Impact of Physician Counseling and Perception of Teratogenic Risks: A Survey of 96 Nonpregnant Women With Anxiety
Objective: To examine the impact of physician counseling on perceived risks, benefits, and likelihood of use of anxiolytic pharmacotherapy during pregnancy among women with a history of anxiety.
Method: We surveyed 96 nonpregnant women, aged 21-45 years, with panic disorder and/or generalized anxiety disorder (DSM-IV criteria) recruited by their family physicians to participate in an anxiety treatment trial from 7 primary care practices in Pittsburgh, Pennsylvania. Trained research assistants telephoned study participants to assess sociodemographics, psychiatric history, comorbidities, and anxiety severity. Respondents were asked to assess risks, benefits, and likelihood of taking a prescribed anxiolytic during pregnancy using 3 Likert scales at baseline. Respondents were then asked to indicate whether their perceptions would change with (1) a US Food and Drug Administration (FDA) warning reporting a 5% chance of birth defects with use and (2) physician counseling that the medication was safe during pregnancy despite the warning. Data were collected from January 1, 2005, through December 30, 2007.
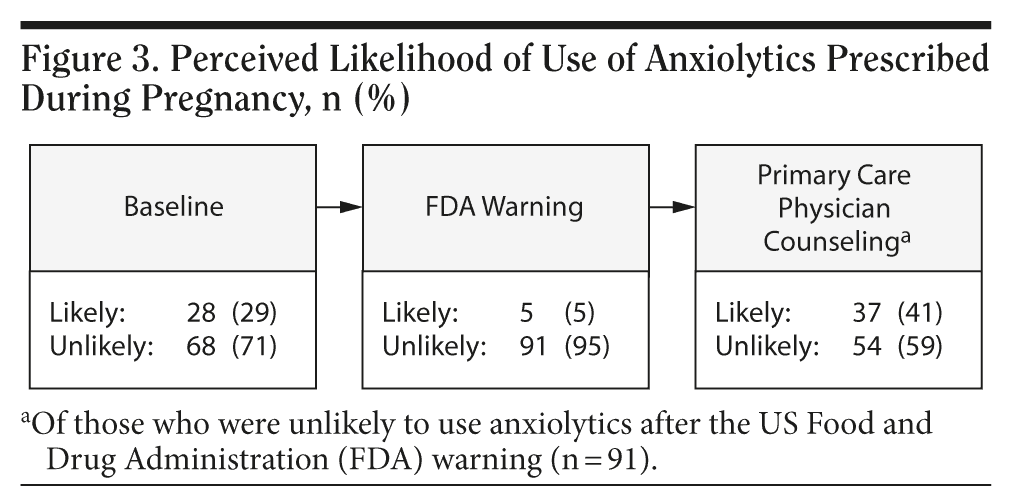
Results: In this study, 46% (44/96) of respondents had generalized anxiety disorder, 14% (14/96) had panic disorder, and 40% (38/96) had both generalized anxiety disorder and panic disorder. The mean baseline Hamilton Anxiety Rating Scale score was 25.6 (SD = 8.4). Respondents were less likely to perceive risk if counseled by their primary care physicians that medication use was safe despite FDA warning. They also saw more benefit in use and reported being more likely to take anxiolytic medications during pregnancy if counseled that doing so was safe. Age, ethnicity, and severity of anxiety did not modify the effect of physician counseling. However, college educated women were less likely to be reassured by primary care physician counseling (P = .05) that anxiolytic use during pregnancy was safe.
Conclusions: Women with anxiety disorders are often hesitant to use anxiolytic medications during pregnancy. Physician counseling may change some women’s perceptions of risk and decisions regarding use during pregnancy.
Trial Registration: Clinicaltrials.gov Identifier: NCT00158327
Primary Care Companion CNS Disord 2011;13(2):e1-e5
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: May 28, 2010; accepted September 1, 2010.
Published online: March 17, 2011 (doi:10.4088/PCC.10m01028).
Corresponding author: Tiffany Behringer, MS, 230 McKee Pl, Ste 600, Pittsburgh, PA 15213 ([email protected]).
Anxiety disorders are the most commonly diagnosed psychiatric disorders in the United States. Generalized anxiety disorder, panic disorder, and other anxiety disorders have an estimated prevalence of 18.1% among adults aged 18 years or older.1 Women are 2 times more likely to be diagnosed with an anxiety disorder than men. As a result, use of benzodiazepines to treat anxiety during pregnancy is relatively common.2 It has been estimated that up to one-third of all pregnant women use a psychotropic medication at some point during their pregnancy.3,4
For women with generalized anxiety disorder and/or panic disorder who become pregnant, decisions about whether or not to use medications, and in particular, benzodiazepines and selective serotonin reuptake inhibitors (SSRIs), can be challenging. SSRIs have been associated with increased risk of congenital malformations including septal heart defects in children whose mothers were prescribed paroxetine, sertraline, and citalopram during pregnancy.5-7 There has also been concern about an increased risk of palate defects among infants whose mothers used benzodiazepines; studies from the 1970s indicated that benzodiazepine use in the first trimester of pregnancy affected embryonic palate formation through their influence on γ-aminobutyric acid channels.8-10
Clinical Points
- Women with anxiety disorders are hesitant to use medications during pregnancy.
- Clinicians should discuss the safety profiles of these medications when pharmacologic treatment for anxiety is indicated.
- Clinicians can have a significant effect on women’s decision making.
However, more recent studies have not confirmed any clear teratogenic risk, including refuting associations between these medications and congenital heart and palatal defects.11-17 Researchers have also demonstrated that anxiolytic use during pregnancy, in particular with benzodiazepines, is associated with an increased incidence of low birth weight and spontaneous abortion as well as low Apgar scores, increased newborn intensive care unit admissions, floppy infant syndrome, respiratory distress syndrome, and, in the case of SSRIs, preterm delivery and fetal growth restriction.18-24 As a result, the question of what risk anxiolytic use during pregnancy may pose remains unclear. While the risks may appear low to some, for individuals predisposed to anxiety, questions about any risk may be alarming. In addition, the burden to a pregnant woman whose anxiety disorder is not adequately treated can be significant. Perinatal anxiety has been associated with many of the same complications as anxiolytic treatment including preterm delivery, low birth weight, and even increased incidence of childhood illnesses and attention-deficit/hyperactivity disorder.25-29 Thus, women must weigh conflicting information about the effects of these medications with the risks of untreated maternal anxiety.
In making decisions about whether or not to use a benzodiazepine during pregnancy, women may seek guidance from the clinician who prescribed the medication.30 However, clinicians who face ongoing time pressures and must juggle a variety of clinical issues may not make discussions of teratogenic risks a priority.31 In this secondary analysis of data collected as part of a treatment trial for individuals with anxiety, we therefore sought to examine the effects of hypothetical physician counseling on perceived risks, benefits, and likelihood of use of anxiolytic pharmacotherapy during pregnancy among women with a history of anxiety.
METHOD
We surveyed 96 nonpregnant women, aged 21-45 years, with panic disorder and/or generalized anxiety disorder (DSM-IV criteria) referred by their primary care physicians to participate in Reducing Limitations From Anxiety in Primary Care (RELAX trial; Clinicaltrials.gov Identifier: NCT00158327), a National Institute of Mental Health (NIMH)-funded anxiety treatment trial from 7 primary care practices in Pittsburgh, Pennsylvania. Details on study recruitment have been described previously.32 Briefly, subjects were excluded if they had a history of bipolar or manic depressive disorder or were receiving treatment for another psychiatric disorder. Trained research assistants telephoned study participants to assess sociodemographic variables, psychiatric history, comorbid medical conditions, and severity of anxiety. For the purposes of this ancillary study, respondents were also asked to rate their perceived risks, benefits, and likelihood of taking a prescribed anxiolytic during pregnancy using 3 Likert scales at baseline. Respondents were then asked to indicate whether their perceptions would change with (1) a US Food and Drug Administration (FDA) warning stating that there was a 5% chance of birth defects with use and (2) physician counseling that the medication was safe despite the FDA warning.
To assess the effect of age, ethnicity, education, and severity of anxiety on the effect of physician counseling on women’s perceptions, the Likert scales were collapsed into 2 categories, and bivariate analyses were then performed. Statistical significance was assessed using P < .05. All analyses were conducted using SAS software (SAS Institute Inc, Cary, North Carolina). This study was approved by the University of Pittsburgh Institutional Review Board, Pittsburgh, Pennsylvania. Data were collected from January 1, 2005, through December 30, 2007.
RESULTS
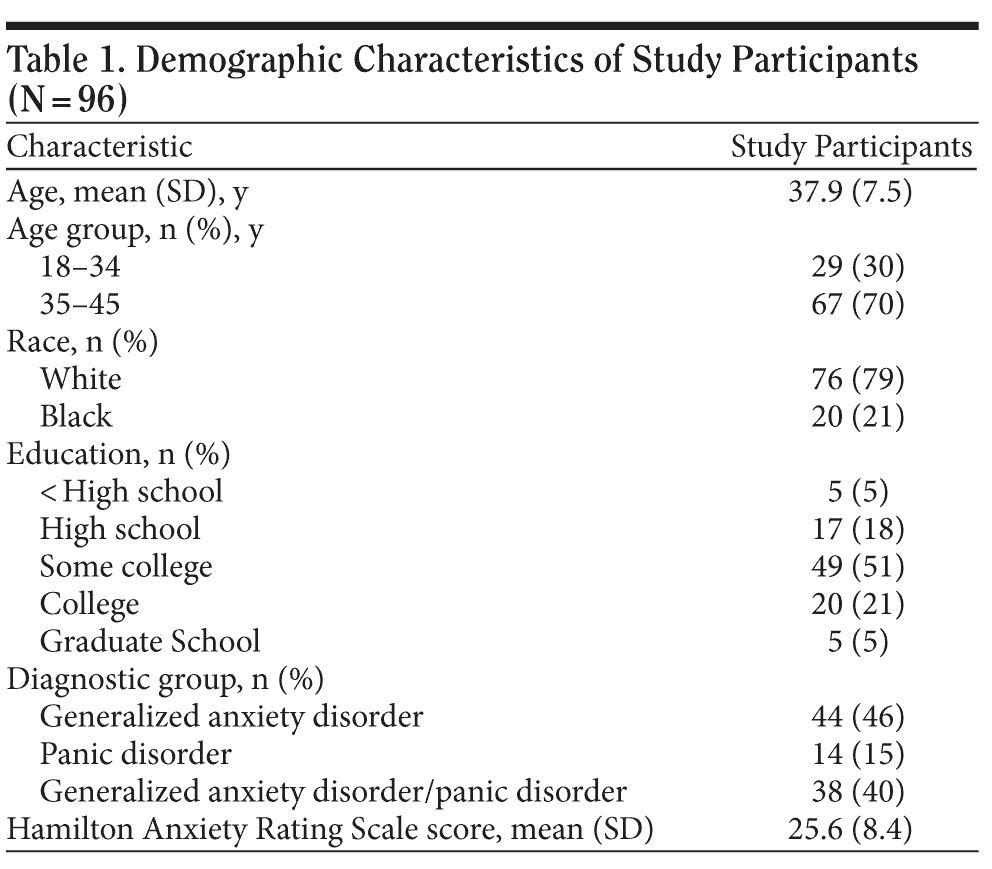
The mean age of the respondents was 38 years (SD = 8 years); 79% (n = 76) were white and 21% (n = 20) were black; 46% (n = 44) had generalized anxiety disorder, 14% (n = 14) had panic disorder, and 40% (n = 38) had both disorders (Table 1). The mean baseline Hamilton Anxiety Rating Scale score was 25.6 (SD = 8.4).
Of the respondents, 74% (71/96) perceived anxiolytic medication use during pregnancy as risky, and once aware of the FDA warning, 90% (86/96) felt this use was risky. However, following primary care physician counseling, 34% (29/86) of these women no longer perceived such risk (Figure 1).
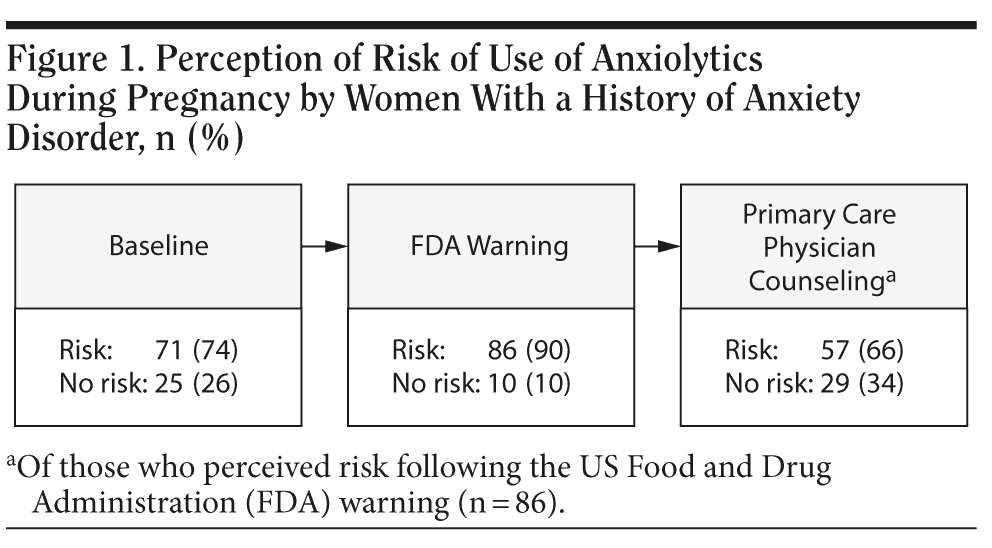
Similarly, 52% (50/96) of respondents initially felt that there would be no benefit to taking an anxiolytic prescribed during pregnancy, and once aware of the FDA warning, 83% (80/96) felt such medication use would have no benefit. However, if counseled by their primary care physician that the medication was safe to use during pregnancy, 43% (34/80) of these women indicated their opinion would change (Figure 2).
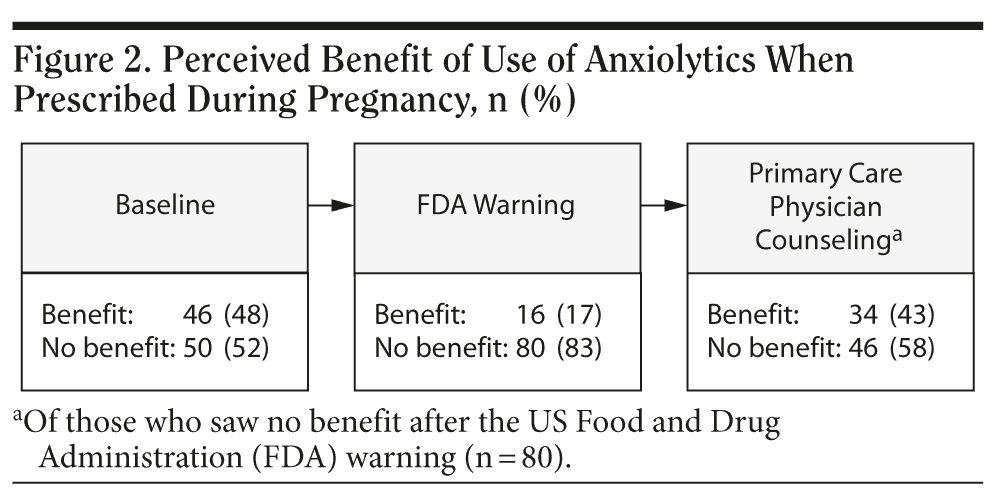
While 71% (68/96) of women initially reported they were unlikely to use an anxiolytic medication if prescribed during pregnancy, once aware of the FDA warning, 95% (91/96) reported that they were unlikely to use such medication. However, 41% (37/91) indicated that counseling from a primary care physician that the medication was safe might result in medication use (Figure 3).
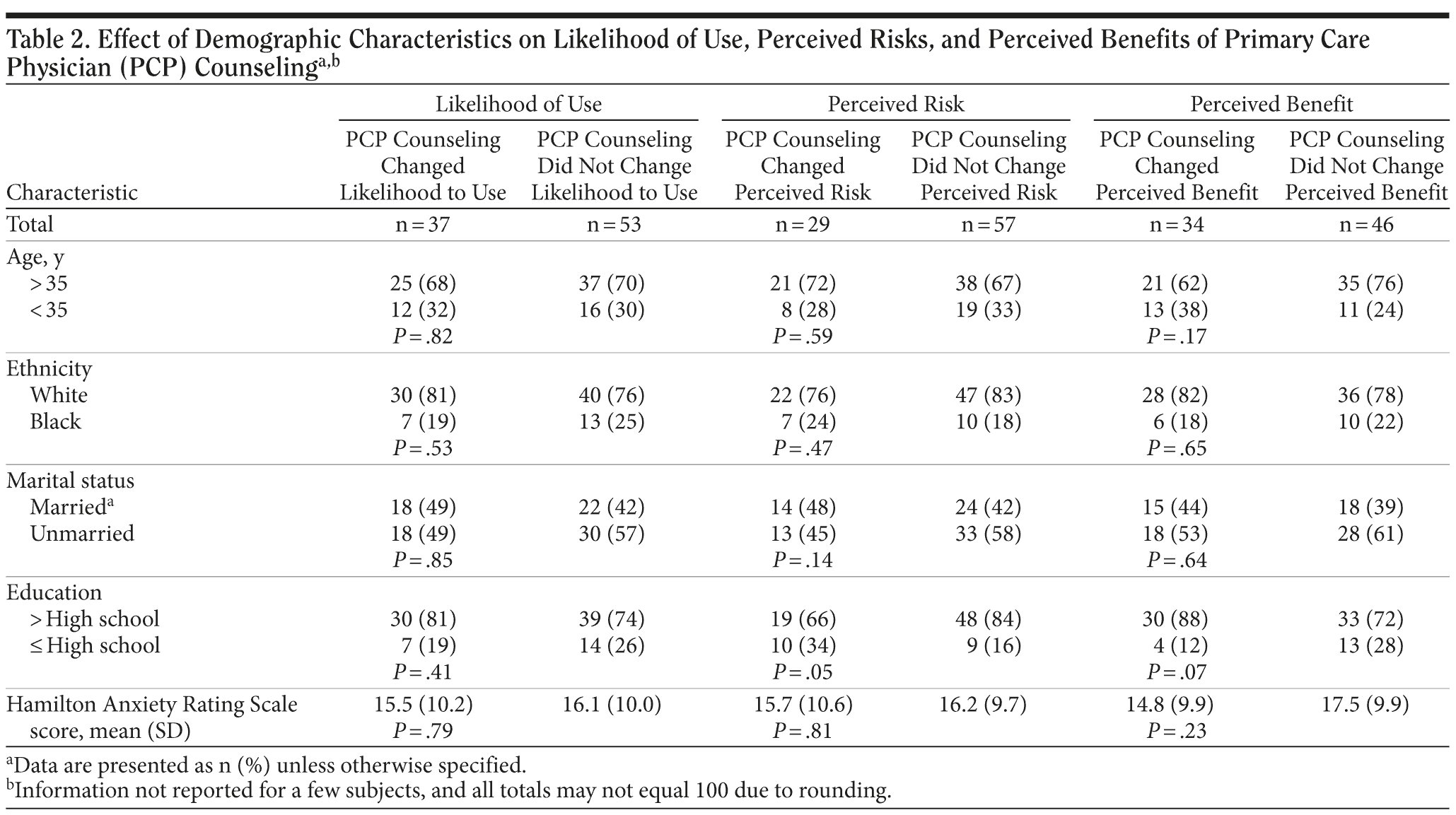
Age, ethnicity, and severity of anxiety did not modify the effect that physician counseling had on the decision choice of participants (Table 2). However, college-educated women were less likely than women with less education to be reassured by primary care physician counseling that anxiolytic use during pregnancy did not pose a significant risk (28% vs 53%, P = .05).
DISCUSSION
This study of attitudes toward anxiolytic use during pregnancy among women with anxiety found that most women were hesitant to use these medications during pregnancy. In addition, many women reported that physician counseling would have a significant effect on their perceptions of risk and decisions regarding use of medications during pregnancy. Interestingly, less educated women were more likely to report that information provided by their health care provider would affect their decisions about whether or not to use an anxiolytic medication during pregnancy.
Previous studies have found that many women expect their physicians to discuss medications’ teratogenic risks regardless of the woman’s current pregnancy intentions.30 Studies have also found that physicians often feel ill-equipped for these conversations due to lack of available sources of information, lack of time per patient, and difficulty addressing pregnancy plans in daily practice.31 In addition, clinicians who do not provide prenatal care may be less likely to discuss the effects that anxiolytic medications might have on a pregnancy. This study highlights the need for discussion of information about teratogenic risks with women who have a history of anxiety and particularly for such women with lower levels of education regardless of the woman’s current pregnancy intentions or status.
Less educated women may be more likely to rely on their physicians for information because they have less access to resources such as Internet databases of teratogenic risk. For those women who are able to find reliable sources of information, the FDA labeling of benzodiazepines as pregnancy risk categories D or X and SSRIs as category C may be difficult to contextualize and may complicate decision making regarding medical treatment and pregnancy plans. It is therefore important for physicians to engage patients in shared decision making about these risks and for health systems to provide clinicians with the resources they need to inform such discussions. For instance, having clinic staff routinely assess women’s plans for pregnancy and contraceptive use may be helpful. In addition, information about hotlines such as those operated by the Organization of Teratology Information Specialists (1-866-626-6847) may be appreciated.
Limitations of this study include the fact that women’s perceptions of risks, benefits, and likelihood of use of medication when imagining pregnancy may differ from their feelings during an actual pregnancy. Additional study is therefore needed to confirm these findings among women who are currently pregnant. We did not have information on prepregnancy intention or pregnancy status. This information would have provided further context for considering the interplay between provider and patient in real-world prepregnancy therapy discussions. We chose to provide a scenario whereby anxiolytics were associated with a 5% risk of birth defects on the basis of prior estimates that birth defects affect 3%-5% of US births, irrespective of medication use. If women were asked to consider a possible 3% chance of birth defects, in line with more recent estimates, the effect of primary care physician counseling on women’s perceptions may have been less visible.33,34
Given the importance of highlighting the risks of not treating their anxiety disorder during pregnancy, ideally our study would have also explored the effect of this variable on women’s decisions regarding medication use. Future studies should also focus on how to most effectively provide counseling about teratogenic risks and how to ensure such counseling is provided to all women and in particular to those with less than a college education, who may depend more on their physicians for this information. Larger studies may detect smaller differences in perceptions than those identified by this relatively small sample size.
In conclusion, this study found that women with anxiety disorders are often hesitant to use anxiolytic medications during pregnancy. As these medications may be helpful in treating women’s anxiety, physicians who feel strongly that their patients would benefit from use of pharmacologic treatment should make time to explicitly discuss the relative safety of such medications with patients who are or may become pregnant, as this counseling appears to have a significant effect on women’s decision making regarding medication use during pregnancy.
Drug names: citalopram (Celexa and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others).
Author affiliations: Division of General Internal Medicine, Center for Research on Health Care (Drs Rollman, Herbeck-Belnap, and Schwarz); Department of Psychiatry (Ms Houck); and Departments of Epidemiology and Obstetrics and Gynecology (Dr Schwarz), University of Pittsburgh School of Medicine (Ms Behringer); and Department of Biostatistics, University of Pittsburgh Graduate School of Public Health (Dr Mazumdar), Pittsburgh, Pennsylvania.
Potential conflicts of interest: Dr Rollman has received grant research support from National Institute of Mental Health and National Heart, Lung and Blood Institute. Dr Schwarz has received grant/research support from the National Institute of Child Health and Development. Drs Herbeck-Belnap and Mazumdar and Mss Behringer and Houck report no conflicts of interest.
Funding/support: The RELAX clinical trial was supported by National Institute of Mental Health grant R01 MH0593953 (principal investigator: Dr Rollman). Dr Schwarz was supported by National Institute of Child Health and Development grant K23HD051585.
Previous presentation: Presented as an abstract at the Society of General Internal Medicine conference; April 30, 2010; Minneapolis, Minnesota.
REFERENCES
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. PubMed doi:10.1001/archpsyc.62.6.593
2. Ververs T, Kaasenbrood H, Visser G, et al. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62(10):863-870. PubMed doi:10.1007/s00228-006-0177-0
3. Doering PL, Stewart RB. The extent and character of drug consumption during pregnancy. JAMA. 1978;239(9):843-846. PubMed doi:10.1001/jama.239.9.843
4. Marchetti F, Romero M, Bonati M, et al; Collaborative Group on Drug Use in Pregnancy (CGDUP). Use of psychotropic drugs during pregnancy: A report of the International Co-Operative Drug Use in Pregnancy (DUP) study. Eur J Clin Pharmacol. 1993;45(6):495-501. PubMed doi:10.1007/BF00315304
5. Pedersen LH, Henriksen TB, Vestergaard M, et al. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339(Sept 23):b3569. PubMed doi:10.1136/bmj.b3569
6. Bakker MK, Kerstjens-Frederikse WS, Buys CH, et al. First-trimester use of paroxetine and congenital heart defects: a population-based case-control study. Birth Defects Res A Clin Mol Teratol. 2010;88(2):94-100. PubMed
7. GlaxoSmithKline. Use of Paxil CR tablets or Paxil tablets during pregnancy. http://www.gsk.com/media/paroxetine/mi_letter_paroxetine_pregnancy.pdf. Accessed January 18, 2011.
8. Aarskog D. Letter: association between maternal intake of diazepam and oral clefts. Lancet. 1975;2(7941):921. PubMed doi:10.1016/S0140-6736(75)92153-4
9. Kellogg CK. Benzodiazepines: influence on the developing brain. Prog Brain Res. 1988;73:207-228. PubMed doi:10.1016/S0079-6123(08)60506-3
10. Saxén I. Associations between oral clefts and drugs taken during pregnancy. Int J Epidemiol. 1975;4(1):37-44. PubMed doi:10.1093/ije/4.1.37
11. Einarson A. The safety of psychotropic drug use during pregnancy: a review. MedGenMed. 2005;7(4):3. PubMed
12. Alwan S, Reefhuis J, Rasmussen SA, et al; National Birth Defects Prevention Study. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684-2692. PubMed doi:10.1056/NEJMoa066584
13. Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749-752. PubMed doi:10.1176/appi.ajp.2007.07060879
14. Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol. 2005;106(6):1289-1296. PubMed doi:10.1097/01.AOG.0000187302.61812.53
15. Pastuszak A, Schick-Boschetto B, Zuber C, et al. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac). JAMA. 1993;269(17):2246-2248. PubMed doi:10.1001/jama.269.17.2246
16. Chambers CD, Johnson KA, Dick LM, et al. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335(14):1010-1015. PubMed doi:10.1056/NEJM199610033351402
17. Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159(12):2055-2061. PubMed doi:10.1176/appi.ajp.159.12.2055
18. Ornoy A, Arnon J, Shechtman S, et al. Is benzodiazepine use during pregnancy really teratogenic? Reprod Toxicol. 1998;12(5):511-515. PubMed doi:10.1016/S0890-6238(98)00035-5
19. Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Am J Obstet Gynecol. 2009;201(6):579, e1-e8. PubMed doi:10.1016/j.ajog.2009.06.061
20. Wikner BN, Stiller CO, Bergman U, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16(11):1203-1210. PubMed doi:10.1002/pds.1457
21. Eros E, Czeizel AE, Rockenbauer M, et al. A population-based case-control teratologic study of nitrazepam, medazepam, tofisopam, alprazolum and clonazepam treatment during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2002;101(2):147-154. PubMed doi:10.1016/S0301-2115(01)00545-0
22. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. PubMed doi:10.1016/0890-6238(94)90029-9
23. Dolovich LR, Addis A, Vaillancourt JM, et al. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ. 1998;317(7162):839-843. PubMed
24. Toh S, Mitchell AA, Louik C, et al. Antidepressant use during pregnancy and the risk of preterm delivery and fetal growth restriction. J Clin Psychopharmacol. 2009;29(6):555-560. PubMed doi:10.1097/JCP.0b013e3181bf344c
25. Menon SJ. Psychotropic medication during pregnancy and lactation. Arch Gynecol Obstet. 2008;277(1):1-13. PubMed doi:10.1007/s00404-007-0433-2
26. Cohen LS, Rosenbaum JF. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry. 1998;59(suppl 2):18-28. PubMed
27. Beijers R, Jansen J, Riksen-Walraven M, et al. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126(2):e401-e409. PubMed doi:10.1542/peds.2009-3226
28. Bhagwanani SG, Seagraves K, Dierker LJ, et al. Relationship between prenatal anxiety and perinatal outcome in nulliparous women: a prospective study. J Natl Med Assoc. 1997;89(2):93-98. PubMed
29. Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75(4):1085-1097. PubMed doi:10.1111/j.1467-8624.2004.00727.x
30. Santucci AK, Gold MA, Akers AY, et al. Women’s perspectives on counseling about risks for medication-induced birth defects. Birth Defects Res A Clin Mol Teratol. 2010;88(1):64-69. PubMed
31. Schwarz EB, Santucci A, Borrero S, et al. Perspectives of primary care clinicians on teratogenic risk counseling. Birth Defects Res A Clin Mol Teratol. 2009;85(10):858-863. PubMed doi:10.1002/bdra.20599
32. Rollman BL, Fischer GS, Zhu F, et al. Comparison of electronic physician prompts versus waitroom case-finding on clinical trial enrollment. J Gen Intern Med. 2008;23(4):447-450. PubMed doi:10.1007/s11606-007-0449-0
33. Centers for Disease Control. Updated overall prevalence of major birth defects: Atlanta, Georgia 1978-2005. MMWR. 2008;57(01):1-5. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a2.htm. Accessed January 18, 2011.
34. Russo CA, Elixhauser A. Hospitalizations for birth defects, 2004. HCUP Statistical Brief #24. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb24.pdf. Accessed January 18, 2011.