Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Memory Disorders Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows.
BANNER ALZHEIMER’ S INSTITUTE
The Banner Alzheimer’s Institute located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2012;14(2):doi:10.4088/PCC.12alz01370
© Copyright 2012 Physicians Postgraduate Press, Inc.
Received: February 27, 2012; accepted February 27, 2012.
Published online: April 26, 2012.
AUTHORS
Roy Yaari, MD, MAS, a neurologist, is associate director of the Memory Disorders Clinic of Banner Alzheimer’s Institute and a clinical professor of neurology at the College of Medicine, University of Arizona, Tucson.
Helle Brand, PA, is a physician assistant at the Memory Disorders Clinic of Banner Alzheimer’s Institute.
James D. Seward, PhD, ABPP, is a clinical neuropsychologist at Banner Alzheimer’s Institute.
Anna D. Burke, MD, is a geriatric psychiatrist and dementia specialist at the Memory Disorders Clinic of Banner Alzheimer’s Institute.
Adam S. Fleisher, MD, MAS, is associate director of Brain Imaging at the Banner Alzheimer’s Institute, a neurologist at the Institute’s Memory Disorders Clinic, and an associate professor in the Department of Neurosciences at the University of California, San Diego.
Jan Dougherty, RN, MS, is director of Family and Community Services at Banner Alzheimer’s Institute.
Pierre N. Tariot, MD, a geriatric psychiatrist, is director of Banner Alzheimer’s Institute and a research professor of psychiatry at the College of Medicine, University of Arizona, Tucson.
Corresponding author: Roy Yaari, MD, MAS, 901 E. Willetta St, Phoenix, AZ 85006 ([email protected]).
CME Background
Original material is selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME activities on a variety of topics from volume to volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute Staff. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the material and go to PrimaryCareCompanion.com to complete the Posttest and Evaluation online.
CME Objective
After studying this case, you should be able to:
- Evaluate and treat an elderly outpatient who has frequent falls, long-term depression and anxiety, progressive cognitive decline, medical illnesses, and somnolence
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Date of Original Release/Review
This educational activity is eligible for AMA PRA Category 1 Creditâ„¢ through April 30, 2015. The latest review of this material was March 2012.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for AstraZeneca, Pfizer, Takeda, and Trovis; and has been a member of the speakers/advisory boards for Forest and Merck. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Ms A, an 82-year-old woman, presented for a new patient evaluation at Banner Alzheimer’s Institute with her son, who provided the clinical history. She had been diagnosed with and treated for a major depressive disorder and a generalized anxiety disorder much of her adult life.
Changes from her baseline cognitive profile were first observed by her son 6 to 7 years ago when Ms A hired a housekeeper to help with chores. She had always been meticulous about cleaning, and it was unusual for her to request assistance. Ms A became less social, was in bed most of the time, and rarely left her home. She presented to multiple doctors for treatment of her ongoing psychiatric illnesses, which she felt were not adequately treated, and received multiple prescriptions for medications including benzodiazepines and a variety of antidepressants. She continued to “shake with anxiety” and “worry when there is nothing to worry about.” Ms A’s son stated that, “She can’ t tell you what she is worrying about—she doesn’ t know.” If a medication did not provide immediate improvement in her symptoms, Ms A would go to another doctor for another medication and would “keep a stash” of her pharmaceutical agents. She did not take them as instructed and would take them when she felt it was necessary, resulting in overmedication, especially with diazepam (her most preferred medication). Her son stated that she was “overusing” the benzodiazepines and was “doped up all the time.”
During this time, Ms A’s husband took over preparing meals and the shopping. Ms A was generally “zoned out,” in that she was somnolent and sleeping much of the time. She could not attend to conversations and would fall asleep. She developed a foggy memory. Two years ago, Ms A started falling frequently, and her husband would call the son or 911 to help her up at these times. These falls gradually escalated over time. Her son reported that she had no balance and would simply collapse.
Over the past 2 to 3 years, Ms A has had continued and progressive cognitive decline with significantly worsening short-term memory. She was seen by a neurologist who performed a brief cognitive test in the clinic (records were not available) and was subsequently diagnosed with dementia. Donepezil and memantine were consecutively initiated without perceptible clinical improvement or side effects. One year ago, Ms A and her husband moved from their home to an independent living facility with a paid caregiver. Since then, Ms A’s son has started to help manage her affairs, and her medications were consolidated and administered according to the prescribed instructions.
Ms A’s recent medical history has been further complicated by multiple hospitalizations for various medical conditions such as endocarditis, recurrent pneumonia, and recurrent urinary tract infections. With each hospitalization, she became weaker and developed even more difficulty ambulating. Her most recent hospitalization was 4 months prior to her evaluation at the Banner Alzheimer’s Institute. At that time, she had stopped ambulating, although she currently attempts to get out of her wheelchair, resulting in frequent falls for which 911 is called.
Currently, Ms A is repetitive with questions and statements. She sleeps much of the day and night and has developed pressure ulcers on her bilateral heels, which began after her hospitalization in May 2011. It is not apparent that she is aware that she has them, and they don’ t seem to cause distress. She is awakened for meals and sometimes falls asleep during the meal. She has word-finding difficulty and is not oriented to time and place. She is depressed and occasionally states that, “I don’ t want to live anymore,” but she does not have a specific suicide plan. Visual hallucinations or delusions are not present. Ms A requires full assistance with most of her personal activities of daily life and is verbally aggressive if someone tries to get her to do something that she does not want to do. She has urinary incontinence. She receives physical therapy twice a week through a home health care agency. The physical therapy has helped slow down the rate of decline of Ms A’s physical debility, as this is the only time that she attempts to ambulate.
PAST MEDICAL HISTORY
Ms A has a history of chronic obstructive pulmonary disease (COPD) requiring 2 L of oxygen 24 hours a day (her oxygen saturation is 93% with 2 L of oxygen), hypertension, hyperlipidemia, anxiety and depression, glaucoma, constipation, urinary incontinence, recurrent pneumonia, recurrent urinary tract infection, Clostridium difficile infection after treatment with antibiotics, a hospitalization for endocarditis in 2009, and hearing loss. She also had a myocardial infarction with a stent placement in 2000 (11 years prior to presenting at Banner Alzheimer’s Institute); this is not an active issue.
MEDICATIONS
Ms A’s current medications include alprazolam 0.5 mg 5 times daily, amitriptyline 10 mg twice daily, memantine 10 mg twice daily, citalopram 10 mg daily, donepezil 10 mg daily, and risperidone 0.5 mg twice daily. She is also taking spironolactone, potassium, magnesium oxide, and a multivitamin.
ALLERGIES
Ms A is allergic to sulfa medications.
SOCIAL HISTORY
Ms A has 14 years of education and worked as a dental hygienist. She raised 4 children. She lives in an independent living facility with her husband and has a paid caregiver 40 hours per week. There is no significant history of alcohol intake. She quit smoking cigarettes 2 years ago after smoking one-half of a pack per day for over 50 years.
FAMILY HISTORY
There is no known history of dementia in Ms A’s family.
PHYSICAL EXAMINATION
Ms A’s vital signs included blood pressure: 120/80 mm Hg, pulse: 80 bpm, respiratory rate: 17 on 2 L of oxygen via nasal canula, and weight: 121 lb. Her general physical examination was unremarkable. She had decreased bilateral airflow in her lungs with expiratory wheezes. Ms A had bilateral foot pressure sores that were bandaged.
NEUROLOGIC EXAMINATION
The neurologic examination was unremarkable except for restricted upgaze and broken smooth pursuits. Ms A had no hearing in her left ear and decreased hearing in her right ear. Coordination testing was slow and arrhythmic bilaterally, worse on the left upper extremity as compared to the right. Ms A was wheelchair bound and required full assistance to stand. When standing, she had what appeared to possibly be an apractic or magnetic gait, as it was quite difficult for her to move her feet. Ms A had 5/5 strength in the bilateral upper extremities, but 4/5 in her bilateral hip flexors and 4/5 in her left ankle dorsiflexion. She had increased tone with cogwheeling in the upper extremities bilaterally and a bilateral upper extremity essential tremor.
The apractic or magnetic gait refers to the appearance of the patient’s feet being “stuck” to the floor like a magnet on a metal surface. Steps are characteristically short, with decreased stride length and height, diminished cadence, and a broadened base with outwardly rotated feet. Postural stability is impaired, and falls are common. Patients turn slowly with several steps.
LABORATORY TESTS/RADIOLOGY
Recent laboratory tests included complete blood count (CBC), comprehensive metabolic panel (CMP), vitamin B12, and thyroid-stimulating hormone (TSH), all of which were unremarkable.
Based on the information thus far, what tests, if any, should be ordered to complete the dementia workup?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. No further tests | 0% |
| B. EEG | 0% |
| C. Structural brain scan (CT or MRI) | 100% |
| D. B and C | 0% |
Guidelines for a routine dementia workup include CBC, CMP, vitamin B12, TSH, and structural brain imaging with either an MRI or CT (Knopman et al, 2001). Even though all attendees at the conference opted for choice “C” in order to complete the routine workup suggested by the guidelines, an argument was made that given Ms A’s comorbidities, it is highly unlikely that brain imaging will change medical management or outcome and will create extra burden on both the patient and caregiver. Although MRIs of the brain provide much better resolution, the treating physician chose a head CT in this relatively frail patient given that CTs are quicker and generally easier for patients to tolerate. Additionally, a head CT scan is useful for diagnosing subdural hematoma, which given the history of multiple falls, should be considered, and if present, could change medical management.
Based on the clinical history alone, do you think a dementia is present?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Yes | 90% |
| B. No | 0% |
| C. Not enough information | 10% |
One conference attendee argued that more information is necessary in order to rule out delirium given the history of recurrent urinary tract infections and polypharmacy with medications that could affect cognition. Most in attendance believed that a dementia is present given the 6 to 7 years of progressive cognitive decline, although polypharmacy could be additionally compromising cognition.
A MMSE (Folstein et al, 1975) score generally correlates with disease severity. Scores ≤ 9 points can indicate severe dementia, between 10-20 points can indicate moderate dementia, and a score > 20 can indicate mild dementia (Mungas, 1991).
Based on the information thus far, what would you expect the MMSE score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 26-30 | 0% |
| B. 21-25 | 20% |
| C. 16-20 | 50% |
| D. 11-15 | 30% |
| E. ≤ 10 | 0% |
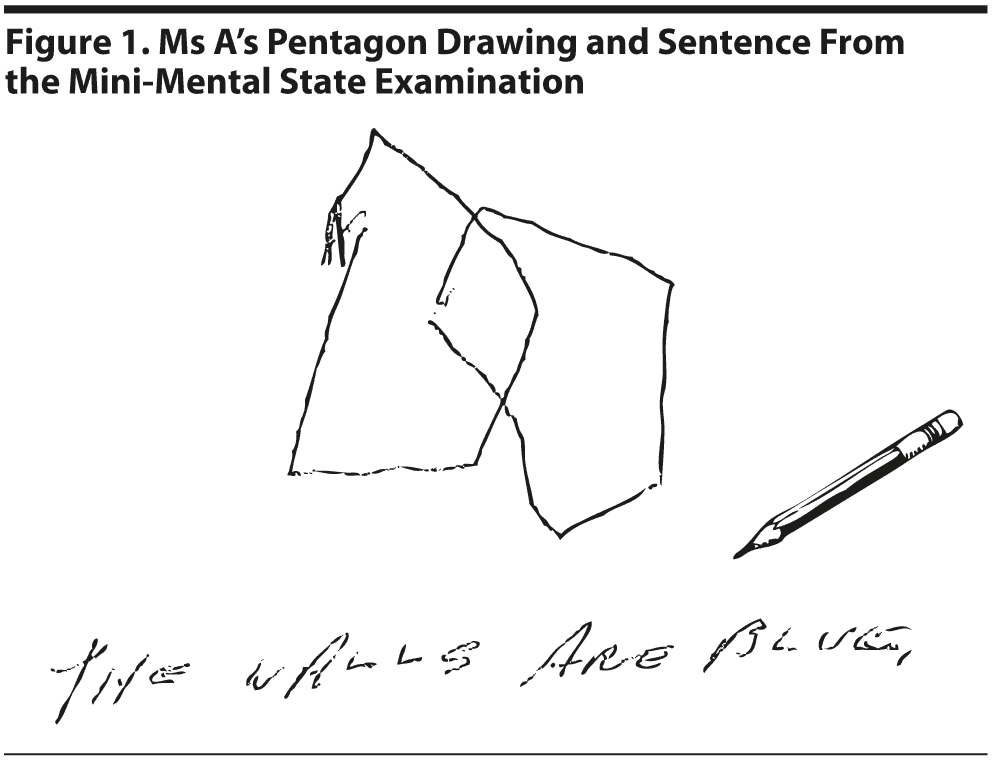
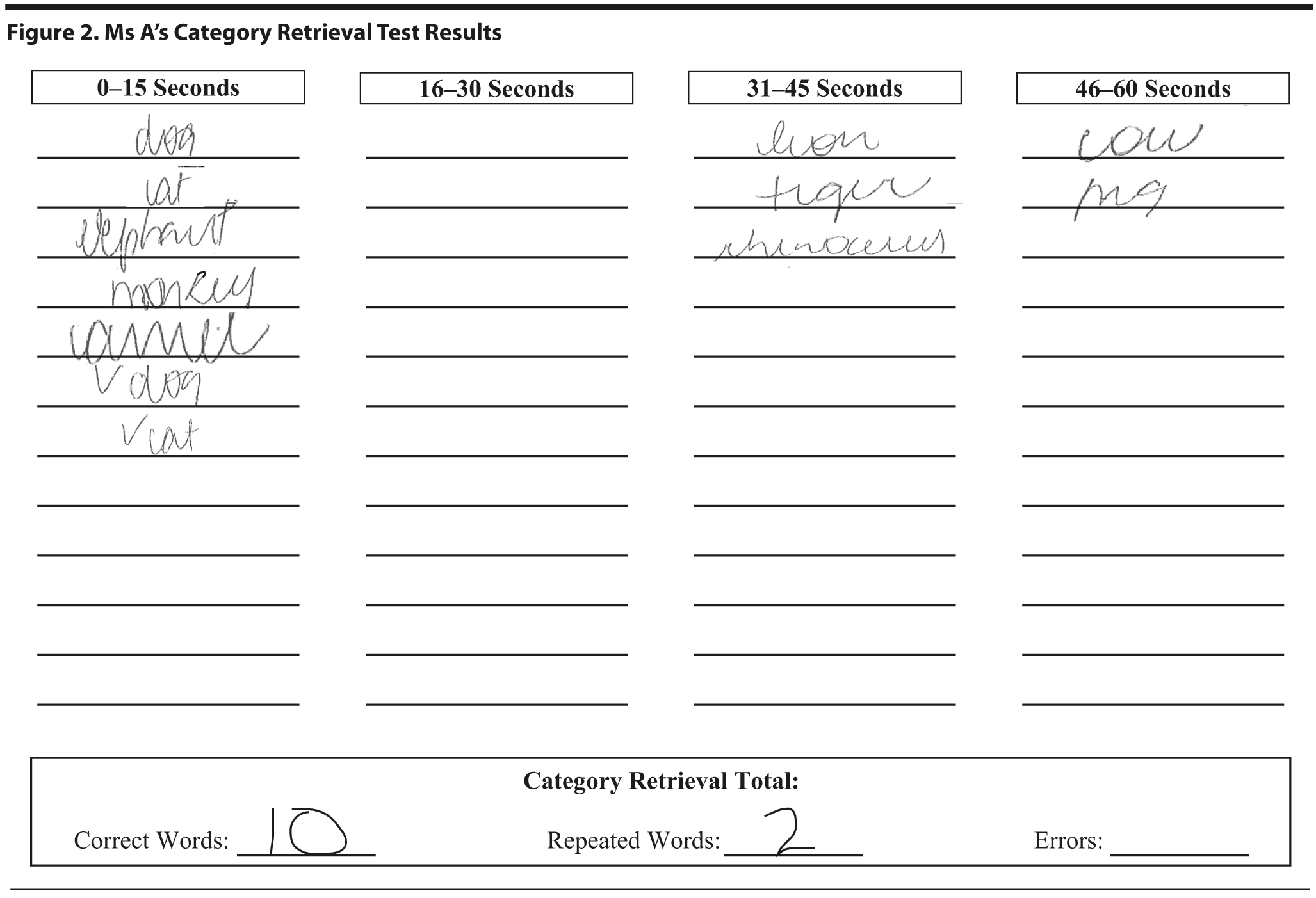
Ms A scored 15 points on the MMSE. She missed 7 points on orientation, 4 points on attention, 1 on construction, and 3 on delayed recall. The intersecting pentagon figure copy and sentence from the MMSE are shown in Figure 1. On the Category Retrieval Test, Ms A listed 10 different animals with 2 repetitions (Figure 2).
In the Category Retrieval Test, the examiner asks the patient to name as many animals as possible in 1 minute. The examiner records the responses. Performance on this measure is influenced by age; unimpaired people in their 60s should name about 18 animals, whereas people in their 80s should name about 15 (Mitrushina et al, 2005). There is no hard-and-fast cutoff for impairment. However, patients who name 4 or more animals less than expected raise concerns. Bilingual individuals are at a disadvantage on this and other measures of verbal fluency (Gollan et al, 2002).
Based on the information thus far, what would you expect the Montreal Cognitive Assessment (MoCA) score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 0 | 0% |
| B. 21-25 | 0% |
| C. 16-20 | 0% |
| D. 11-15 | 50% |
| E. ≤ 10 | 50% |
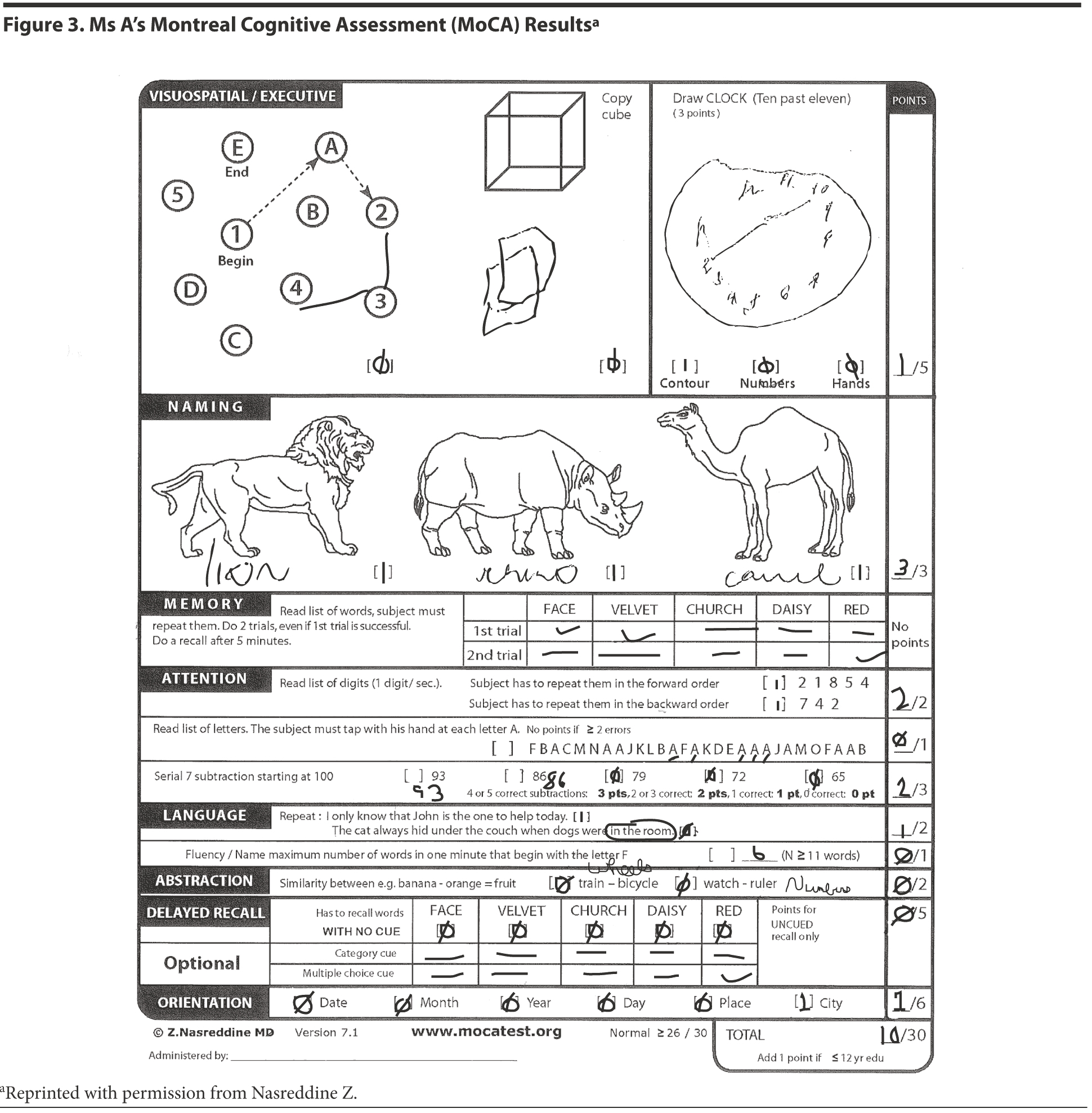
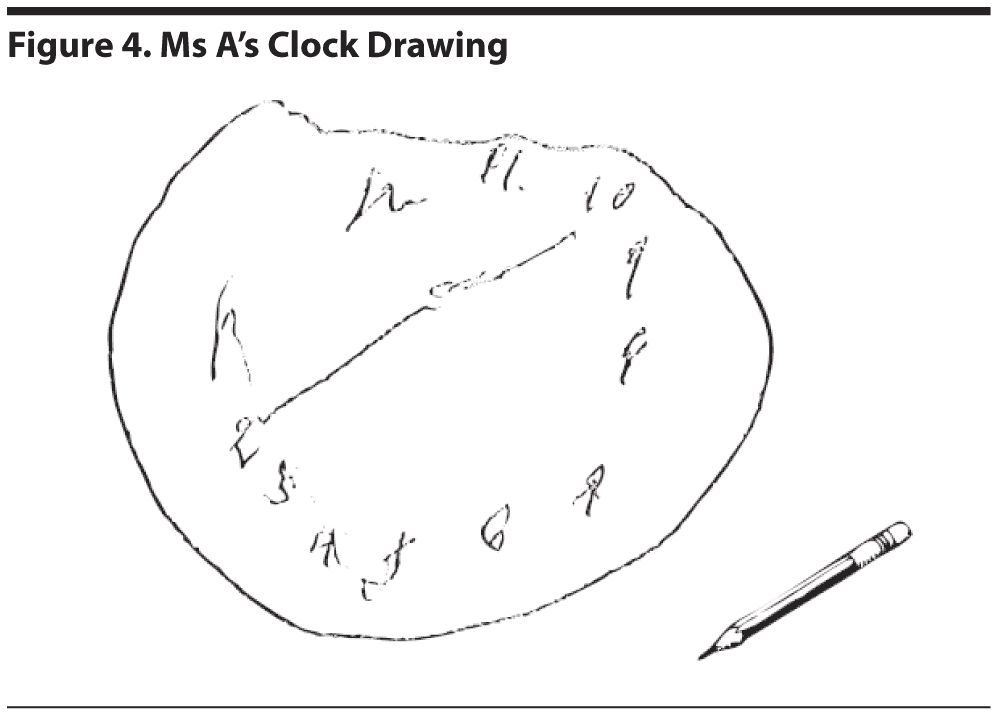
The results of Ms A’s MoCA test are provided in Figure 3. Figure 4 shows Ms A’s clock drawing.
The MoCA is a 30-point test that assesses several cognitive domains. Because it is more challenging than the MMSE, it has greater sensitivity for mild cognitive impairment and early stages of dementia. With a cutoff score < 26, the sensitivity for detecting mild cognitive impairment (N = 94) was 90% and the specificity was 87% (Nasreddine et al, 2005). This test is available online at http://mocatest.org/.
Based on the information thus far, what should be considered as a possible etiology for this patient’s cognitive changes?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Polypharmacy | 0% |
| B. Alzheimer’s disease | 0% |
| C. Normal pressure hydrocephalus | 0% |
| D. Vascular dementia | 0% |
| E. A and C only | 0% |
| F. A, B, C, and D | 100% |
Most attendees believed that polypharmacy, although not likely the cause of her cognitive changes, is certainly contributing to Ms A’s symptoms and should be considered as a potential etiology. Alzheimer’s disease was thought to be the most likely possibility, but without more information at this time, both normal pressure hydrocephalus and vascular dementia should be considered.
Normal pressure hydrocephalus is a communicating hydrocephalus causing ventriculomegaly that is either idiopathic or secondary to a subarachnoid hemorrhage, head trauma, tumor, central nervous system infection, or a complication of cranial surgery. Normal pressure hydrocephalus may exhibit a triad of clinical findings of urinary incontinence, gait disturbance, and dementia. Gait disturbance is typically the initial and most prominent symptom of the triad (Relkin et al, 2005). Vascular dementia is due to single or multiple cerebrovascular events in brain areas involved with cognition or is a result of chronic brain changes due to significant small vessel ischemic disease. Often, strokes may occur without noticeable or obvious clinical symptoms. Both normal pressure hydrocephalus and vascular dementia would be evident on brain imaging.
One attendee commented on hypoxia due to poorly controlled COPD as a possible factor. Studies have shown that hypoxemia in COPD can lead to cognitive impairment (Thakur et al, 2010). Ms A is compliant with her oxygen regimen, and she is seen by her pulmonologist regularly. It is unlikely that the COPD is causing her cognitive symptoms.
What medications are most likely to be contributing to somnolence and/or confusion and should be attempted to be reduced/discontinued?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Donepezil, memantine, and amitriptyline | 0% |
| B. Amitriptyline, alprazolam, and risperidone | 100% |
| C. Citalopram, alprazolam, and donepezil | 0% |
| D. Citalopram, memantine, and risperidone | 0% |
| E. All her medications are likely to be causing cognitive decline; the patient should have an immediate drug holiday | 0% |
The most common side effects of cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) include cholinergic side effects of diarrhea, nausea, vomiting, and bradycardia (can be symptomatic with syncope). Somnolence and confusion are relatively rare side effects of donepezil (Farlow et al, 2008). In rare cases, memantine can worsen baseline confusion or induce somnolence (Farlow et al, 2008). Citalopram can sometimes induce somnolence, but this is not common (Muldoon, 1996).
Amitriptyline, a tricyclic antidepressant, has relatively high anticholinergic properties and could worsen confusion in dementia patients as well as diminish the therapeutic effect of cholinesterase inhibitors such as donepezil. Amitriptyline also has a relatively high possibility of causing sedation, relative to other antidepressants, and these effects could be potentiated when used with other sedative drugs. Due to these anticholinergic and sedative effects, this medication should be used with caution in the elderly (National Institutes of Health, 2009).
Side effects associated with benzodiazepines (such as alprazolam, lorazepam, and diazepam) in dementia patients include ataxia, falls, confusion, anterograde amnesia, and sedation (Yaari et al, 2009). Benzodiazepines are generally not recommended beyond a brief period of use because of risks of dependence, tolerance, sedation, cognition deterioration, and delirium (Patel and Tariot, 1995). For these reasons, use of benzodiazepines in dementia patients is typically limited for agitation associated with procedures or time-limited acute or as-needed use, with chronic use only in those patients for whom other agents have proven ineffective. Benzodiazepines are recommended for use with only minimum effective doses given that side effects are dose related. Also, because of withdrawal risks, agents that are prescribed for an extended period should not be stopped abruptly, but rather tapered (Yaari et al, 2009).
The most common side effects seen with risperidone include somnolence, fatigue, and sedation (among others including parkinsonism) (Risperdal package insert, 2011). In Ms A, it is not clear why risperidone was initiated but may have been related to aggression with personal care and/or agitation related to delirium. It is important to note that no medication is US Food and Drug Administration (FDA)-approved for treatment of behavioral symptoms in dementia, and there are no clinical trial data guiding best practices in this situation. There are specific warnings regarding use of atypical antipsychotics in the elderly (FDA, 2008).
An additional consideration, apart from effects of medications, is daytime somnolence related to apathy, depression, and boredom sometimes seen in patients with Alzheimer’s disease, resulting in gradually increasing periods of sleep as the disease progresses.
The Treating Physician’s Impression
The patient is an 82-year-old woman who presents for a cognitive evaluation. The clinical history and cognitive findings are consistent with a dementia. The most likely etiology of the patient’s dementia is Alzheimer’s disease; however, further workup will be initiated to rule out other possibilities such as stroke, tumor, or normal pressure hydrocephalus. The patient’s dementia is further complicated by depression, anxiety, somnolence, and gait disturbance with multiple falls. She also has polypharmacy with several medications that could negatively affect cognition including alprazolam, amitriptyline, and risperidone.
The Treating Physician’s Plan
- Complete the dementia workup; order a head CT scan.
- Continue donepezil 10 mg/day.
- Continue memantine 10 mg twice daily.
- Continue citalopram 10 mg/day.
- Reduce risperidone to 0.5 mg/day, working toward discontinuation. There is no history of delusions, visual hallucinations, or other psychosis.
- For the time being, continue alprazolam 0.5 mg 5 times daily with the intent to gradually reduce the dose and hopefully discontinue it if possible in the near future.
- Discontinue amitriptyline 10 mg twice daily in the near future.
- Referred the patient’s son to a Banner Alzheimer’s Institute caregiver class. He already attends the Men Who Care support group. The other attendees in this support group encouraged him to bring his mother in to be seen as a patient at Banner Alzheimer’s Institute.
- Referred the patient to a 1-on-1 visit with a social worker with the Family and Community Services team for family psychotherapy. Issues to discuss include optimizing the current living situation and making sure that the patient has optimal care, as her elderly husband has difficulty caring for her, especially lifting her when she falls, and they have utilized 911 numerous times.
- The patient will follow up in approximately 2 to 3 weeks.
Banner Alzheimer’s Institute has free classes for caregivers that address topics such as understanding dementia, current treatment strategies, resources to help meet the ever-changing needs of the patient, improved communication skills to avoid arguments and behavior upsets, and finding in-home and community assistance.
Men Who Care is a support group for men at Banner Alzheimer’s Institute. This group meets monthly to share educational and problem-focused solutions for men involved in caring for someone with Alzheimer’s disease or a related dementia.
UPDATE
Two-Week Follow-Up
The head CT was unremarkable; there was no evidence of prior strokes or normal pressure hydrocephalus. Risperidone was further reduced to 0.25 mg at bedtime for 1 week and then discontinued, at which time alprazolam was reduced to 0.5 mg 3 times daily. Because of the overutilization of 911 services, Ms A’s son was informed of a community partnership first responder program.
Four-Week Follow-Up
As these changes were made, Ms A became significantly “more alert,” and there was “more life in her.” A repeat MMSE improved to 19/30. She is walking better and has had fewer falls (1 fall without injury). Citalopram was increased to 20 mg, and alprazolam was further reduced to 0.5 mg twice daily.
Seven-Week Follow-Up
The reduction of alprazolam resulted in increased anxiety, and citalopram was increased to 30 mg/day. Amitriptyline will be tapered off. Ms A had a fall without injury but was found to have pneumonia and was treated with antibiotics. Her pneumonia was subsequently complicated by large left pleural effusion that is to be drained (the procedure was pending at the time of writing this article). In light of repeated infections, oxygen-dependent COPD, multiple falls, and dependence for most activities of daily life, hospice services were discussed but not ordered.
A nurse specialist present at the conference stressed the importance of having continued discussions with Ms A and the family about health care treatments that may pose increased burden in the face of multiple system declines. Unless the medical team engages Ms A (when appropriate) and family in these discussions, treatments that are not consistent with the patient’s expressed wishes may occur and take away from the ultimate goal of comfort. Ms A’s current medications are donepezil 10 mg/day, memantine 10 mg twice daily, citalopram 30 mg/day, alprazolam 0.5 mg twice daily, and amitriptyline 10 mg twice daily. She is also taking spironolactone, potassium, magnesium oxide, and a multivitamin.
Disclosure of off-label usage
The authors have determined that, to the best of their knowledge, alprazolam, citalopram, diazepam, lorazepam, risperidone, and amitriptyline are not approved by the US Food and Drug Administration for the treatment of psychiatric symptoms in dementia.
Faculty FINANCIAL DISCLOSURE
Dr Yaari is a consultant for Amedisys Home Health. Dr Tariot has served as a consultant for Acadia, AC Immune, Allergan, Eisai, Epix, Forest, Genentech, MedAvante, Memory Pharmaceuticals, Myriad, Novartis, Sanofi-Aventis, Schering-Plough, and Worldwide Clinical Trials; has received consulting fees and grant/research support from Abbott, AstraZeneca, Avid, Baxter, Bristol-Myers Squibb, GlaxoSmithKline, Elan, Eli Lilly, Medivation, Merck, Pfizer, Toyama, and Wyeth; has received educational fees from Alzheimer’s Foundation of America; has received other research support from Alzheimer’s Association, Arizona Department of Health Services, GE, Institute for Mental Health Research, Janssen, National Institute of Mental Health, and National Institute on Aging; has received honoraria from AstraZeneca, Eisai, Eli Lilly, and Pfizer; is a stock shareholder in Adamas and MedAvante; and holds a patent for “Biomarkers of Alzheimer’s Disease.” Drs Seward, Burke, and Fleisher and Mss Brand and Dougherty have no personal affiliations or financial relationships with any commercial interest to disclose relative to the activity.
FUNDING/SUPPORT
None reported.
DISCLAIMER
The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press.
For Clinical Use
- Careful review of medications taken by a patient with dementia should be performed to eliminate untoward side effects and improve quality of life.
- Benzodiazepine use in patients with dementia could result in ataxia, falls, confusion, and sedation and should be limited if possible.
- Tricyclic antidepressants have anticholinergic properties and could worsen confusion in patients with dementia as well as diminish the therapeutic effect of cholinesterase inhibitors that are used to treat Alzheimer’s disease.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
Farlow MR, Miller ML, Pejovic V. Treatment options in Alzheimer’s disease: maximizing benefit, managing expectations. Dement Geriatr Cogn Disord. 2008;25(5):408-422. PubMed doi:10.1159/000122962
Gollan TH, Montoya RI, Werner GA. Semantic and letter fluency in Spanish-English bilinguals. Neuropsychology. 2002;16(4):562-576. PubMed doi:10.1037/0894-4105.16.4.562
Gollan TH, Montoya RI, Werner GA. Semantic and letter fluency in Spanish-English bilinguals. Neuropsychology. 2002;16(4):562-576. PubMed doi:10.1037/0894-4105.16.4.562
Knopman DS, DeKosky ST, Cummings JL, et al. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153. PubMed
Mitrushina M, Boone KB, Razani J, et al. Handbook of Normative Data for Neuropsychological Assessment. Second edition. New York, NY: Oxford University Press; 2005.
Mitrushina M, Boone KB, Razani J, et al. Handbook of Normative Data for Neuropsychological Assessment. Second edition. New York, NY: Oxford University Press; 2005.
Muldoon C. The safety and tolerability of citalopram. Int Clin Psychopharmacol. 1996;11(suppl 1):35-40. PubMed doi:10.1097/00004850-199603001-00007
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. PubMed doi:10.1111/j.1532-5415.2005.53221.x
National Institutes of Health. Daily Med. Amitriptyline. Revised: 2009. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15582. Accessed February 28, 2012.
Patel S, Tariot PN. Use of benzodiazepines in behaviorally disturbed patients: risk-benefit ratio. In: Lawler BA, ed. Behavioral Complications of Alzheimer’s Disease. Washington, DC: American Psychiatric Association Press; 1995:153-170.
Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(suppl 3):S4-S16, discussion ii-v. PubMed
Risperdal [package insert]. Titusville, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2011. http://www.risperdal.com/prescribing.html. Accessed February 28, 2012.
Thakur N, Blanc PD, Julian LJ, et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis. 2010;5:263-269. PubMed
US Food and Drug Administration. Safety: Antipsychotics, Conventional and Atypical. Posted June 16, 2008. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm110212.htm. Accessed February 28, 2012.
Yaari R, Tariot PN, Richards D. Pharmacological treatment of neuropsychiatric symptoms. In: Weiner MF, Lipton AM, eds. The American Psychiatric Publishing Textbook of Alzheimer Disease and Other Dementias. 1st ed. Arlington, VA: American Psychiatric Publishing, Inc; 2009:285-300.
Please sign in or purchase this PDF for $40.00.