ABSTRACT
With the ongoing evolution in genetics, recent evidence highlights the role of circulatory microRNA (miRNA) for schizophrenia. The objective of this article is to explore the role of blood/serum miRNA expression in schizophrenia management and to review the expression of different miRNAs before and after treatment with antipsychotics. miRNAs can help increase the accuracy of diagnosis, identify patients at risk of developing schizophrenia, and possibly predict drug response. The collective evidence from this review showed that several miRNAs are promising candidates for schizophrenia diagnosis, management, and prognosis.
Prim Care Companion CNS Disord 2022;24(2):21nr03048
To cite: Vadukapuram R, Trivedi C, Shah K, et al. The promise of predictive biomarkers for antipsychotic efficacy: a review of peripheral microRNAs to evaluate schizophrenia treatment response. Prim Care Companion CNS Disord. 2022;24(2):21nr03048.
To share: https://doi.org/10.4088/PCC.21nr03048
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York
bSt David Medical Center, Austin, Texas
cDepartment of Psychiatry, Griffin Memorial Hospital, Norman, Oklahoma
dDepartment of Psychiatry, Boston Children’s Hospital/Harvard Medical School, Boston, Massachusetts
eDepartment of Psychiatry, Virginia Tech Carilion School of Medicine, Roanoke, Virginia
‡Drs Mansuri and Reddy share equal credits for senior authorship.
*Corresponding author: Ramu Vadukapuram, MD, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl, New York, NY 10029 ([email protected]).
Schizophrenia is a clinical syndrome that involves a range of cognitive, behavioral, and emotional dysfunctions, which vary significantly among patients.1 Schizophrenia is one of the leading causes of disability worldwide.2 The median incidence of schizophrenia is 1.5 per 10,000 people, and the rate ratio for males: females is 1.4:1.3 While the global prevalence of schizophrenia has been reported to range from 0.33% to 0.75%, in the United States the estimated prevalence of schizophrenia and related psychotic disorders ranges between 0.25% and 0.64%.3,4 The age at onset is typically early to mid-20s for males and the late 20s for females, with slightly more males than females developing the disorder.1,3 Genetics is shown to have a strong influence in the development of schizophrenia. First-degree relatives of a schizophrenia patient have an increased risk of approximately 10% compared to 3% in second-degree relatives.5 With the etiology and pathogenesis still evolving and under investigation, the diagnosis of this complex disease at present is mainly based on clinical symptoms. Schizophrenia patients may experience delusions, hallucinations, disorganized speech, grossly disorganized or catatonic behavior, or negative symptoms. According to the DSM-5 diagnostic criteria for schizophrenia, at least 2 of these symptoms must occur for 6 months, with active symptoms exhibiting for at least 1 month.1 It is a clinical diagnosis that depends primarily on self-reports from patients, collateral information from family members, mental state examination, and clinical interviews. Due to the lack of objective laboratory tests, misdiagnosis is common.6
Standard laboratory tests are not yet available to diagnose psychiatric disorders, as most can be attributable to poor understanding of their biological mechanisms or difficulty in routinely accessing brain tissue in live diseased patients.7,8 The lack of tests highlights the role of biological markers, which can aid in the understanding of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.9 Biomarkers have been shown to help researchers observe the disease’s biological state, can be measured accurately, and have reproducible results.10
Several research studies10–17 have shown the important role that microRNA (miRNA) plays in various human diseases, and growing evidence has shown that miRNAs are valuable biomarkers in disease pathogenesis, diagnosis, treatment efficacy, and prognosis prediction. The objective of this article is to review and summarize the most important findings of experimental investigations aimed at identifying the role of human miRNAs in schizophrenia management. We also aimed to review the expression of different miRNAs before and after treatment with antipsychotics, which could serve as a biomarker and help in determination of therapeutic intervention measures and pharmacologic targets.
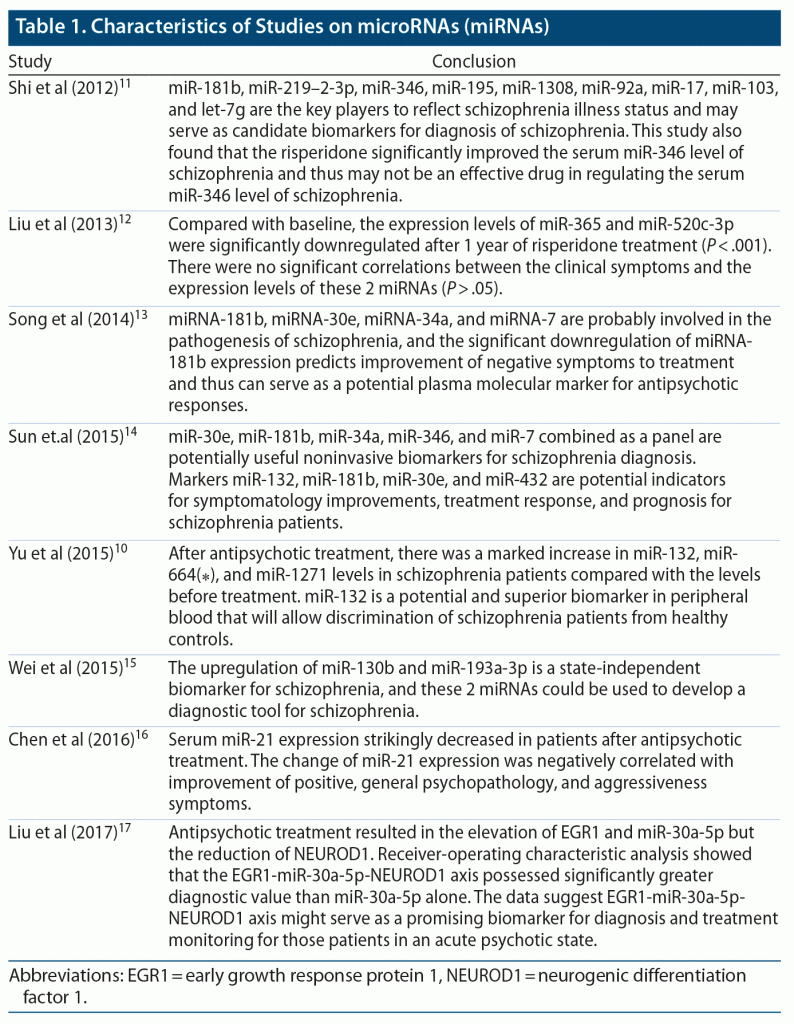
We searched peer-reviewed published articles using PubMed, PubMed Central, and MEDLINE databases. A wide range of key terms were used, such as blood, miRNA, schizophrenia, biomarkers, and antipsychotics. The search process uncovered 8 peer-reviewed articles published from 2010 to 2020 with a single filter that included only human studies (Table 1).10–17 We excluded articles that did not mention miRNAs associated with schizophrenia and alteration of expression with antipsychotics, which is the topic of interest.
miRNAs and SCHIZOPHRENIA
After introduction in the early 1990s, miRNAs became a special focus due to their unique functions and mechanism of action. miRNAs are present in various tissues and body fluids such as blood, cerebrospinal fluid, urine, and saliva.18 So far, more than 2,500 human miRNAs are reported in miRBase version 22.19 miRNAs are a class of short noncoding RNAs consisting of 20 to 22 nucleotides in length. They undergo a series of steps within the nucleus to form primary miRNA transcribed by RNA polymerase II, which is further processed by Drosha (RNase III enzyme), ultimately creating pre-miRNA. This pre-miRNA is transported to the cytoplasm and undergoes further processing to form a miRNA duplex containing mature miRNA by exportin-5, where it encounters a further transformation by Dicer (RNase III protein). It then changes into a miRNA duplex that includes the mature miRNA.20 After unwinding from this duplex, the mature single-stranded miRNA is incorporated into a multiprotein complex RNA-induced silencing complex, wherein it assumes the job of inducing the silencing of messenger RNAs (mRNAs).21 A single miRNA can regulate the expression of more than one messenger RNA, and each messenger RNA can be regulated by multiple miRNAs.22
Approximately ~50% of all protein-coding genes are regulated by miRNA, and studies23 suggest that they contribute to the regulation of nearly all cellular processes. The mutations of underlying miRNA biogenesis are associated with the pathophysiology of neuropsychiatric disorders like schizophrenia.24 Researchers have noticed neuropathologic differences in the miRNA expression in postmortem studies of schizophrenia.25 In contrast to other blood molecules, miRNAs have several unique properties, such as they are cell or tissue specific and steady in the RNase-rich blood environment, are stable in extremes of pH, and can sustain prolonged storage at room temperature and have freeze-thaw cycles.26,27 Peripheral miRNAs are easy to access through noninvasive and straightforward processes and are readily identified by quantitative reverse transcription-polymerase chain reaction (qRT-PCR).28 Use of peripheral miRNA as biomarkers could help improve the diagnosis of schizophrenia. In some cases, miRNAs can also fast track the diagnosis, which allows earlier interventions, especially in remote areas where access to mental health services is limited.15
ALTERATION IN miRNA BIOMARKER EXPRESSION WITH ANTIPSYCHOTICS
Antipsychotics are the medication of choice in managing the symptoms of schizophrenia, and depending on the symptoms, it could be either typical/first-generation or atypical/second-generation antipsychotics.29 The world’s first antipsychotic, chlorpromazine, was introduced into clinical practice in France in 1952.30 Thereafter, atypical/second-generation antipsychotics were introduced in 1990 in the United States, and since then atypical antipsychotics have been used as first-line treatment for schizophrenia because of their safety side effect profile, which includes decreased extrapyramidal symptoms and increased efficacy on negative symptoms.29 Few studies were conducted to understand the effect of antipsychotic medications on miRNAs before and after taking them in patients with schizophrenia.
To discover the role of miRNAs as diagnostic and prognostic biomarkers, Wei et al15 used Solexa sequencing, TaqMan Low-Density Assay to profile circulating miRNAs in plasma (n = 351, scz = 164, ctl = 187). They observed 8 upregulated miRNAs, and on validation (n = 775, scz = 400 ctl = 213, non-scz disorder = 162) with qRT-PCR, they found upregulation of miR-130b and miR-193a-3p in patients with schizophrenia compared to control subjects. They also observed suppressed levels of miR-130b and miR-193a-3p on follow-up after 1 year of treatment with antipsychotics.15
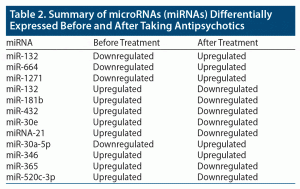
To understand the role of miRNA as a therapeutic marker in schizophrenia, a study10 (n = 235, scz = 105, ctl = 130) using microarray and qPCR found decreased expression of miR-132, miR-134, miR-1271, miR-664(⁎), miR-200c, and miR-432 levels in patients with schizophrenia before taking antipsychotics compared to control subjects (Table 2). The study also found increased expression of miR-132 (P < .01), miR-664(⁎) (P < .05), and miR-1271 (P < .05) after treatment compared to the expression level prior to taking medications.10
Chen et al16 used microarray analysis on blood mononuclear cells from 125 schizophrenia patients prior to taking any antipsychotics and from control subjects and found 9 miRNAs with differential expression on validation by qRT-PCR (scz = 82, ctl = 43). When these 9 miRNAs were compared in patients before and after taking antipsychotics, they found miRNA-21 decreased expression and negatively associated clinical symptomatology.16 On the other hand, in a study by Gardiner et al,31 miRNA-21 was found to be significantly downregulated in patients with schizophrenia compared to control subjects. Also, in a study by Miller et al,32 miRNA-132 was significantly downregulated in the prefrontal cortices of patients with schizophrenia compared to controls.
Liu et al17 witnessed significant downregulation of transcription factor (early growth response protein 1 [EGR1]) and miR-30a-5p and upregulation of neurogenic differentiation factor 1 (NEUROD1) using RT-qPCR in peripheral blood mononuclear cells in schizophrenia patients (n = 38) compared to control subjects (n = 50). However, patients who underwent treatment with antipsychotics had increased expression of EGR1 and miR-30a-5p and decreased expression of transcription factor NEUROD1.
To explore the effect of antipsychotics on miRNAs, Sun et al14 analyzed 10 miRNAs in plasma by qPCR in 148 subjects (scz = 61, ctl = 62, 6-week drug t/t group = 25) and observed significantly increased expression (area under the curve: 0.713, sensitivity: 35.5%, specificity: 90.2%) of a cluster of miRNAs such as miR-30e, miR-181b, miR-34a, miR-346, and miR-7. In the treatment group, they found notably decreased expression levels of miR-132, miR-181b, miR-432, and miR-30e, which were associated with improved symptoms in the patients.14
On the other hand, Song et al13 observed significant downregulation of miR-181b after treatment (n = 40, scz = 20, ctl = 20) (P < .05) compared to the level before treatment. This change was significantly correlated to symptomatic improvement (P < .001).13
Shi et al11 quantified 9 miRNAs using qRT-PCR and semi-nested qRT-PCR and found the expression levels of miR-181b, miR-219–2-3p, miR-1308, let-7g, miR-346, and miR-92a were considerably higher in schizophrenia patients compared to healthy controls. Also, the expression levels of miR-195 and miR-17 were significantly downregulated in schizophrenia patients compared to healthy controls. In comparison, the expression level of miR-346 was significantly higher in the risperidone treatment group than in healthy controls.11 Gardiner et al31 showed the downregulation of miR-181b in peripheral blood mononuclear cells of patients with schizophrenia compared to control subjects, which is inconsistent with Shi et al,11 Song et al,13 and Sun et al.14
Liu et al17 quantified expression of 7 miRNAs in plasma using qRT-PCR in patients with schizophrenia before and after taking antipsychotics (n = 40). They17 observed decreased expression levels of miR-365 and miR-520c-3p after antipsychotic treatment (P < .001), but there was no correlation with the symptoms (P > .05). Overall, these research studies have acknowledged that the peripheral miRNA patterns would have value as biomarkers for schizophrenia disorder.
Although the results of these studies are promising, one of the limitations of our review is the sample size of the studies we included. Large-sample, longitudinal studies are needed in this field.
In summary, miR-664, miR-1271, and miR-30a-5p were downregulated in patients with schizophrenia before they started taking antipsychotics and upregulated after the intake of antipsychotic medications. In contrast, miRNAs such as miR-181b, miR-432, miR-30e, miRNA-21, miR-346, miR-365, and miR-520c-3p were upregulated in patients with schizophrenia before taking antipsychotics and downregulated after intake of antipsychotics. Yu et al10 found that miR-132 was downregulated before antipsychotic intake and upregulated after antipsychotics in patients with schizophrenia, which contrasts with Miller et al32 whose results were opposite. This expression of different miRNAs before and after treatment with antipsychotics could serve as a biomarker and help in determination of therapeutic intervention measures and pharmacologic targets.
CONCLUSION
Although the role of miRNAs in the diagnosis, management, and prognosis of schizophrenia is not yet understood, collective evidence from this review shows that several miRNAs are promising candidate biomarkers in this regard. They can help increase the accuracy of diagnosis, identify patients at risk of developing schizophrenia, and possibly predict drug response. Further large-scale controlled clinical studies are required to determine the sensitivity and specificity of biomarkers, which could be a turning point for schizophrenia management.
Submitted: June 13, 2021; accepted September 21, 2021.
Published online: April 7, 2022.
Relevant financial relationships: None.
Funding/support: None.
Clinical Points
- Several microRNAs (miRNAs) are promising candidate biomarkers for schizophrenia diagnosis, management, and drug response.
- Schizophrenia diagnosis might be enhanced by use of peripheral miRNAs as biomarkers.
- In rare situations, use of peripheral miRNAs as biomarkers can expedite the diagnosis, allowing for quicker intervention, particularly in rural areas with few mental health resources.
References (32)

- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. PubMed CrossRef
- McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30(1):67–76. PubMed CrossRef
- NIMH ” Schizophrenia. National Institute of Mental Health Information Resource Center website. Accessed February 9, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia
- Multani PK, Saini N, Kaur R, et al. Biomarkers for Drugs of Abuse and Neuropsychiatric Disorders: Models and Mechanisms. In: Gupta RC, ed. Biomarkers in Toxicology. Academic Press; 2014:983–1001.
- Wakefield JC. Misdiagnosing normality: psychiatry’s failure to address the problem of false positive diagnoses of mental disorder in a changing professional environment. J Ment Health. 2010;19(4):337–351. PubMed CrossRef
- Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17(12):1174–1179. PubMed CrossRef
- Vargas G. Biomarkers in schizophrenia. Biomarkers Med. 2014;8(1):1–3. PubMed CrossRef
- Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–466. PubMed CrossRef
- Yu HC, Wu J, Zhang HX, et al. Alterations of miR-132 are novel diagnostic biomarkers in peripheral blood of schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2015;63:23–29. PubMed CrossRef
- Shi W, Du J, Qi Y, et al. Aberrant expression of serum miRNAs in schizophrenia. J Psychiatr Res. 2012;46(2):198–204. PubMed CrossRef
- Liu S, Yuan YB, Guan LL, et al. MiRNA-365 and miRNA-520c-3p respond to risperidone treatment in first-episode schizophrenia after a 1 year remission. Chin Med J (Engl). 2013;126(14):2676–2680. PubMed
- Song HT, Sun XY, Zhang L, et al. A preliminary analysis of association between the down-regulation of microRNA-181b expression and symptomatology improvement in schizophrenia patients before and after antipsychotic treatment. J Psychiatr Res. 2014;54:134–140. PubMed CrossRef
- Sun XY, Zhang J, Niu W, et al. A preliminary analysis of microRNA as potential clinical biomarker for schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2015;168(3):170–178. PubMed CrossRef
- Wei H, Yuan Y, Liu S, et al. Detection of circulating miRNA levels in schizophrenia. Am J Psychiatry. 2015;172(11):1141–1147. PubMed CrossRef
- Chen SD, Sun XY, Niu W, et al. A preliminary analysis of microRNA-21 expression alteration after antipsychotic treatment in patients with schizophrenia. Psychiatry Res. 2016;244:324–332. PubMed CrossRef
- Liu S, Zhang F, Shugart YY, et al. The early growth response protein 1-miR-30a-5p-neurogenic differentiation factor 1 axis as a novel biomarker for schizophrenia diagnosis and treatment monitoring. Transl Psychiatry. 2017;7(1):e998. PubMed CrossRef
- Jin XF, Wu N, Wang L, et al. Circulating microRNAs: a novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cell Mol Neurobiol. 2013;33(5):601–613. PubMed CrossRef
- Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47(D1):D155–D162. PubMed CrossRef
- Wahid F, Shehzad A, Khan T, et al. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010;1803(11):1231–1243. PubMed CrossRef
- Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genomics. 2010;11(7):537–561. PubMed CrossRef
- Cai Y, Yu X, Hu S, et al. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7(4):147–154. PubMed CrossRef
- Caputo V, Ciolfi A, Macri S, et al. The emerging role of MicroRNA in schizophrenia. CNS Neurol Disord Drug Targets. 2015;14(2):208–221. PubMed CrossRef
- Beveridge NJ, Cairns MJ. MicroRNA dysregulation in schizophrenia. Neurobiol Dis. 2012;46(2):263–271. PubMed CrossRef
- Beveridge NJ, Tooney PA, Carroll AP, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17(8):1156–1168. PubMed CrossRef
- Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. PubMed CrossRef
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. PubMed CrossRef
- Kichukova TM, Popov NT, Ivanov HY, et al. Circulating microRNAs as a novel class of potential diagnostic biomarkers in neuropsychiatric disorders. Folia Med (Plovdiv). 2015;57(3-4):159–172. PubMed CrossRef
- Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40(6):407–414. PubMed CrossRef
- Haddad PM, Correll CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 2018;8(11):303–318. PubMed CrossRef
- Gardiner E, Beveridge NJ, Wu JQ, et al. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol Psychiatry. 2012;17(8):827–840. PubMed CrossRef
- Miller BH, Zeier Z, Xi L, et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc Natl Acad Sci U S A. 2012;109(8):3125–3130. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!