescribed levetiracetam, especially post dosage adjustment.
Data Sources: PubMed was queried with no time limitation to December 2018 using a combination of controlled terms. Using the Boolean operators "AND" and "OR," the authors searched PubMed for case reports and case series on levetiracetam-related suicidal behavior. The search terms used were [levetiracetam] OR [Keppra] AND in combination with suicidal, suicide, suicidal ideation, suicide attempt, and suicidality.
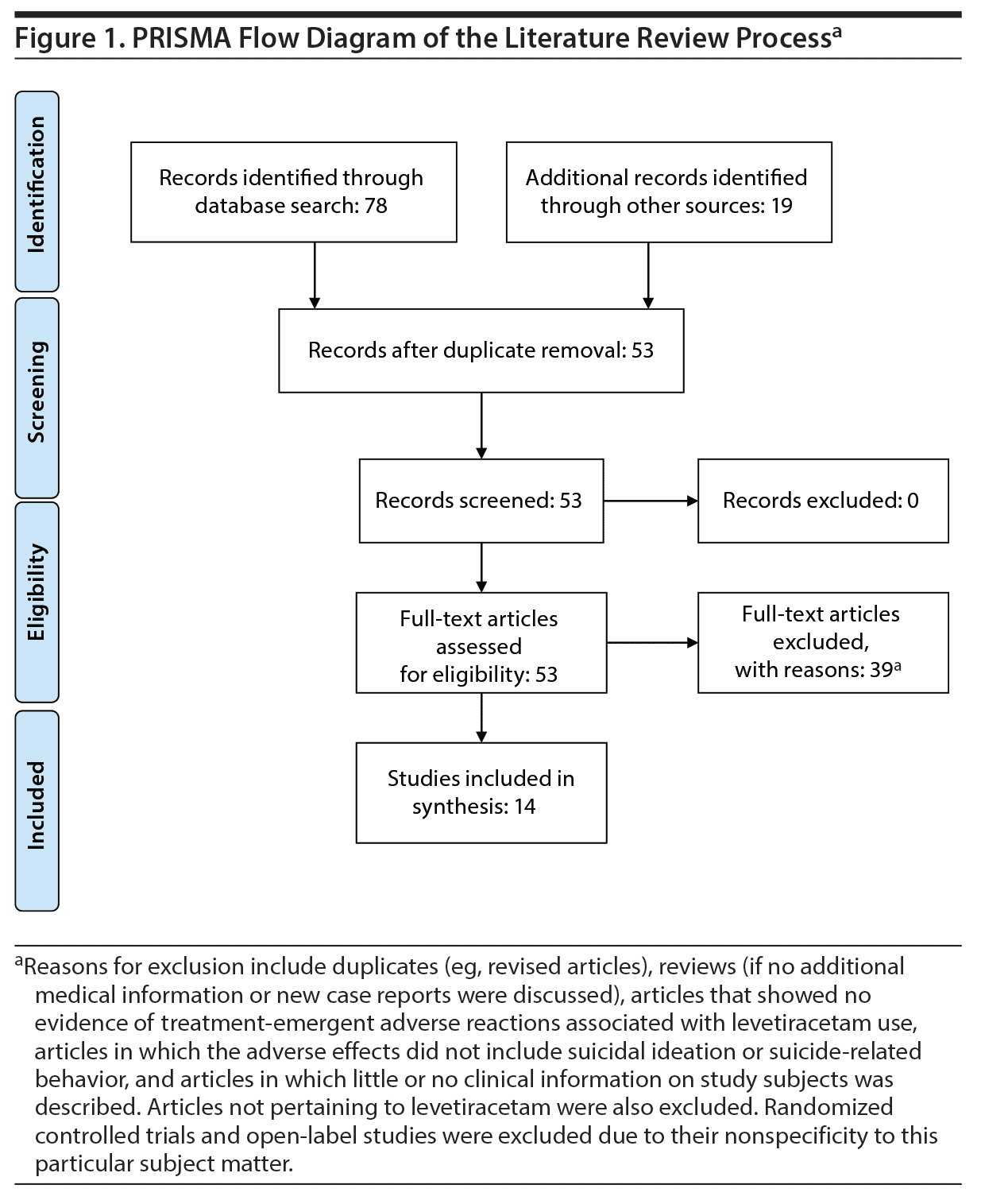
Study Selection: Relevant English-language human studies on levetiracetam and its effect on suicidal behavior were included. The search terms generated 78 results from the databases. After excluding all duplicates and applying the inclusion and exclusion criteria, a total of 14 clinical studies were retained for review.
Data Extraction: Two reviewers independently extracted relevant data and assessed the methodological quality of each study.
Results: The included studies reveal a number of risk factors for suicide ideation, suicide-related behavior, and suicide attempt among individuals taking levetiracetam. These risk factors include a prior psychiatric disorder, a history of traumatic brain injury, a history of substance use disorder, and a structural brain abnormality. Patients with these risk factors constitute a specific subgroup of patients with epilepsy who have an increased vulnerability to suicidal ideation or behavior if prescribed levetiracetam. These patients should, therefore, be monitored closely.
Conclusions: Suicidal behavior in epileptic patients appears to be multifactorial in etiology. Psychiatric disorders are more prevalent in epileptic patients than in the general population and contribute to this risk. In spite of the high risk of suicidal behavior with the use of antiepileptic drugs, studies have shown that the benefits of anticonvulsant therapy often outweigh the risks. Nevertheless, timely consultation with a psychiatrist is invaluable in the care of these patients, particularly those with multiple risk factors, as in the index case. The risk of suicidality should be balanced with the risk of uncontrolled seizures. Specifically, in the case of levetiracetam, it is important to be aware of the subgroup of individuals with prior severe psychiatric illness, a history of traumatic brain injury, or a history of substance use disorder who might be at an increased risk of developing suicide-related behavior and suicidal ideations once levetiracetam is started.

Levetiracetam and Suicidality:
A Case Report and Literature Review
ABSTRACT
Objective: To identify clinical characteristics common among epileptic patients prescribed levetiracetam who report suicidal ideation or who exhibit suicidal behavior. A case is also provided that highlights the need for increased vigilance for neuropsychiatric sequelae in fragile epileptic patients prescribed levetiracetam, especially post dosage adjustment.
Data Sources: PubMed was queried with no time limitation to December 2018 using a combination of controlled terms. Using the Boolean operators “AND” and “OR,” the authors searched PubMed for case reports and case series on levetiracetam-related suicidal behavior. The search terms used were [levetiracetam] OR [Keppra] AND in combination with suicidal, suicide, suicidal ideation, suicide attempt, and suicidality.
Study Selection: Relevant English-language human studies on levetiracetam and its effect on suicidal behavior were included. The search terms generated 78 results from the databases. After excluding all duplicates and applying the inclusion and exclusion criteria, a total of 14 clinical studies were retained for review.
Data Extraction: Two reviewers independently extracted relevant data and assessed the methodological quality of each study.
Results: The included studies reveal a number of risk factors for suicide ideation, suicide-related behavior, and suicide attempt among individuals taking levetiracetam. These risk factors include a prior psychiatric disorder, a history of traumatic brain injury, a history of substance use disorder, and a structural brain abnormality. Patients with these risk factors constitute a specific subgroup of patients with epilepsy who have an increased vulnerability to suicidal ideation or behavior if prescribed levetiracetam. These patients should, therefore, be monitored closely.
Conclusions: Suicidal behavior in epileptic patients appears to be multifactorial in etiology. Psychiatric disorders are more prevalent in epileptic patients than in the general population and contribute to this risk. In spite of the high risk of suicidal behavior with the use of antiepileptic drugs, studies have shown that the benefits of anticonvulsant therapy often outweigh the risks. Nevertheless, timely consultation with a psychiatrist is invaluable in the care of these patients, particularly those with multiple risk factors, as in the index case. The risk of suicidality should be balanced with the risk of uncontrolled seizures. Specifically, in the case of levetiracetam, it is important to be aware of the subgroup of individuals with prior severe psychiatric illness, a history of traumatic brain injury, or a history of substance use disorder who might be at an increased risk of developing suicide-related behavior and suicidal ideations once levetiracetam is started.
Prim Care Companion CNS Disord 2020;22(4):19nr02502
To cite: Esang M, Santos MG, Ahmed S. Levetiracetam and suicidality: a case report and literature review. Prim Care Companion CNS Disord. 2020;22(4):19nr02502.
To share: https://doi.org/10.4088/PCC.19nr02502
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry & Behavioral Sciences, Nassau University Medical Center, East Meadow, New York
*Corresponding author: Michael Esang, MD, Clarion Psychiatric Center, 2 Hospital Dr, Clarion, PA 16214 ([email protected]).
Levetiracetam has been available as an antiepileptic drug (AED) since its US Food and Drug Administration (FDA) approval in 1999. Nine years later, however, the FDA released a postmarketing statement describing an increased risk of suicide (0.43%) in patients taking AEDs, including levetiracetam.1 Levetiracetam is a second-generation AED that has shown clinical effectiveness in generalized and partial epilepsy syndromes, both as monotherapy and as adjunctive treatment.2 As with other second-generation AEDs, levetiracetam is generally better tolerated than the conventional anticonvulsants, with a more favorable side effect profile. Although its exact mechanism of action is unknown, levetiracetam is believed to act via binding to synaptic vesicle protein (SV2A), which seems to be protective against seizure activity.3,4 There is also evidence of levetiracetam’s modulatory activity on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.5 The recommended starting dose of levetiracetam is 500 mg twice daily. It can be titrated by 1,000 mg every 2 weeks as needed to a maximum dose of 3,000 mg daily.6 The most common side effect of levetiracetam was found to be sedation at 10.7%, while mood disturbance was found in 4.8% of patients.7 A possible association has been reported between the use of levetiracetam, topiramate, or lamotrigine and suicidality.8 However, further research is needed, as a review of relevant literature on suicidal behavior in epileptic patients points to a multifactorial etiology. The primary objective of this review is to identify clinical characteristics common among epileptic patients prescribed levetiracetam who report suicidal ideation or who exhibit suicide-related behavior. In this review, suicide-related behavior included suicide attempts, self-harming behavior with a history of suicidal ideation, and actual suicides.
METHODS
Only articles published in English were included. The authors separately conducted a review of relevant literature with no limitation in the timeline until December 2018. Using the Boolean operators “AND” and “OR,” the authors searched PubMed for case reports and case series on levetiracetam-related suicidal behavior. The search terms used were as follows [levetiracetam] OR [Keppra] AND in combination with suicidal, suicide, suicidal ideation, suicide attempt, and suicidality.
The original search returned 78 articles. Articles were reviewed, and duplicates were removed using Mendeley Desktop Software (V-1.17.10). The current study followed the recommendations of the Preferred Items for Reporting of Systematic Reviews and Meta-Analyses (PRISMA) statement.9 In reviewing the references of the 78 original articles, 19 additional articles were identified and added to the study. Two investigators (M.E. and M.G.S.) independently reviewed the articles for eligibility. If either of the reviewers deemed an article as potentially eligible based on title/abstract review, a full-text review was then performed independently. Final decisions regarding the eligibility of the articles were made by consensus following the full-text review.
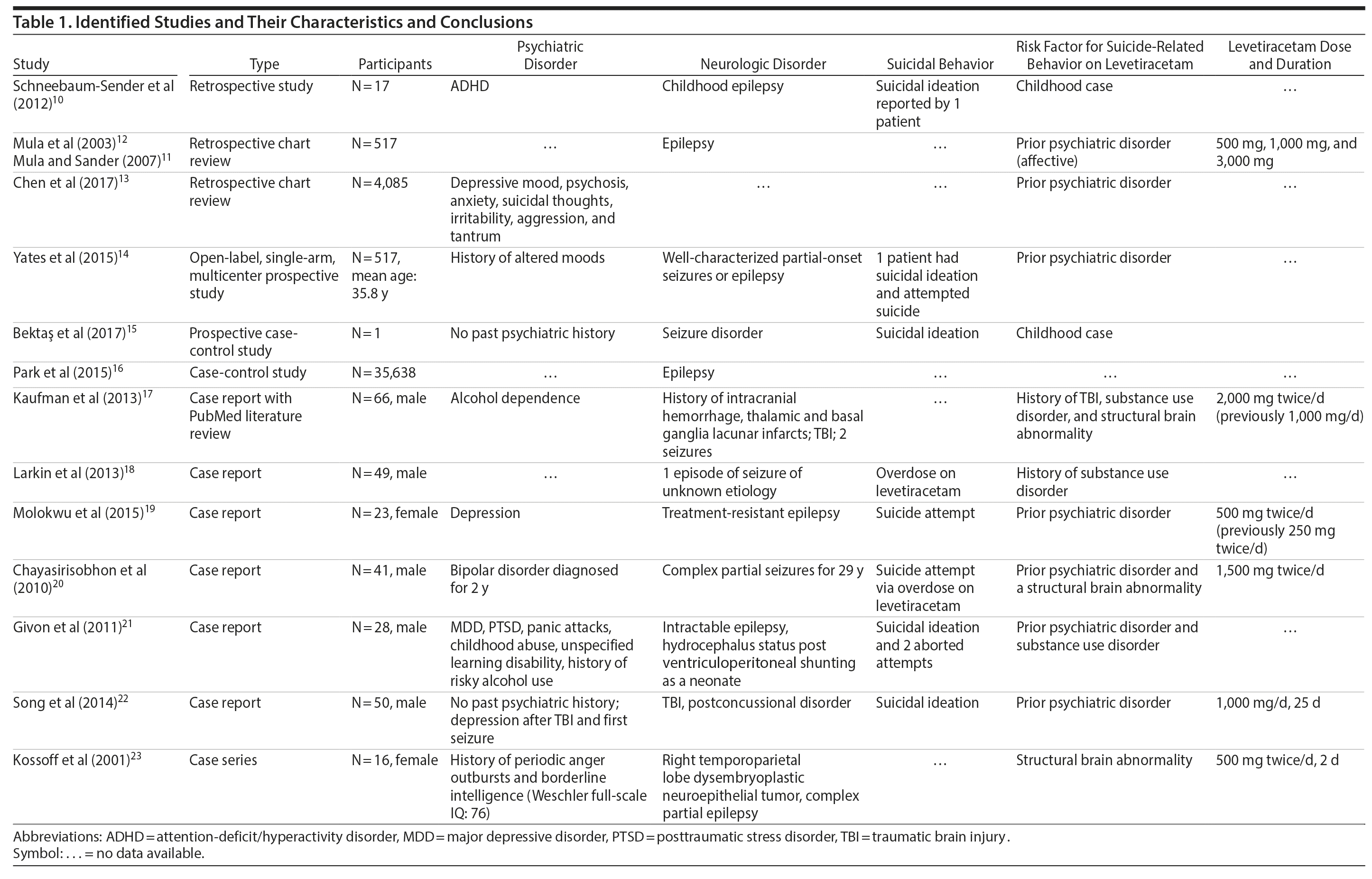
A total of 14 studies met the inclusion criteria: 4 retrospective studies, 3 prospective studies, and 7 case reports/series.10-23 A total of 39 studies were excluded.4,8,24-52 The PRISMA flowchart is shown in Figure 1. A list of articles with their respective characteristics and results are shown in Table 1.
RESULTS
Observational Studies (cohort and case control)
Schneebaum-Sender et al10 published a retrospective study of patients with attention-deficit/hyperactivity disorder and childhood epilepsy. One patient reported suicidal ideation, which the authors attributed to levetiracetam use. This study10 highlights the need for risk factor assessment in the pediatric population prior to levetiracetam use.
Mula et al11,12 conducted a retrospective cohort study of a case registry of 517 patients with epilepsy taking levetiracetam. The study set out to describe the clinical and psychopathological features of patients who developed suicidal ideation during levetiracetam therapy. Suicidal ideation was reported by 4 patients (0.7%). Those patients developed a major depressive episode, with one describing psychotic features. The onset of depressed mood and suicidality presented early during levetiracetam treatment (mean of 46.5 days; range, 18-120 days). The dosages at which this onset occurred were 500 mg for 2 patients, 1,000 mg for 1 patient, and 3,000 mg for 1 patient. These patients had no previous suicide attempts, but each had previous histories of affective disorders. Three of the patients had major depression with marked anergia and depressed mood, and 1 patient had a history of an anxiety disorder. For all 4 cases, once levetiracetam was discontinued or reduced in dosage, symptoms rapidly remitted. From this retrospective study, the authors11,12 concluded that a history of febrile convulsions, status epilepticus, and a previous psychiatric history were significantly correlated with psychiatric adverse effects during levetiracetam treatment.
In a retrospective cohort study of 4,085 adult patients, Chen et al13 compared psychiatric and behavioral side effect profiles of older and newer AEDs in a large sample of patients diagnosed with epilepsy. Levetiracetam showed the greatest psychiatric and behavioral side effects rate at 22.1%. Four patients on levetiracetam developed suicidal thoughts; however, no suicide-related behavior or suicides were reported. The authors concluded that compared to other AEDs, patients on levetiracetam experienced significantly more psychiatric and behavioral side effects including suicidal ideation. They also found that, in general, a history of any psychiatric condition, intractable epilepsy, and static encephalopathy were non-AED factors associated with increased risk of a psychiatric side effect.13
In a 12-week open-label, single-arm, multicenter prospective study, Yates et al14 evaluated possible changes in nonpsychotic behavioral adverse events in patients receiving levetiracetam who switched to brivaracetam with no titration. The patient population mean age was 35.8 years, and they had well-characterized partial-onset seizures or epilepsy. One patient had a history of altered mood and morbid thoughts, which were both attributed to the use of levetiracetam. This patient later reported suicidal ideation and attempted suicide after the switch. The authors14 attributed this change to levetiracetam. The authors suggest that brivaracetam had better tolerability in terms of side effects compared with levetiracetam. However, this study was limited, as the authors14 did not provide information about the patients’ psychiatric history and the sample size was small.
Bektas et al15 conducted a prospective case-control study of children aged 6-16 years with new-onset partial seizures who started treatment with either levetiracetam or valproic acid. This study examined the frequency and timing of treatment-emergent psychosocial and behavioral problems in children receiving levetiracetam irrespective of the seizure variable. One 15-year-old female patient, out of 32 patients taking levetiracetam, with no previous psychiatric history developed suicidal ideation after 1 month of taking levetiracetam. Levetiracetam was switched to valproic acid, and suicidal ideation resolved. This patient’s suicidal ideation was attributed to levetiracetam.15
Finally, in a case-control study of 35,638 epileptic patients, Park et al16 identified the clinical correlates of suicide in patients with epilepsy. Of the patients on levetiracetam, 6.8% committed suicide. The authors16 found that treatment with levetiracetam was associated with a higher risk of completed suicide compared to other AEDs. They also concluded that patients with temporal lobe epilepsy with weekly seizures or more, who lacked aura, who used levetiracetam, or who took an antidepressant were at a higher risk of suicide.16
Descriptive Studies (case reports)
The case was reported of a 66-year-old man with a history of diabetes, diabetic neuropathy, hypertension, alcohol dependence with withdrawal features, and seizures secondary to a traumatic brain injury (TBI) with intracranial hemorrhage.17 The patient had been on levetiracetam 500 mg daily for 5 years before being hospitalized for recurrent seizures. The levetiracetam dose was increased to 1,000 mg twice a day; he reported no depressive symptoms on this dose. However, when the dose was increased to 2,000 mg daily, he complained of suicidal ideation, developed a vegetative affect, and eventually attempted suicide via insulin overdose. His Naranjo Adverse Drug Reaction Probability Scale score was 5, which was probable for levetiracetam as a cause of the suicidal behavior. The authors17 concluded that there was a dose-dependent relationship between levetiracetam and the development of a de novo major depressive episode. In this case, it culminated in a near-fatal suicide attempt following a levetiracetam dose adjustment in a patient with no prior psychiatric history who had already been on levetiracetam for years.17
Givon et al21 reported the case of a 28-year-old man with a past psychiatric history of major depressive disorder, posttraumatic stress disorder, panic attacks, child abuse, and an unspecified learning disability. He also had a history of risky alcohol use and suffered from intractable epilepsy. As a neonate, he had hydrocephalus, which was treated with ventriculoperitoneal shunting. This patient had missed several neurology clinic appointments and had been experiencing breakthrough seizures as well as recurrent falls. After starting levetiracetam, he developed increased irritability, mood swings, and suicidal ideation and had 2 aborted suicide attempts. He also had a violent aggressive episode against a security officer. Violent threats persisted, but subjective improvement in his mood was reported after cessation of levetiracetam.21
Another case described a 50-year-old man with no previous psychiatric history who developed depression and new-onset seizures a few months after a TBI.22 Once he became depressed, he was started on sertraline, and, later, levetiracetam was added to manage his seizures. Levetiracetam was titrated to 1,000 mg daily, with the patient admitting to suicidal ideation 25 days after initiation of levetiracetam. Initially passive, the suicidal ideation progressed to active thoughts (specific plans not specified). Suicidal ideation ceased after levetiracetam was discontinued. The authors22 proposed that suicidality with levetiracetam use was associated more with depression than with anxiety or impulsivity.
Chayasirisobhon et al20 reported the case of a 41-year-old man with bipolar disorder and complex partial seizures. After a right anterior temporal lobectomy, the patient was being treated with carbamazepine extended-release 700 mg twice a day and lamotrigine 300 mg twice a day. He continued to have seizures once every 2 months, so levetiracetam was added to his drug regimen with lamotrigine taper initiated simultaneously. Levetiracetam was gradually titrated to 1,500 mg twice a day. The patient then became depressed and cited family and financial problems as stressors. He subsequently attempted suicide by taking 126 tablets of levetiracetam (500 mg strength each) within 20 minutes.20

- The etiology of suicidal behavior in epileptic patients is multifactorial.
- Neurologic and psychiatric risk factors are involved in the associations/causal relationships of suicide.
- Psychosocial stressors further elevate the risk of suicidal behavior.
- Increased vigilance by psychiatrists and neurologists and early collaboration are vital in reducing morbidity and mortality in patients at high risk of suicidal behavior.
In a report by Larkin et al,18 a 49-year-old man with a past medical history of 1 episode of seizure of unknown etiology, hypertension, chronic obstructive pulmonary disease, and hepatitis C infection overdosed on levetiracetam in a suicide attempt. The patient also presented to the emergency department with elevated blood alcohol levels.18
Kossoff et al23 presented the case report of a 16-year-old female with borderline intelligence (Weschler full-scale IQ of 76), a history of anger outbursts toward her family, and dysembryoplastic neuroepithelial tumor (a brain tumor commonly found in the temporal lobe). The patient was on carbamazepine monotherapy for complex partial epilepsy. Levetiracetam was started at 500 mg twice a day to help control her seizures while a presurgical evaluation was completed. Two days later, she became acutely agitated with pressured speech and attempted to run away from home. She was found to be suicidal and homicidal and to have suddenly developed persecutory delusions. In addition, she became hyperreligious and quoted scripture repeatedly. Electroencephalogram revealed right temporal lobe slowing, but no epileptiform discharges. The consulting psychiatrist concluded that she had developed drug-induced psychosis. In a matter of days after discontinuation of levetiracetam, the patient had returned to near baseline levels with the exception of occasional paranoid thoughts. At 6-month follow-up, there were no clear delusions.
Case Report
Mr A, a 20-year-old Hispanic man with a history of epilepsy, unspecified and multiple substance use disorder (alcohol, benzodiazepines, and cannabinoids), was seen by the psychiatry consultation-liaison service following a suicide attempt by hanging. The patient had abruptly stopped levetiracetam 500 mg twice/day because of new-onset depressive symptoms including deliberate self-cutting behavior soon after treatment commencement. He then developed a seizure and attempted suicide by hanging himself with a belt during a postictal state.
Mr A was found by a family member, transported to the hospital, and stabilized on the trauma service before being transferred to the inpatient medicine service for the management of aspiration pneumonia. On evaluation by our psychiatry consultation-liaison team, he admitted to episodes of cutting himself while taking levetiracetam prior to the hospitalization but denied any previous suicide attempts. When his past psychiatric history was explored, he attested to a chronic history of abusing multiple substances including alcohol, nonprescribed alprazolam, and cannabis, as well as a remote history of lysergic acid diethylamide use. He admitted to drinking, on average, half a liter of vodka daily since he was 18 years of age. His recent history, however, revealed sobriety from these substances (supported by negative urine toxicology screen at admission). He also reported a history of multiple hospitalizations for recurrent seizures and falls due to noncompliance with prescribed AEDs.
A few days after cessation of levetiracetam, Mr A developed a grand mal seizure that was further complicated by postictal psychosis. The psychotic episode manifested in the form of command auditory hallucinations, including a feeling of being physically “controlled by evil spirits.” He followed the directions given by these disembodied voices and attempted suicide via hanging.
Following stabilization on the medical service, the patient was subsequently transferred to inpatient psychiatry where he was continued on an antidepressant (citalopram). His depressive symptoms resolved completely, he was deemed psychiatrically stable, and he was discharged on citalopram 20 mg daily with outpatient psychiatric follow-up care.
During his inpatient hospital course, Mr A was also followed by the neurology consulting team who prescribed oral divalproex sodium delayed release 500 mg 2 times/day to address the seizure disorder. The neurology service followed his progress in inpatient psychiatry, maintaining his divalproex sodium delayed release regimen at the same dosage throughout his inpatient stay. At the time of discharge from the hospital, Mr A was also given an appointment to follow-up with a neurologist as an outpatient.
DISCUSSION
To date, the contribution of AEDs, specifically levetiracetam, to suicide risk is debated. Table 2 highlights a number of risk factors associated with suicidal behavior among epileptic patients according to the literature.
A closer examination of the cohort and case-control studies included in our review revealed some pertinent information. Risk factors described for patients on levetiracetam included febrile convulsions, status epilepticus, intractable epilepsy, static encephalopathy, temporal lobe epilepsy, and a previous psychiatric history including concurrent treatment with an antidepressant. Apart from temporal lobe epilepsy, these risk factors are all represented in Table 2. Antidepressant treatment itself could be viewed as a proxy for a psychiatric history of a mood or anxiety disorder. Although Park et al16 did not specify the indication for antidepressant treatment, it was identified as a risk factor for suicide in their study. An interesting concept for further research would be to explore the possibility of the inherent antidepressant risk of increased suicidal ideation being additive to the independent risk posed by levetiracetam.
The case reports we reviewed were particularly helpful in providing greater detail for the individuals who were suicidal on levetiracetam. Unfortunately, only 1 of the 6 reported cases included a female patient. The rest were males, with ages ranging from 28 to 66 years. The 1 reported female patient was a 16-year-old girl with borderline intelligence who had a temporal lobe tumor that was most likely the source of her seizures. Here, again, we see a reference to temporal lobe pathology and its association with suicidal behavior in a patient taking levetiracetam. Among the male cases, a number of risk factors appeared to be relevant including recurrent and poorly controlled seizures, substance use (alcohol), TBI, comorbid affective disorders (including major depressive disorder and bipolar disorder), and use of sertraline (an antidepressant). In addition, 2 of these cases presented with some form of structural compromise to brain architecture. One had hydrocephalus as a neonate, for which he had ventriculoperitoneal shunting. The other had a right anterior temporal lobectomy—another case with temporal lobe involvement. The case with a history of hydrocephalus, however, was most similar to our index case presentation. Both cases had a series of missed neurology clinic appointments and breakthrough seizures. Both had a prior history of multiple falls as well as substance use disorders. Both also reported a history of multiple psychosocial stressors. Following initiation of levetiracetam, both patients developed increased irritability, mood swings, and suicidal behavior. Additional risk factors were present in the case presented by Givon et al,21 such as a history of an unspecified learning disability as well as neonatal hydrocephalus treated by ventriculoperitoneal shunting.
A qualitative evaluation of our selected study findings, as presented in Table 1, therefore revealed a number of risk factors for suicide-related behavior and suicidal ideation among individuals who took levetiracetam. Among these, the following emerged as recurring and prominent themes: prior psychiatric disorder, a history of substance use disorder, a history of TBI, and a structural brain abnormality (tumor, infarcts).
We propose that patients with these risk factors constitute a specific subgroup of patients with epilepsy who have an increased vulnerability to suicidal thoughts or behavior when they take levetiracetam. These patients should thus be monitored closely. There are reports suggesting that a history of psychiatric disorders, specifically affective disorders, can predispose patients on levetiracetam to suicide-related behavior.11 This behavior has been observed even if the patients have not had a history of side effects to levetiracetam.12,54 There are also multiple reports of levetiracetam-associated suicidal ideation/suicide-related behavior in the setting of a history of TBI or a structural brain abnormality.48,51,52 Other articles highlight the role of substance use disorders in predisposing patients on levetiracetam to suicidal ideation/suicide-related behavior.17,18,21 Findings from the Song et al,22 Molokwu et al,19 and Kaufman et al17 studies went further to suggest that there might be a dose-response relationship between levetiracetam and suicide-related behavior. Also of note is the study by Yates et al.14 Their findings suggested that brivaracetam had better tolerability in terms of side effects compared to levetiracetam. Brivaracetam binds more selectively to SV2A than levetiracetam and has no activity on the AMPA receptor.4 This finding may suggest that the psychiatric adverse effects of levetiracetam may be associated with its activity on the AMPA receptor. These adverse effects appear to play a role in our case presentation.
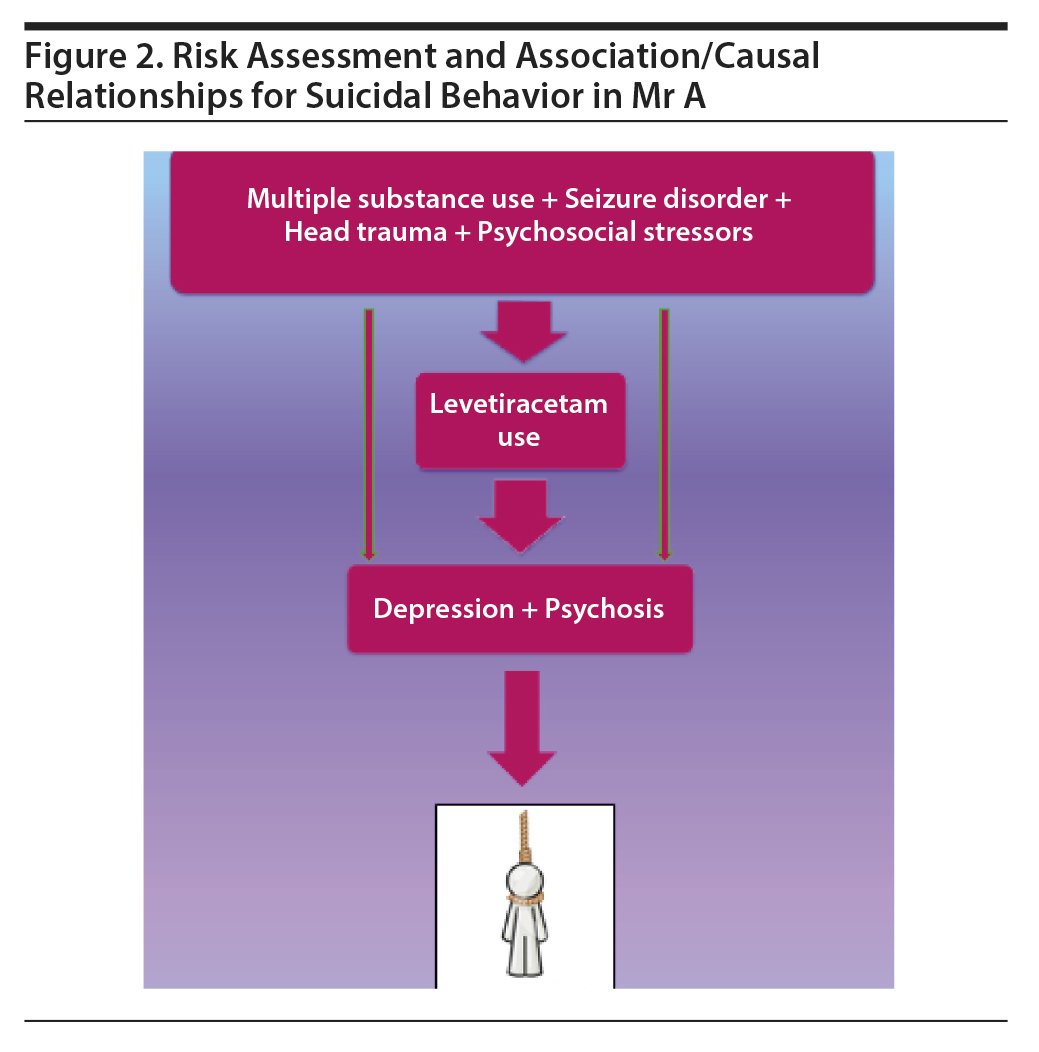
Mr A, the index patient in this report, is a 20-year-old Hispanic man who presented with increasing severity of depression following levetiracetam use for his seizure disorder. In addition to use of levetiracetam, the patient had an elevated risk of suicidal behavior attributable to other risk factors such as comorbid substance use disorder, a poorly controlled seizure disorder, and various psychosocial stressors including job loss and impaired interpersonal relationships (Figure 2). The patient’s onset of depression followed the initiation of levetiracetam for seizure control. He complained of depressive symptoms, but this was not addressed by his care providers. He eventually stopped the medication and as a result had a seizure episode. This episode was followed a few days later by a delusion of demonic control, culminating in his suicide attempt via hanging.
Kanemoto et al55 reported an increased suicide risk in patients experiencing postictal psychosis. A case-control study16 conducted in South Korea linked suicidal behavior to depression arising from levetiracetam use. These 2 factors are likely to have contributed synergistically to the suicide attempt in our patient. A timely and comprehensive psychiatric evaluation and risk assessment may have prevented this near fatality. Mr A’s case can be conceptualized as one in which the brain is already encumbered with almost unrestrained epileptic activity, multiple substance use, and recurrent mechanical falls. Our case and all of the cases reported in our review emphasize the need for psychiatric care and monitoring with the consideration of the “fragility” of the brain in these patients.
In summary, the risk of suicidality needs to be balanced with the risk of uncontrolled seizures. Specifically, for levetiracetam use, it is important to be aware of the subgroup of individuals with prior severe psychiatric illness, history of TBI, or history of substance use disorder who might be at an increased risk of developing suicide-related behavior and suicidal ideation once levetiracetam is started.
Limitations
In this review, only studies published in English were considered. We encourage the use of any studies published in other languages. For our specific case report, we did not have blood levels of levetiracetam.
CONCLUSION
Suicidal behavior in epileptic patients appears to be multifactorial in etiology.54 Psychiatric disorders are more prevalent among patients with epilepsy than in the general population and contribute to an increased risk of suicidal behavior.54 Despite the high risk of suicidal behavior with the use of AEDs, research has shown that the benefits of anticonvulsant therapy often outweigh the risks.54 Nevertheless, timely consultation with a psychiatrist is invaluable in the care of these patients, particularly those with multiple risk factors, as in the index case.
Submitted: June 19, 2019; accepted April 16, 2020.
Published online: July 30, 2020.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Consent was obtained from the index patient in the case report, and information was de-identified to protect anonymity.
REFERENCES
1. Mula M, Bell GS, Sander JW. Suicidality in epilepsy and possible effects of antiepileptic drugs. Curr Neurol Neurosci Rep. 2010;10(4):327-332. PubMed CrossRef
2. Surges R, Volynski KE, Walker MC. Is levetiracetam different from other antiepileptic drugs? levetiracetam and its cellular mechanism of action in epilepsy revisited. Ther Adv Neurol Disorder. 2008;1(1):13-24. PubMed CrossRef
3. Cormier J, Chu CJ. Safety and efficacy of levetiracetam for the treatment of partial onset seizures in children from one month of age. Neuropsychiatr Dis Treat. 2013;9:295-306. PubMed
4. Wood MD, Gillard M. Evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia. 2017;58(2):255-262. PubMed CrossRef
5. Carunchio I, Pieri M, Ciotti MT, et al. Modulation of AMPA receptors in cultured cortical neurons induced by the antiepileptic drug levetiracetam. Epilepsia. 2007;48(4):654-662. PubMed CrossRef
6. Betts T, Waegemans T, Crawford P. A multicentre, double-blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2,000 mg daily and 4,000 mg daily, without titration in patients with refractory epilepsy. Seizure. 2000;9(2):80-87. PubMed CrossRef
7. Nicolson A, Lewis SA, Smith DF. A prospective analysis of the outcome of levetiracetam in clinical practice. Neurology. 2004;63(3):568-570. PubMed CrossRef
8. Siamouli M, Samara M, Fountoulakis KN. Is antiepileptic-induced suicidality a data-based class effect or an exaggeration? a comment on the literature. Harv Rev Psychiatry. 2014;22(6):379-381. PubMed CrossRef
9. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. PubMed CrossRef
10.Schneebaum-Sender N, Goldberg-Stern H, Fattal-Valevski A, et al. Does a normalizing electroencephalogram in benign childhood epilepsy with centrotemporal spikes abort attention deficit hyperactivity disorder? Pediatr Neurol. 2012;47(4):279-283. PubMed CrossRef
11.Mula M, Sander JW. Suicidal ideation in epilepsy and levetiracetam therapy. Epilepsy Behav. 2007;11(1):130-132. PubMed CrossRef
12.Mula M, Trimble MR, Yuen A, et al. Psychiatric adverse events during levetiracetam therapy. Neurology. 2003;61(5):704-706. PubMed CrossRef
13.Chen B, Choi H, Hirsch LJ, et al. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017;76:24-31. PubMed CrossRef
14.Yates SL, Fakhoury T, Liang W, et al. An open-label, prospective, exploratory study of patients with epilepsy switching from levetiracetam to brivaracetam. Epilepsy Behav. 2015;52(Pt A):165-168. PubMed CrossRef
15.Bektaş G, Tekin U, Özkan MU, et al. The influence of levetiracetam on psychosocial and behavioral functioning in children: a case-control and follow-up study. Epilepsy Behav. 2017;72:39–42. PubMed CrossRef
16.Park S-J, Lee HB, Ahn MH, et al. Identifying clinical correlates for suicide among epilepsy patients in South Korea: a case-control study. Epilepsia. 2015;56(12):1966-1972. PubMed CrossRef
17.Kaufman KR, Bisen V, Zimmerman A, et al. Apparent dose-dependent levetiracetam-induced de novo major depression with suicidal behavior. Epilepsy Behav Case Rep. 2013;1:110-112. PubMed CrossRef
18.Larkin TM, Cohen-Oram AN, Catalano G, et al. Overdose with levetiracetam: a case report and review of the literature. J Clin Pharm Ther. 2013;38(1):68-70. PubMed CrossRef
19.Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81. PubMed CrossRef
20.Chayasirisobhon S, Chayasirisobhon WV, Tsay CC. Acute levetiracetam overdose presented with mild adverse events. Acta Neurol Taiwan. 2010;19(4):292-295. PubMed
21.Givon L, Porter S, Padmanabhan B, et al. Levetiracetam, seizures, and suicidality. Harv Rev Psychiatry. 2011;19(1):47-55. PubMed CrossRef
22.Song HR, Woo YS, Wang H-R, et al. How does antiepileptic drug induce suicidality? a case associated with levitracetam use. Gen Hosp Psychiatry. 2014;36(3):360.e1-360.e2. PubMed CrossRef
23.Kossoff EH, Bergey GK, Freeman JM, et al. Levetiracetam psychosis in children with epilepsy. Epilepsia. 2001;42(12):1611-1613. PubMed CrossRef
24.Born C, Dittmann S, Post RM, et al. Newer prophylactic agents for bipolar disorder and their influence on suicidality. Arch Suicide Res. 2005;9(3):301-306. PubMed CrossRef
25.Bishop-Freeman SC, Kornegay NC, Winecker RE. Postmortem levetiracetam (Keppra) data from North Carolina. J Anal Toxicol. 2012;36(6):422-428. PubMed CrossRef
26.Marino S, Vitaliti G, Marino SD, et al. Pyridoxine add-on treatment for the control of behavioral adverse effects induced by levetiracetam in children: a case-control prospective study. Ann Pharmacother. 2018;52(7):645-649. PubMed CrossRef
27.Chua-Tuan JL, Cao D, Iwanicki JL, et al. Cardiac sodium channel blockade after an intentional ingestion of lacosamide, cyclobenzaprine, and levetiracetam: case report. Clin Toxicol (Phila). 2015;53(6):565-568. PubMed CrossRef
28.Pugh MJV, Copeland LA, Zeber JE, et al. Antiepileptic drug monotherapy exposure and suicide-related behavior in older veterans. J Am Geriatr Soc. 2012;60(11):2042-2047. PubMed CrossRef
29.Raju Sagiraju HK, Wang CP, Amuan ME, et al. Antiepileptic drugs and suicide-related behavior: is it the drug or comorbidity? Neurol Clin Pract. 2018;8(4):331-339. PubMed CrossRef
30.Barrueto F Jr, Williams K, Howland MA, et al. A case of levetiracetam (Keppra) poisoning with clinical and toxicokinetic data. J Toxicol Clin Toxicol. 2002;40(7):881-884. PubMed CrossRef
31.Deeb S, McKeown DA, Torrance HJ, et al. Simultaneous analysis of 22 antiepileptic drugs in postmortem blood, serum and plasma using LC-MS-MS with a focus on their role in forensic cases. J Anal Toxicol. 2014;38(8):485-494. PubMed CrossRef
32.Lee J-J, Song H-S, Hwang Y-H, et al. Psychiatric symptoms and quality of life in patients with drug-refractory epilepsy receiving adjunctive levetiracetam therapy. J Clin Neurol. 2011;7(3):128-136. PubMed CrossRef
33.Gottzein AK, Musshoff F, Madea B. Systematic toxicological analysis revealing a rare case of captan ingestion. J Forensic Sci. 2013;58(4):1099-1103. PubMed CrossRef
34.Theochari E, Cock H, Lozsadi D, et al. Brivaracetam in adults with drug-resistant epilepsy and psychiatric comorbidities. Epilepsy Behav. 2019;90:129-131. PubMed CrossRef
35.Chong DJ, Bazil CW. Update on anticonvulsant drugs. Curr Neurol Neurosci Rep. 2010;10(4):308-318. PubMed CrossRef
36.Tao K, Wang X. The comorbidity of epilepsy and depression: diagnosis and treatment. Expert Rev Neurother. 2016;16(11):1321-1333. PubMed CrossRef
37.Glauser TA, Pellock JM, Bebin EM, et al. Efficacy and safety of levetiracetam in children with partial seizures: an open-label trial. Epilepsia. 2002;43(5):518-524. PubMed CrossRef
38.Tekg×¼l H, Gencpinar P, ÇavuÅŸoÄŸlu D, et al. The efficacy, tolerability and safety of levetiracetam therapy in a pediatric population. Seizure. 2016;36:16-21. PubMed CrossRef
39.Steinhoff BJ, Eckhardt K, Doty P, et al. A long-term noninterventional safety study of adjunctive lacosamide therapy in patients with epilepsy and uncontrolled partial-onset seizures. Epilepsy Behav. 2016;58:35-43. PubMed CrossRef
40.Wen X, Meador KJ, Loring DW, et al. Is antiepileptic drug use related to depression and suicidal ideation among patients with epilepsy? Epilepsy Behav. 2010;19(3):494-500. PubMed CrossRef
41.Pisani LR, Nikanorova M, Landmark CJ, et al. Specific patient features affect antiepileptic drug therapy decisions: focus on gender, age, and psychiatric comorbidities. Curr Pharm Des. 2017;23(37):5639-5648. PubMed CrossRef
42.Kalinin VV. Suicidality and antiepileptic drugs: is there a link? Drug Saf. 2007;30(2):123-142. PubMed CrossRef
43.Ferrer P, Ballar×n E, Sabaté M, et al. Antiepileptic drugs and suicide: a systematic review of adverse effects. Neuroepidemiology. 2014;42(2):107-120. PubMed CrossRef
44.Devinsky O, Patel AD, Thiele EA, et al; GWPCARE1 Part A Study Group. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204-e1211. PubMed CrossRef
45.Awaad Y. Accidental overdosage of levetiracetam in two children caused no side effects. Epilepsy Behav. 2007;11(2):247. PubMed CrossRef
46.Wood H. Epilepsy: psychiatric adverse effects of levetiracetam linked to genetic variation in dopamine signalling. Nat Rev Neurol. 2012;8(10):532. PubMed CrossRef
47.Wood MD, Sands ZA, Vandenplas C, et al. Further evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia. 2018;59(9):e147-e151. PubMed CrossRef
48.Gibbons RD, Hur K, Brown CH, et al. Relationship between antiepileptic drugs and suicide attempts in patients with bipolar disorder. Arch Gen Psychiatry. 2009;66(12):1354-1360. PubMed CrossRef
49.Grant R, Shorvon SD. Efficacy and tolerability of 1,000-4,000 mg per day of levetiracetam as add-on therapy in patients with refractory epilepsy. Epilepsy Res. 2000;42(2-3):89-95. PubMed CrossRef
50.Kang BS, Moon HJ, Kim YS, et al. The long-term efficacy and safety of levetiracetam in a tertiary epilepsy centre. Epileptic Disord. 2013;15(3):302-310. PubMed CrossRef
51.White DA. Catatonia and the neuroleptic malignant syndrome—a single entity? Br J Psychiatry. 1992;161(4):558-560. PubMed CrossRef
52. Physicians’ Desk Reference. 65th ed. Montevale, NJ: PDR Network; 2010.
53.Mula M, Hesdorffer DC. Suicidal behavior and antiepileptic drugs in epilepsy: analysis of the emerging evidence. Drug Healthc Patient Saf. 2011;3:15-20. PubMed CrossRef
54.Mula M, Sander JW. Suicide risk in people with epilepsy taking antiepileptic drugs. Bipolar Disord. 2013;15(5):622-627. PubMed CrossRef
55.Kanemoto K, Kawasaki J, Mori E. Violence and epilepsy: a close relation between violence and postictal psychosis. Epilepsia. 1999;40(1):107-109. PubMed CrossRef
56.Hesdorffer DC, Kanner AM. The FDA alert on suicidality and antiepileptic drugs: fire or false alarm? Epilepsia. 2009;50(5):978-986. PubMed CrossRef
57.LaFrance WC, Kanner AM, Hermann B. Psychiatric comorbidities in epilepsy. In: Gidal B, Harden C, eds. Epilepsy in Women: The Scientific Basis for Clinical Management, Chapter 20. Elsevier; 2008:347-383.
58.Nilsson L, Ahlbom A, Farahmand BY, et al. Risk factors for suicide in epilepsy: a case control study. Epilepsia. 2002;43(6):644-651. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!