Treatment outcomes for bulimia nervosa in type 1 diabetes are worse than those for conventional bulimia nervosa. These outcomes may be a consequence of late detection and subsequent management. The combination of these disorders has been referred to as diabulimia; however, this is not an official diagnosis and is a colloquial term used by patients and the media to describe the associated maladaptive pattern of compensatory behaviors. Early intervention is required to prevent short- and longer-term complications, with intensive treatment approaches having the best current evidence. Collaboration is required between specialist services for patients to receive optimal care. This narrative review summarizes the latest published evidence in the formulation, detection, and subsequent management of bulimia nervosa in type 1 diabetes, while highlighting the need for higher-quality research in the assessment and treatment of these comorbidities.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. This activity is free.
CME Objective
After studying this article, you should be able to:
- Employ a collaborative approach in the treatment of patients with bulimia nervosa and type 1 diabetes
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in December 2020 and is eligible for AMA PRA Category 1 Creditâ„¢ through December 31, 2022. The latest review of this material was December 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for Acadia, Allergan, Eisai, Merck, Supernus, and Takeda; has been a stock shareholder of M-3 Information; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Treatment outcomes for bulimia nervosa in type 1 diabetes are worse than those for conventional bulimia nervosa. These outcomes may be a consequence of late detection and subsequent management. The combination of these disorders has been referred to as diabulimia; however, this is not an official diagnosis and is a colloquial term used by patients and the media to describe the associated maladaptive pattern of compensatory behaviors. Early intervention is required to prevent short- and longer-term complications, with intensive treatment approaches having the best current evidence. Collaboration is required between specialist services for patients to receive optimal care. This narrative review summarizes the latest published evidence in the formulation, detection, and subsequent management of bulimia nervosa in type 1 diabetes, while highlighting the need for higher-quality research in the assessment and treatment of these comorbidities.
Prim Care Companion CNS Disord 2020;22(6):20nr02707
To cite: Yahya AS, Khawaja S, Chukwuma J, et al. Early diagnosis and management of bulimia nervosa in type 1 diabetes. Prim Care Companion CNS Disord. 2020;22(6):20nr02707.
To share: https://doi.org/10.4088/PCC.20nr02707
© Copyright 2020 Physicians Postgraduate Press, Inc.
aWaltham Forest Older Adults Mental Health Team, North East London NHS Foundation Trust, Red Oak Lodge, London, United Kingdom
bBarnet, Enfield and Haringey Mental Health Trust, London, United Kingdom
cBabylon Health Office, London, United Kingdom
*Corresponding author: Ahmed Saeed Yahya, MBBS, MRCPsych, Waltham Forest Older Adults Mental Health Team, North East London NHS Foundation Trust, Red Oak Lodge, London, E11 4HU, United Kingdom ([email protected]).
Estimates indicate that 3.7 million people are living with diabetes in the United Kingdom, and approximately 10% have a diagnosis of type 1 diabetes (T1D).1 Eating disorders are more frequent in T1D and are associated with significant risk.2-6 The mortality and morbidity rates are increased in those with both diagnoses.7-12 Eating disorders tend to be more common in females with T1D, and studies13-17 indicate they are twice as likely to develop an eating disorder compared to those without T1D. A meta-analysis7 reported a 3-fold increase of bulimia nervosa (BN) in patients with T1D compared to controls.
Eating disorders can manifest with intentional insulin restriction and omission, which can cause life-threatening complications including diabetic ketoacidosis and hypoglycemia.10-14 Glycemic control is poor in this patient group (as reflected by raised glycated hemoglobin [HbA1c]), and hospitalization is frequent, which is distressing for both patients and their families. Rapid correction of glucose control can itself increase the risk of microvascular disease.13
Diabulimia is a term used by patients and the media to describe BN in T1D and is not an official diagnosis.1,3,4 Up to 20% of women with T1D may have this disorder.1 Diabulimia is intentional insulin omission or manipulation to achieve weight loss or prevent weight gain manifest in the form of purging, whereby hyperglycemia is induced with the aim to lose glucose calories through urine.6-9 There are associated binging episodes wherein patients normally report temporary loss of control over their eating. Those who omit insulin have been found to be 3 times more likely at risk of premature death than those who cooperate with their prescribed treatment. Cases of T1D and BN are difficult to manage and understandably provoke anxiety in health care professionals.6,7,10,11 These disorders are especially challenging given the difficulties in detection and diagnosis, which could be related to lack of awareness but are also linked to the clinical invisibility of BN in T1D.
BN in T1D can take over the patient’s life with primacy and central importance attached to stringently monitoring dietary intake and enforcing extreme measures to control weight. Additional measures aside from insulin manipulation can include dietary restriction, overexercising, and misusing laxatives. The importance of good communication and collaboration between family, primary care, diabetes, and specialist psychiatric services is essential. This collaboration would ensure prompt recognition, which has been associated with better outcomes and the use of a robust, proactive interdisciplinary approach in managing these potentially devastating comorbidities.2,8,18
This review summarizes the latest published evidence in the formulation, detection, and subsequent management of BN in T1D. We recommend regular screening for disordered eating to be incorporated into consultations, particularly for those considered to be at higher risk, which would assist in the earlier identification of BN in T1D. We highlight possible benefits of using a biopsychosocial model of care, with focus on psychoeducation, motivational interviewing, and use of an adapted form of cognitive-behavioral therapy (CBT) in the treatment of this challenging condition.
METHODS
We conducted a comprehensive search for the relevant literature published in the last 10 years and base our recommendations on these findings. Our search was restricted to articles published in the English language. We searched various databases including MEDLINE, PubMed, Cochrane Library, Trip Database, NICE Healthcare Database Advanced Search, DynamedPlus, North East London NHS Foundation Trust (NELFT) Discovery, and the NELFT library catalog. Our search terms included bulimia nervosa, type 1 diabetes mellitus, feeding disorders, eating disorders, insulin, drug misuse, and prescription drug misuse. We screened the bibliography of all relevant articles as part of the evidence search.
The search identified 47 articles,1-47 2 national guidelines,48,49 and 1 book chapter50 relevant for the review. The results included 1 recent systematic review and meta-analysis,2 which reported on the efficacy of interventions for people with T1D and general disordered eating. Another systematic review5 provided a brief overview of the literature pertaining to insulin restriction as a disordered eating behavior in T1D. The authors5 proposed a novel maintenance model that adapted and integrated concepts from well-known accepted theories by Treasure and colleagues12 and Cooper and Fairburn.21 These models may already guide current structured CBT for BN in T1D. These models comprise the 3 disordered eating behaviors specific to T1D and BN, which include dietary restriction and binging and purging behaviors.
On the basis of the evidence gathered and our clinical experience, we provide practical recommendations for the assessment, diagnosis, formulation, and management of BN in T1D.
RESULTS
Formulation of BN in T1D

- Early recognition and treatment of bulimia nervosa in type 1 diabetes results in better outcomes.
- Efficacy of recognized treatments for bulimia nervosa in type 1 diabetes is equivocal.
- A collaborative effort between diabetes and eating disorder services appears to be the best approach in the treatment of patients with bulimia nervosa and type 1 diabetes.
There are 2 established models for the etiology of BN in T1D: the transdiagnostic model for eating disorders and the dual pathway model. Both models address the psychopathology of dysfunctional ideation about the control of weight and shape through eating, poor coping mechanisms, and the impact of dysregulated emotional states. We have integrated concepts from both models to provide a concise and more readable synopsis.5,9,12,19-21
A diagnosis of T1D is life changing and requires considerable adjustment, leading to the negative cognitions of denial, anger, and frustration. In 90% of cases, the onset of T1D is before the eating disorder.12 Multiple interacting factors may lead to the development of BN including predisposing personality traits (ie, emotional instability, interpersonal conflicts, and individual genetic vulnerabilities). There is significant focus on diet, in particular carbohydrate counting, and exercise in T1D. The advised lifestyle management may instill a preoccupation with weight in vulnerable patients and promote dysfunctional compensatory measures.5,9,12,19,21
The close monitoring of blood sugar levels and scrutiny associated with the timing, quantity, and nutritional content of meals may further reinforce existing perfectionist, obsessional traits and the adoption of rigid dietary rules.5,9,12,19,21 Patients with T1D may experience difficulty recognizing hunger and satiety cues because of underlying hormonal dysregulation, which may evoke physiologic and psychological deprivation states predisposing to an episodic and addictive pattern of binge eating. There also may be episodes of disinhibited eating in response to misperceived or actual episodes of hypoglycemia because of overexercising, inappropriate insulin use, and regulation of blood sugar levels.5,9,12,19,21
Additionally, those with T1D are prone to weight gain and obesity, with females being at higher risk. Insulin encourages fat storage, and patients gradually become more aware of this effect. Prior to the initial diagnosis of T1D, individuals may have experienced weight loss as a consequence of endogenous insulin cessation from damage to pancreatic β cells.5,9,12,19,21 Diabetic patients find it more challenging to maintain an optimal weight, with a tendency to be more self-conscious. They may misinterpret comments from professionals, and the public/media perception of an “ideal body weight” can be triggering and also a perpetuating factor. Body image concerns may exacerbate low self-esteem, leading to negative emotionality and subsequent dietary restraint.5,9,12,19,21,22 Both dietary restraint and negative affect are thought to be predictive of binge eating in BN. When the patient expectably breaks these extreme dietary rules, an episode of binge eating may result. The difficulties associated with BN may be a manifestation of or precipitate depressive and anxiety disorders in some individuals.8
Assessment of BN in T1D
In consultations, there should be emphasis on good communication and establishing a rapport to elicit pertinent details (Table 1 summarizes potential warning signs). A relevant clinical history and examination allow the exclusion of other potential causal factors. There may be objective clinical signs including calluses on the hand (also called Russell’s sign), parotid and submandibular lymph gland enlargement, poor dentition, gastrointestinal symptoms, and hematologic/biochemical abnormalities in blood tests.1,6,18,19,23
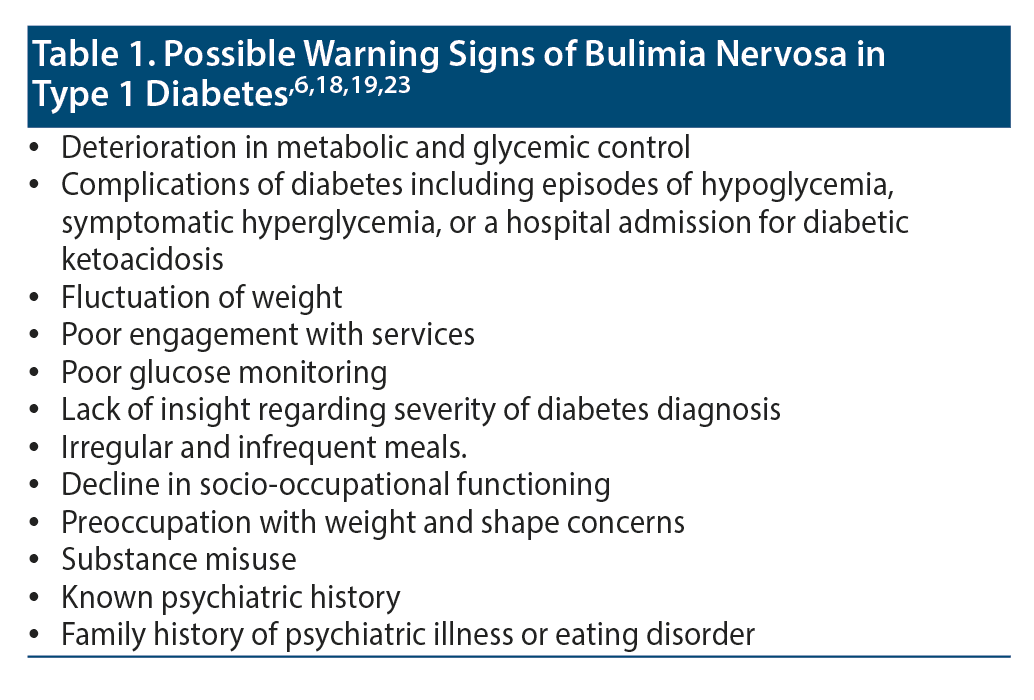
Deterioration in metabolic and glycemic control confirmed by a raised HbA1c level is a red flag. Levels of HbA1c provide an indication of diabetic control. Those who have had complications of diabetes including episodes of hypoglycemia, symptomatic hyperglycemia, or an admission for diabetic ketoacidosis necessitate further investigation. The fluctuation of weight in some patients may be a sign of illness. Poor engagement with services including missed appointments, inadequate glucose monitoring, and minimization of the degree of illness should raise concern.24-28
The consumption of irregular and infrequent meals may be a sign of dysfunction, although this is fairly common in adolescents with T1D. A study15 found that 28% of female and 7% of male adolescents with T1D were skipping some meals. The study15 also reported that 22% of healthy school children were missing breakfast. The omission and restriction of caloric intake may be associated with poor academic and occupational performance, which is an indication for further investigation. Patients with BN may express unusual dissatisfaction/sensitivity about their weight/shape in consultations, refuse to be weighed, have excessive preoccupation with their diet, or adopt unusual dietary practices. Individuals who misuse illicit substances, are socially withdrawn, present with affective/anxiety symptoms, or have a psychiatric history or positive family history for specific eating disorders/mental disorder may be at greater risk of developing BN.29-34
The Diabetes Eating Problem Survey-Revised (DEPS-R)51 and the modified SCOFF (mSCOFF)52 eating disorder questionnaire are available as an adjunct in detecting eating disorders in T1D. The DEPS-R is a standardized, reliable, specific, and concise 16-item self-report diabetes screening measure.6,35,36 It is a modification of the longer DEPS53 and is used to identify at-risk individuals who warrant further evaluation.35,36 The mSCOFF is a 5-item questionnaire, altered from the original validated SCOFF54 questionnaire, to assess the possible presence of an eating disorder in diabetes.6,19
Management of BN in T1D
An eclectic and holistic model of care is required in the treatment of BN in T1D and consists of several modalities. A concurrent treatment approach is recommended, with several areas that need to be addressed at one time.
Motivational Interviewing
The implementation of simple techniques such as motivational interviewing cannot be underestimated. Motivational interviewing has been found to be a useful intervention in BN and T1D. The utilization of a person-centered approach, demonstrating curiosity and interest may help to alleviate initial resistance, while promoting acceptance and change.37-40 A fundamental concept of motivational interviewing is demonstrating appropriate empathy and reinforcing autonomy and self-efficacy. It facilitates self-reflection and ultimately creates discrepancy in deep-seated values and current behaviors.37-39,48 Motivational interviewing works synchronously with the transtheoretical model of change, which breaks down the concept of readiness to change into distinct stages.37 It is important to avoid confrontation and solving problems on behalf of the patient. Control forms a significant element of the psychopathology of BN, and it is therefore crucial to foster empowerment and guide self-discovery.48
Psychoeducation and Family Support
Psychoeducation by professionals from the multidisciplinary team is advised. This also forms a component of the structured CBT model, which is a recognized treatment for BN.48 Information should be conveyed about the harmful effects of insulin manipulation. The patient should be informed that improved adherence with insulin treatment can cause insulin-related edema and thus transient weight gain. The improvement in glycemic control may cause short-term bloating and abdominal discomfort as a result of water retention.6,18,19
NICE guidelines48 recommend that family therapy should be offered to a young person with BN. It is important to educate family members about the psychopathology of BN and encourage them to avoid high expressed emotion during interactions, while providing adequate behavioral and emotional support through listening and validation. Their presence at key moments can prevent purging and binging behaviors, ie, offering to stand outside the bathroom, as these are usually private acts performed in secrecy.40-42,48,49 A study41 found that insulin restriction is less prevalent in the morning and additional support may be required during late afternoon.
Psychoeducation may improve restrictive eating practices, body dissatisfaction, and preoccupation with food. However, evidence suggests this does not appear to improve metabolic control, treatment adherence, or insulin manipulation in those with BN in T1D.6,18,19
Adapted CBT Model
The CBT model used to treat BN in T1D is an adapted, specialized treatment. It employs a variety of strategies to encourage adherence and thereby achieve positive outcomes. It can be administered as either group or individual therapy (Table 2 provides a summary of CBT).19,21A study43 reported that group CBT led to improvement in glycemic control and eating habits of 6 female patients with T1D and comorbid BN. CBT comprises 2 forms: 1 form focuses primarily on the eating disorder psychopathology, while the other focuses on addressing external barriers to change including clinical perfectionism, core low self-esteem, and interpersonal problems. The therapist is vigilant for mental health comorbidity during the course of therapy.10,12,18,21,43
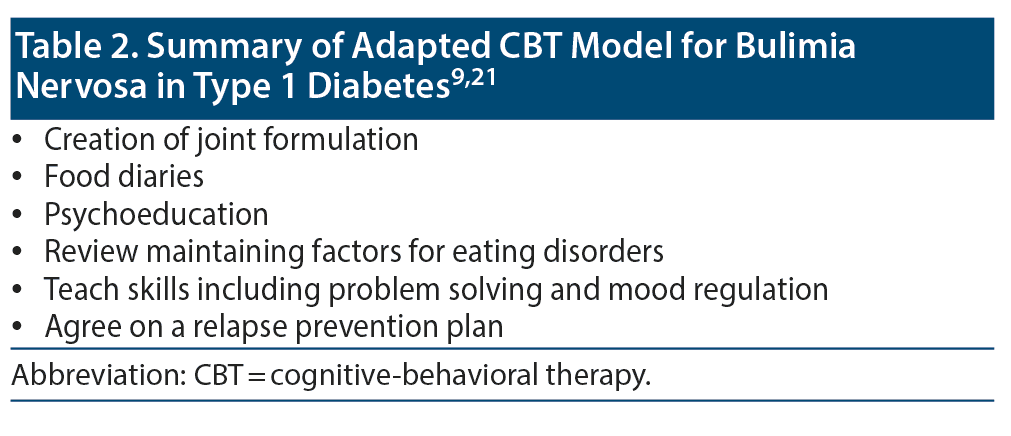
Treatment consists of 4 stages and is time limited (usually 20 sessions). Components include jointly creating a formulation; monitoring of eating and linking this to underlying thoughts, emotions, and behaviors (diaries are encouraged to monitor and record thoughts and feelings during binge-eating episodes, times of insulin misuse, when monitoring blood sugar levels, and before other compensatory behaviors); and psychoeducation directed toward weight regulation, consistent and regular eating, a balanced diet with a variety of foods, the effects of misusing insulin, and the physical complications and adverse effects associated with current disturbed behaviors.19,21
The therapist explores the maintaining factors of BN with emphasis on addressing maladaptive thoughts, feelings, and behaviors, including overevaluation of body shape/weight and understanding the mechanism of dietary restraint and the events that trigger disruptive behaviors. Patients are taught skills that include problem solving and methods to regulate mood patterns. As the therapy comes to an end, both the patient and therapist would concentrate on devising an appropriate relapse prevention plan.19,21
Nutritional Management
The aim should be to normalize and stabilize the diet by introducing regular meals (having 3 meals and 3 snacks daily), introducing a variety of foods, and encouraging a balanced diet. The frequency of snacking should correlate with the timing of the insulin injections. There should be some flexibility with the diet and initial emphasis on ensuring patient safety.6,17-19
Insulin Treatment
Patient safety and stabilization is paramount. A flexible and realistic insulin treatment and blood glucose monitoring regimen, suited to the individual’s lifestyle, is recommended. Continuous blood glucose monitoring and the use of an insulin pump may be considered. These interventions may reduce binge-eating episodes, as they would allow the individual to distinguish and control episodes of hypoglycemia, which may be an antecedent. They may promote dietary change and support adherence to a lower glycemic diet.2,6,12,18,19,44,49
Pharmacologic Treatments
Antidepressants have proven efficacy in treating symptoms of BN. The selective serotonin reuptake inhibitor fluoxetine at a dose of 60 mg daily is an option, although NICE guidelines48 recommend drug treatment should not be the only intervention.45,47 Antidepressants are particularly beneficial when there are comorbid depressive or anxiety disorders. In a study45 of 387 subjects treated with fluoxetine, there were notable improvements in both binge-eating and purging behaviors. There is reasonable evidence that the anticonvulsant topiramate decreases binge-eating and purging behaviors. Topiramate is associated with weight loss, and this potential effect should be carefully considered when prescribing this medication.45,50 Systematic reviews have suggested benefit for lisdexamfetamine, which is approved in the United States for the treatment of binge-eating disorder. The starting dose of lisdexamfetamine is usually 30 mg daily and the maintenance dose between 50 and 70 mg daily.50
DISCUSSION
The evidence for the efficacy of recognized treatments for BN in T1D is equivocal. The treatment outcomes are poorer than for conventional eating disorders. A systematic review2 including 6 studies concluded there were insignificant effects in glycemic control with current regimens. However, in some studies there was some improvement noted in eating disorder symptoms and insulin misuse.2 Inpatient therapy appeared to be the superior treatment, which may be due to a multifaceted and intensive holistic model of care employed in these facilities. The authors2 add that the quality of the data retrieved from these studies is variable and open to question, and their review suggests that an intensive and integrated treatment is required. Treatment ideally should comprise tailored diabetes management, nutritional management, and psychological/pharmacologic treatment for BN.
Further research is indicated in the development of more effective treatments while obtaining more valid data on outcomes with current approaches. Even for BN without T1D, a Cochrane review46 summarized that there is only a small body of evidence for the effectiveness of psychological therapies.
Early recognition and management of BN results in better outcomes.2,18,19 However, the secrecy enshrined with BN and the lack of willingness or ability of the person to disclose their weight/shape concerns and subsequent disruptive behaviors is a complicating factor. Families can be oblivious to the disordered eating and the compensatory behaviors employed by the patient. In rarer cases, they may be accommodating or enabling these behaviors. It is imperative to detect this disorder in time before these maladaptive beliefs and disruptive behaviors become entrenched and resistant to treatment. A joint effort from both diabetic and eating disorder services appears to be the way forward to ensure early detection and implementation of a timely comprehensive personalized management plan.
CONCLUSIONS
There is generally a lack of high-quality published research in the etiology and management of comorbid BN and T1D. There are already efforts to direct more attention toward the recognition and management of this disorder. Given the complexity of BN in T1D and the nature of the risks with these disorders, further research is required with regard to detection and also in the development of efficacious treatments.
Submitted: June 3, 2020; accepted July 17, 2020.
Published online: December 17, 2020.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial disclosure: Drs Yahya, Khawaja, J. Chukwuma, and C. Chukwuma have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: None.
Acknowledgments: The authors thank Sherin Francis, MSc, and Folu Ojutalayo, MSc (clinical librarians, Audrey Keep Library and Knowledge Service, North East London NHS Foundation Trust, Goodmayes Hospital site, Ilford, England) for their support with the literature search. They report no conflicts of interest related to the subject of this review.
REFERENCES
1.Chelvanayagam S, James J. What is diabulimia and what are the implications for practice? Br J Nurs. 2018;27(17):980-986. PubMed CrossRef
2.Clery P, Stahl D, Ismail K, et al. Systematic review and meta-analysis of the efficacy of interventions for people with type 1 diabetes mellitus and disordered eating. Diabet Med. 2017;34(12):1667-1675. PubMed CrossRef
3.Staite E, Zaremba N, Macdonald P, et al. ‘ Diabulima’ through the lens of social media: a qualitative review and analysis of online blogs by people with type 1 diabetes mellitus and eating disorders. Diabet Med. 2018;35(10):1329-1336. PubMed CrossRef
4.Falc×£o MA, Francisco R. Diabetes, eating disorders and body image in young adults: an exploratory study about “diabulimia”. Eat Weight Disord. 2017;22(4):675-682. PubMed CrossRef
5.De Paoli T, Rogers PJ. Disordered eating and insulin restriction in type 1 diabetes: a systematic review and testable model. Eat Disord. 2018;26(4):343-360. PubMed CrossRef
6.Candler T, Murphy R, Pigott A, et al. Fifteen-minute consultation: diabulimia and disordered eating in childhood diabetes. Arch Dis Child Educ Pract Ed. 2018;103(3):118-123. PubMed CrossRef
7.Wisting L, Fr׸island DH, Skrivarhaug T, et al. Disturbed eating behavior and omission of insulin in adolescents receiving intensified insulin treatment: a nationwide population-based study. Diabetes Care. 2013;36(11):3382-3387. PubMed CrossRef
8.Berger G, Waldhoer T, Barrientos I, et al. Association of insulin-manipulation and psychiatric disorders: a systematic epidemiological evaluation of adolescents with type 1 diabetes in Austria. Pediatr Diabetes. 2019;20(1):127-136. PubMed CrossRef
9.Peterson CM, Fischer S, Young-Hyman D. Topical review: a comprehensive risk model for disordered eating in youth with type 1 diabetes. J Pediatr Psychol. 2015;40(4):385-390. PubMed CrossRef
10.Baechle C, Castillo K, Straןburger K, et al; German Paediatric Surveillance Unit (ESPED) and the DPV-Science Initiative. Is disordered eating behavior more prevalent in adolescents with early-onset type 1 diabetes than in their representative peers? Int J Eat Disord. 2014;47(4):342-352. PubMed CrossRef
11.Macdonald P, Kan C, Stadler M, et al. Eating disorders in people with type 1 diabetes: experiential perspectives of both clients and healthcare professionals. Diabet Med. 2018;35(2):223-231. PubMed CrossRef
12.Treasure J, Kan C, Stephenson L, et al. Developing a theoretical maintenance model for disordered eating in type 1 diabetes. Diabet Med. 2015;32(12):1541-1545. PubMed CrossRef
13.Ryman B, MacIsaac J, Robinson T, et al. Assessing the clinical utility of the Diabetes Eating Problem Survey-Revised (DEPS-R) in adolescents with type 1 diabetes. Endocrinol Diabetes Metab. 2019;2(3):e00067. PubMed CrossRef
14.Nip ASY, Reboussin BA, Dabelea D, et al; SEARCH for Diabetes in Youth Study Group. Disordered eating behaviors in youth and young adults with type 1 or type 2 diabetes receiving insulin therapy: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2019;42(5):859-866. PubMed CrossRef
15.Peducci E, Mastrorilli C, Falcone S, et al. Disturbed eating behavior in pre-teen and teenage girls and boys with type 1 diabetes. Acta Biomed. 2019;89(4):490-497. PubMed
16.Wisting L, Skrivarhaug T, Dahl-J׸rgensen K, et al. Prevalence of disturbed eating behavior and associated symptoms of anxiety and depression among adult males and females with type 1 diabetes. J Eat Disord. 2018;6(1):28. PubMed CrossRef
17.Toni G, Berioli MG, Cerquiglini L, et al. Eating disorders and disordered eating symptoms in adolescents with type 1 diabetes. Nutrients. 2017;9(8):906. PubMed CrossRef
18.Pinhas-Hamiel O, Hamiel U, Levy-Shraga Y. Eating disorders in adolescents with type 1 diabetes: challenges in diagnosis and treatment. World J Diabetes. 2015;6(3):517-526. PubMed CrossRef
19.Gagnon C, Aimé A, Bélanger C, et al. Comorbid diabetes and eating disorders in adult patients: assessment and considerations for treatment. Diabetes Educ. 2012;38(4):537-542. PubMed CrossRef
20.Rome ES, Strandjord SE. Eating disorders. Pediatr Rev. 2016;37(8):323-336. PubMed
21.Cooper Z, Fairburn CG. Management of bulimia nervosa and other binge eating problems. Adv Psychiatr Treat. 2009;15(2):129-136. CrossRef
22.Deeb A, Akle M, Al Ozairi A, et al. Common issues seen in paediatric diabetes clinics, psychological formulations, and related approaches to management. J Diabetes Res. 2018;2018:1684175. PubMed CrossRef
23.Cherubini V, Skrami E, Iannilli A, et al. Disordered eating behaviors in adolescents with type 1 diabetes: a cross-sectional population-based study in Italy. Int J Eat Disord. 2018;51(8):890-898. PubMed CrossRef
24.Hastings A, McNamara N, Allan J, et al. The importance of social identities in the management of and recovery from ‘ diabulimia’ : a qualitative exploration. Addict Behav Rep. 2016;4:78-86. PubMed CrossRef
25.Cecilia-Costa R, Volkening LK, Laffel LM. Factors associated with disordered eating behaviours in adolescents with type 1 diabetes. Diabet Med. 2019;36(8):1020-1027. PubMed CrossRef
26.Balfe M, Doyle F, Smith D, et al. Dealing with the devil: weight loss concerns in young adult women with type 1 diabetes. J Clin Nurs. 2013;22(13-14):2030-2038. PubMed CrossRef
27.Larra×±aga A, Docet MF, Garc×a-Mayor RV. Disordered eating behaviors in type 1 diabetic patients. World J Diabetes. 2011;2(11):189-195. PubMed CrossRef
28.Reinehr T, Dieris B, Galler A, et al. Worse metabolic control and dynamics of weight status in adolescent girls point to eating disorders in the first years after manifestation of type 1 diabetes mellitus: findings from the Diabetes Patienten Verlaufsdokumentation Registry. J Pediatr. 2019;207:205-212.e5. PubMed CrossRef
29.Goebel-Fabbri AE, Uplinger N, Gerken S. From research to practice/eating disorders and diabetes: outpatient management of eating disorders in type 1 diabetes. Diabetes Spectr. 2009;22(3):147-152. CrossRef
30.Snyder LL, Truong YKN, Law JR. Evaluating substance use and insulin misuse in adolescents with type 1 diabetes. Diabetes Educ. 2016;42(5):529-537. PubMed CrossRef
31.Pinhas-Hamiel O, Hamiel U, Greenfield Y, et al. Detecting intentional insulin omission for weight loss in girls with type 1 diabetes mellitus. Int J Eat Disord. 2013;46(8):819-825. PubMed CrossRef
32.Bächle C, Stahl-Pehe A, Rosenbauer J. Disordered eating and insulin restriction in youths receiving intensified insulin treatment: results from a nationwide population-based study. Int J Eat Disord. 2016;49(2):191-196. PubMed CrossRef
33.Eisenberg Colman MH, Quick VM, Lipsky LM, et al. Disordered eating behaviors are not increased by an intervention to improve diet quality but are associated with poorer glycemic control among youth with type 1 diabetes. Diabetes Care. 2018;41(4):869-875. PubMed CrossRef
34.Wisting L, Reas DL, Bang L, et al. Eating patterns in adolescents with type 1 diabetes: associations with metabolic control, insulin omission, and eating disorder pathology. Appetite. 2017;114:226-231. PubMed CrossRef
35.Custal N, Arcelus J, Ag×¼era Z, et al. Treatment outcome of patients with comorbid type 1 diabetes and eating disorders. BMC Psychiatry. 2014;14(1):140. PubMed CrossRef
36.Pinna F, Diana E, Sanna L, et al. Assessment of eating disorders with the Diabetes Eating Problems Survey-Revised (DEPS-R) in a representative sample of insulin-treated diabetic patients: a validation study in Italy. BMC Psychiatry. 2017;17(1):262. PubMed CrossRef
37.Treasure J. Motivational interviewing. Adv Psychiatr Treat. 2004;10(5):331-337. CrossRef
38.Eisenberg MH, Lipsky LM, Dempster KW, et al. I should but i can’ t: controlled motivation and self-efficacy are related to disordered eating behaviors in adolescents with type 1 diabetes. J Adolesc Health. 2016;59(5):537-542. PubMed CrossRef
39.Schwartz SA, Weissberg-Benchell J, Perlmuter LC. Personal control and disordered eating in female adolescents with type 1 diabetes. Diabetes Care. 2002;25(11):1987-1991. PubMed CrossRef
40.Troncone A, Cascella C, Chianese A, et al. Parental assessment of disordered eating behaviors in their children with type 1 diabetes: a controlled study. J Psychosom Res. 2019;119:20-25. PubMed CrossRef
41.Merwin RM, Moskovich AA, Honeycutt LK, et al. Time of day when type 1 diabetes patients with eating disorder symptoms most commonly restrict insulin. Psychosom Med. 2018;80(2):222-229. PubMed CrossRef
42.Merwin RM, Dmitrieva NO, Honeycutt LK, et al. Momentary predictors of insulin restriction among adults with type 1 diabetes and eating disorder symptomatology. Diabetes Care. 2015;38(11):2025-2032. PubMed CrossRef
43.Peveler RC, Fairburn CG. The treatment of bulimia nervosa in patients with diabetes mellitus. Int J Eat Disord. 1992;11(1):45-53. CrossRef
44.Pinhas-Hamiel O, Graph-Barel C, Boyko V, et al. Long-term insulin pump treatment in girls with type 1 diabetes and eating disorders—is it feasible? Diabetes Technol Ther. 2010;12(11):873-878. PubMed CrossRef
45.Crow SJ. Pharmacologic treatment of eating disorders. Psychiatr Clin North Am. 2019;42(2):253-262. PubMed CrossRef
46.Hay PPJ, Bacaltchuk J, Stefano S, et al. Psychological treatments for bulimia nervosa and binging. Cochrane Database Syst Rev. 2009;(4):CD000562. PubMed
47.Bacaltchuk J, Hay P, Trefiglio R. Antidepressants versus psychological treatments and their combination for bulimia nervosa. Cochrane Database Syst Rev. 2001;2001(4):CD003385. PubMed
48.Eating disorders: recognition and treatment. NICE guideline [NG69]. National Institute for Health Care Excellence website. www.nice.org.uk/guidance/ng69. May 23, 2017.
49.Type 1 diabetes in adults: diagnosis and management. NICE guideline [NG17]. National Institute for Health Care Excellence website. www.nice.org.uk/guidance/ng17. August 26, 2015.
50.Taylor D, Barnes TRE, Young AH. Drug treatment of other psychiatric conditions. In: Maudsley Prescribing Guidelines. 13th Edition. Hoboken, NJ: John Wiley & Sons, Inc; 2018:669.
51.Wisting L, Wonderlich J, Skrivarhaug T, et al. Psychometric properties and factor structure of the diabetes eating problem survey – revised (DEPS-R) among adult males and females with type 1 diabetes. J Eat Disord. 2019;7(1):2. PubMed CrossRef
52.Zuijdwijk CS, Pardy SA, Dowden JJ, et al. The mSCOFF for screening disordered eating in pediatric type 1 diabetes. Diabetes Care. 2014;37(2):e26-e27. PubMed CrossRef
53.Markowitz JT, Butler DA, Volkening LK, et al. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care. 2010;33(3):495-500. PubMed CrossRef
54.Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: a new screening tool for eating disorders. West J Med. 2000;172(3):164-165. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!




