LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord. 2023;25(6):23f03544
Author affiliations are listed at the end of this article.
Have you puzzled over how to screen for mild cognitive impairment (MCI)? Have you wondered whether MCI invariably leads to Alzheimer’s disease (AD)? Would you like to understand the role that new treatments (eg, monoclonal antibodies) might play in slowing or halting the progression to more severe cognitive impairment? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Ms B, a 76-year-old retired teacher, presents to her annual wellness examination accompanied by her adult daughter with concerns about her memory. She shares that her adult children have told her that she repeats questions during conversations and that she has trouble remembering dates. Recently, she was taken by surprise when her son and his family showed up at her door for a planned visit. Ms B’s daughter shares that she has noticed increasing forgetfulness in her mom; for example, when the family was recently together for Thanksgiving, Ms B went out to the grocery store for some last-minute items and came back without most of them, having forgotten that she had written a list and carried it in her purse. Ms B’s daughter has taken on more of the planning, organizing, and meal preparation for family holidays over the past several years, but this year she noticed that her mother seemed to have more trouble following a familiar recipe for pumpkin pie. She and her 2 other siblings are concerned that their mother seemed more irritable when the family engaged in their usual holiday banter about current political issues. She also worries that her mother has not been getting out of the house as much to socialize or attend church groups, citing low energy and lack of interest over the past few years. Ms B reports feeling low at times but enjoys spending time with her grandchildren and going out to eat with friends, although she has been doing this a bit less due to some difficulty following conversations. She denies feeling overly anxious, but she is starting to feel more concerned about her memory, and she has started to feel like her children are quizzing her on her daily activities when they call to check on her. She lives alone, grocery shops for herself, drives, manages her appointments and medications, and prepares her meals. She denies recent motor vehicle accidents or getting lost driving to familiar places. She describes her diet as generally healthy, although she has relied more on frozen dinners for the past few years. She denies recent weight loss, and her body mass index (BMI) is stable at 27 kg/m2. She reports sleeping approximately 7 hours/night and feeling rested when she wakes up. Her daughter and granddaughters spent the night recently, and they did not notice any snoring.
Ms B is a former smoker and faithfully takes a low-dose statin for hyperlipidemia diagnosed in her 50s. She has never used illicit drugs or alcohol to excess. She is not currently taking medication for mood or anxiety, although she was prescribed sertraline in her 40s for anxiety and depression. Her physician administers screening tests for depression, anxiety, and cognition. She scores a 5 on the Geriatric Depression Scale (≥ 5 suggests depression),1 9 on the Geriatric Anxiety Scale (7–9 suggest mild anxiety),2 and 24/30 on the Montreal Cognitive Assessment (MoCA),3 with deficits in naming, fluency, delayed recall, and orientation (to the day of the week).
Ms B’s physician explains that given her education and prior occupation, her cognitive screening score is lower than he would have expected, and he is concerned that she has something called MCI. Ms B and her daughter look at each other and ask, “What does this mean, and what should we do?”
DISCUSSION
What Is MCI?
According to the DSM-5, MCI is synonymous with “mild neurocognitive disorder.” It is defined by evidence of modest cognitive decline in 1 or more cognitive domains that does not interfere with independence in everyday activities. MCI is a broad term that defines a clinical state; it is not specific to any underlying condition. Medical illnesses, including reversible etiologies or neurodegenerative processes, may account for cognitive decline. The most common neurodegenerative processes associated with MCI are AD, α-synuclein diseases (including Parkinson’s disease and dementia with Lewy bodies), and early stages of frontotemporal dementia as well as cerebrovascular disease, including strokes and small vessel ischemic disease. Each process may have unique clinical signs, neuroimaging and cerebrospinal fluid (CSF) findings, and a distinct clinical timeline.
How Might a Primary Care Clinician Diagnose MCI?
Cognitive decline is often brought to the attention of the primary care clinician (PCC) by patients and their families who have noticed an apparent decline in the patient’s cognition. Many individuals wonder whether their cognitive decline is a normal part of aging. The challenge to the clinician is establishing meaningfulness or value in diagnostics while providing sufficient perspective to alleviate unnecessary patient and family concerns. This might be accomplished at a Medicare annual wellness visit. Indeed, the rate of dementia diagnoses increases with annual wellness visit utilization for this purpose.4 However, the many competing priorities at this visit, from cancer screenings to vaccines and other geriatric screenings as well as the nuances and sensitivities of patient and family concerns surrounding cognitive impairment, may warrant a dedicated memory evaluation at a follow-up visit.
Given the impact of MCI on multiple clinical domains, several guidelines for diagnosing MCI can be applied. A meta-analysis of many of these guidelines is referenced.5 Guidelines from the National Institute on Aging, the Alzheimer’s Association, the American Psychiatric Association, and the American Academy of Neurology include diagnostic recommendations that involve a combination of history taking with the patient and close associates along with careful consideration of contributing comorbidities or conditions that may contribute to cognitive decline (eg, depression, medication-induced cognitive deficits, vitamin B12 deficiency, hypothyroidism, sleep disturbances). Formal neuropsychological testing, a physical examination, blood testing for metabolic derangements, brain magnetic resonance imaging (MRI), and CSF biomarker testing (eg, β-amyloid and phosphorylated tau biomarkers of AD) are recommended to varying extents in consensus guidelines.
A practical approach in the primary care setting should include history taking with the patient and their family or close associates, including a review of challenges in activities of daily living (ADLs), a physical and neurologic examination, cognitive screening, consideration of possible medical contributions to the cognitive decline, blood testing for metabolic derangement, and a non-contrast brain MRI given sufficient clinical concerns. Outside referral for neuropsychological testing is not always practical; however, it is often extremely helpful in identifying strengths and weaknesses in various domains of cognitive functioning, which assist in diagnosis, treatment planning, and developing targeted coping strategies. Referral to a neurologist is prudent when gait, movement, significant speech or language, or visuospatial difficulties complicate the patient’s presentation. Too often, memory disorder specialists are unavailable in communities, leaving the PCC with greater responsibility for diagnosing and treating cognitive decline related to neurodegenerative disease. In this setting, practical considerations, such as the utility of MRI brain imaging only when clinical uncertainty is present (such as in early disease or rapidly progressive disease) may be warranted. As detailed below, emphasis on ADLs, and instrumental ADLs (IADLs) alongside abbreviated cognitive testing may provide meaningful documentation as a baseline for follow-up appointments and aid the PCC in distinguishing disease severity and need for resource support.
The MoCA is a 10 to 15–minute cognitive screening tool that assesses cognition across several cognitive domains, including perceptual-motor function, language, executive function, complex attention, and encoding and retrieval of information. Developed to help physicians differentiate MCI from normal cognitive aging, the MoCA provides excellent value in screening for MCI when used in the proper setting. Overall scores < 26 out of a possible 30 suggest MCI or dementia, although a recent systematic review and meta-analysis suggested a cutoff of 23 as more helpful in limiting false positive results.6 In a recent guide to dementia screening tools, Molnar et al7 provided rationale and criteria for test selection based on US Preventative Task Force recommendations8 that excluded tests that charge for use, training, and/or resources to apply the test (eg, Mini Mental State Examination9 or MoCA, which charges for clinical certification), avoidance of tests with challenges to scoring, and ensuring the test challenges short-term memory. The tests meeting these criteria include the Memory Impairment Screen,10 the Informant Questionnaire on Cognitive Decline in the Elderly (26 or 12 item versions),11 the Saint Louis University Mental Status Examination,12 the Mini-Cog,13 the Lawton Instrumental Activities of Daily Living (IADL),14 the Washington University Dementia Screening Test (AD8),15 and the Functional Activities Questionnaire (FAQ).16 Of these, the tests that take less than 5 minutes include the Mini-Cog, Lawton IADL, AD8, and FAQ.
How Does MCI Compare to Dementia?
A key characteristic that distinguishes MCI from dementia is that individuals with MCI remain independent with basic ADLs and IADLs. These activities may take the patient more time and effort, but they are able to do them. Excellent resources for reviewing and documenting basic and instrumental ADLs are available.17,18 While an individual with MCI would not require significant assistance, they may have developed lifestyle adaptations to help them remain independent. For example, compared to earlier in life, an individual may rely more on lists to remind oneself of daily routines. Frequently, patients with MCI develop social impairments, and many individuals also have mood symptoms. These mood symptoms are sometimes recognized as prodromes for dementia, but more studies are needed to validate this.
In contrast, individuals with dementia—also known as major neurocognitive disorder—demonstrate significant cognitive decline in 1 or more cognitive domains and require some assistance in everyday activities. Dementia is classified as mild, moderate, or severe, depending on the type and amount of assistance needed. In mild dementia, a person requires assistance with 1 or more IADLs (eg, managing finances, shopping for groceries, or preparing meals). In moderate dementia, a person requires assistance with 1 or more basic ADLs (eg, bathing, dressing, or toileting). In severe dementia, an individual is wholly dependent regarding ADLs. AD is the most common cause of dementia, present in 60%–80% of all cases.
Does Having MCI Mean That the Patient Will Develop AD?
When a patient is diagnosed with the syndrome of MCI, the differential diagnosis is broad, including many reversible causes. Examples include affective symptom exacerbations (previously referred to as pseudodementia), underlying metabolic changes, or other medical etiologies. MCI may also progress to a non-Alzheimer’s dementia. Still, not all individuals with MCI develop dementia. One meta-analysis found MCI to dementia conversion rates between 10.2% and 33.6% (median 19%) over 1 year of follow-up, with higher conversion rates noted in clinics compared to community-based samples.19 However, MCI increases a person’s lifetime risk of dementia even if it resolves, suggesting that diagnosis of MCI at any time may have prognostic value.20 Recent estimates suggest that approximately 22% of US adults over age 65 years are living with MCI,21–23 and recent imaging studies estimate that roughly half of these individuals have biomarker evidence of Alzheimer’s-related brain changes.24 Overall, one-third of individuals with MCI due to AD progress to dementia within 5 years.19
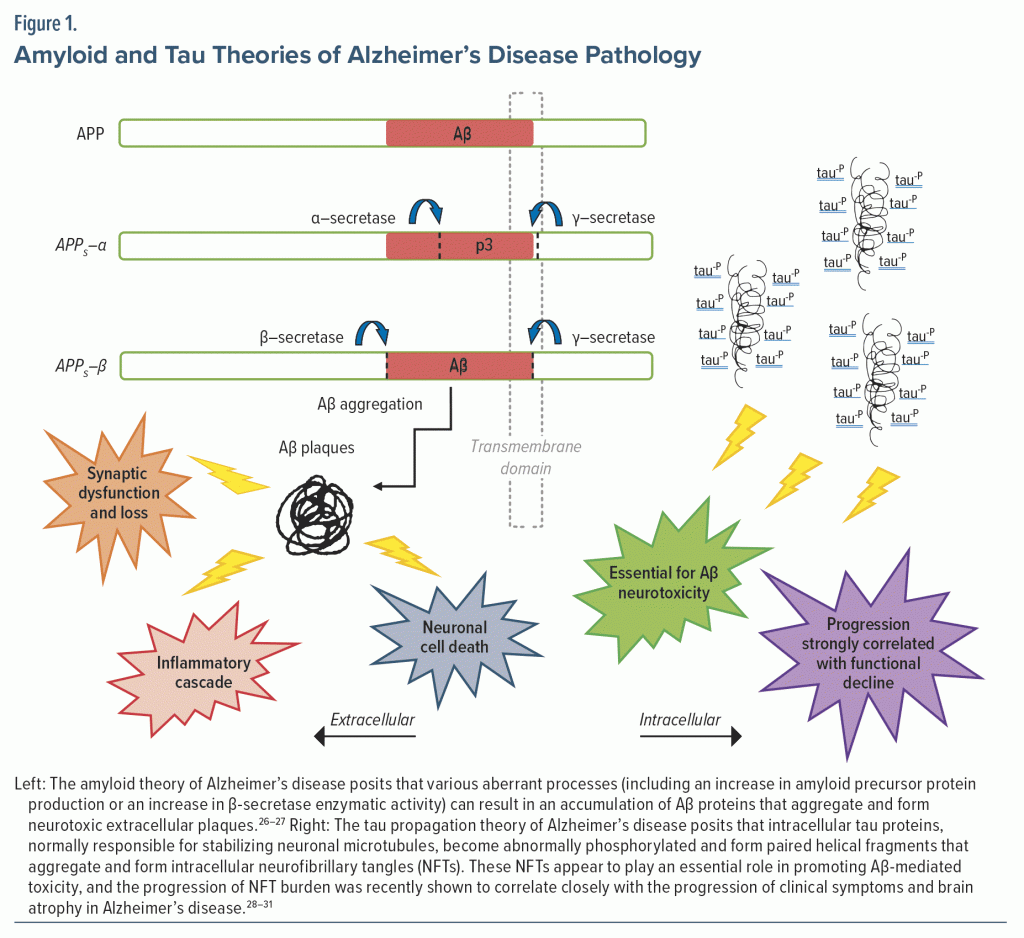
AD (in contrast to Alzheimer’s-type MCI or dementia) is definitively diagnosed by histologic examination of brain pathology (with evidence of cerebral neurofibrillary tangles and amyloid β plaques) on postmortem examination. However, advances in CSF biomarkers and positron emission tomography (PET) molecular neuroimaging now provide tools for diagnosis during life, with accuracy approaching histologic examination. From these biomarker studies, we have learned that it is not uncommon for a patient to harbor the amyloid and tau biological features of AD for many years (eg, while displaying minimal or no symptoms). Figure 1 provides an abbreviated introduction to the amyloid and tau hypotheses of AD. If a patient presents with symptoms of dementia and diagnostic testing aligns these symptoms and laboratory or imaging-based findings as consistent with AD (eg, MoCA scoring, ADL dependence, and CSF biomarkers consistent with AD), the next steps include disclosure of the appropriate diagnosis (eg, probable AD) to the patient and their support system. This anxiety-provoking and often life-changing disclosure requires the utmost thoughtfulness and experience. One method of disclosure, particularly in the context of emerging biomarker-based testing, includes a 5-step process that involves (1) determining the appropriateness of testing and planning ahead for the returning of results to individuals, (2) providing pretesting education and obtaining consent, (3) testing, (4) sharing of results with the patient and their support system, and (5) following up on the patient’s and caregiver’s well-being.32 This may be practically challenging given limited access to imaging (eg, amyloid-PET) or to the lumbar puncture procedure. As new treatments for AD become accessible, such as monoclonal antibody–based treatments, biomarker-based techniques or alternative blood-based biomarker techniques will by necessity become more readily available. At present, the PCC may appreciate fragmentation and inequality in their patients’ access to biomarker-based diagnostics for AD.
How Can MCI and AD Be Treated?
The current treatment of MCI and AD can be categorized into the prevention of further cognitive decline by controlling risk factors, use of cognitive enhancers, and treatment of behavioral and psychological symptoms that arise as a result of dementia.
Prevention. It may be helpful to inform patients diagnosed with MCI that there are potentially modifiable risk factors that can lead to dementia. Some factors are less within an individual’s control, such as those linked with socioeconomic hardships and past experiences. Modifiable risk factors include hypertension, diabetes, obesity, smoking, hearing loss, and sedentary lifestyle. Undiagnosed, but suspected, sleep apnea is worth considering, given its close relationship with cardiovascular risk factors of AD. The overlap between cardiovascular risk factors and AD is so great that the burden of mixed dementia, which denotes contributions of both vascular disease and AD-specific pathology to cognitive decline, is estimated to apply to the majority of dementia cases, particularly among the very old.33 Other risk factors include unstable or inadequately treated depression, alcohol use, poor air quality, and social isolation.34
Most, if not all, of these risk factors can be mitigated within the PCC setting. For instance, screening for hearing loss results in increased utilization of hearing aids, and use of hearing aids in individuals with MCI leads to reduced progression to dementia (based on the annual rate of change of the Clinical Dementia Rating Scale, hazard ratio of 0.73, with 95% confidence intervals of 0.61 and 0.8935).
Since patients with MCI due to AD (with biomarkers) often progress to dementia, practical and meaningful steps toward modifying any of these risk factors may be considered secondary prevention for dementia. In the context of a specific dementia etiology, patients are not immune to other causes of dementia or compounding factors (eg, vascular disease) that could increase cognitive decline. Further, strong social supports and adequate attention to mental health support patient resilience and prevent the development of behavioral and psychological symptoms of dementia (BPSD).
Cognitive enhancers. The primary cognitive enhancers (donepezil, galantamine, rivastigmine, and memantine) have been available and studied in AD and related dementias for over 25 years. Guidelines suggest that once a patient is diagnosed with AD, regardless of their staging, donepezil is indicated and should only be tapered, transitioned to another in-class medication, or delayed when poorly tolerated.36 The most common side effect of these medications is gastrointestinal (GI) distress, which is often short-lasting, but it can make initiating these oral medications challenging for some individuals. A transdermal patch form of rivastigmine (Exelon) and donepezil (Adlarity) are available, which have proved more tolerable in many individuals. Currently, insurance coverage of transdermal patches varies, and 1 or more trials of oral acetylcholinesterase inhibitors with documented intolerance to side effects and/or with prior authorization may be necessary for drug initiation and maintenance. Once a patient has reached the moderate stage of AD, memantine is also indicated. Both donepezil and memantine can help with BPSD by delaying their onset.37 Cognitive enhancers can be used concurrently and may work synergistically.38 There is also support for the efficacy of donepezil in α-synuclein diseases.39 Rivastigmine is indicated for use in all stages of dementia in both AD and Parkinson’s dementia.40
BPSD. BPSD describes a constellation of symptoms that frequently arise in patients with dementia. Common symptoms include agitation, aggression, yelling out, irritability, crying, and tearfulness. Some of the most common and earliest BPSD symptoms are affective (eg, anxiety and depression). Clinicians should consider the underlying psychiatric diagnosis that explains the clinical state, such as major depressive disorder (MDD), generalized anxiety disorder (GAD), or an adjustment disorder with anxious or depressive features. Once the diagnosis is made, it is appropriate to use evidence-based medications that include selective serotonin receptor inhibitors (SSRIs). Application of geriatric psychiatry–based guidelines in prescribing medications (ie, start low, go slow) is always prudent. Additional caution is advised when prescribing citalopram. While citalopram is an effective SSRI, dosing that may be necessary for efficacy is often above the US Food and Drug Administration (FDA)–recommended maximal dose of 20 mg/d for adults over the age of 65, which is linked to onset of cardiac arrhythmias.41 Caution is also advised when prescribing venlafaxine, a serotonin-norepinephrine reuptake inhibitor (SNRI), as this medication leads to dose-related hypertension,42 which can also exacerbate the risk for vascular dementia. There are also signs and symptoms of venlafaxine withdrawal after a missed dose, which can occur more frequently in older adults, and can be mistaken for an exacerbation of BPSD.
At least half of patients develop BPSD within 10 months of their dementia diagnosis43; expectation setting through early education of caregivers is helpful. When BPSD symptoms occur, an algorithm-based logic (eg, by Chen et al37) can be combined with clinical judgment based on knowledge of the whole patient and their circumstances.
BPSD can be stratified into emergent, urgent, or nonurgent symptoms. Under emergent circumstances, one should assume that the patient cannot take oral medications, and emergency-department–facilitated intramuscular (IM) olanzapine, haloperidol, or benzodiazepines may be necessary. (Note: in general, it is best to avoid use of benzodiazepines, as these can worsen cognition and increase the risk of falls.44) In urgent circumstances, where the use of oral medications is feasible, antipsychotics (such as aripiprazole, quetiapine, or risperidone) can be helpful. Electroconvulsive therapy (ECT) may be extremely helpful in resolving persistent or severe BPSD, although it can be challenging to initiate in the outpatient setting. Under nonurgent circumstances, consideration should be given for use of trazodone, escitalopram, sertraline, or one of the antipsychotic medications recommended for urgent situations.37 A bevy of psychopharmacologic options can be found in the PCC’s toolkit, including some not listed here. Support from a geriatric psychiatrist can be helpful for management of more challenging BPSD.
What Are Monoclonal Antibodies, and How (and when) Might They Be Used in MCI and Early AD?
Monoclonal antibodies are laboratory-engineered immunoglobulins that possess a high degree of specificity for a particular antigen or epitope.45 They are produced through laboratory-based clonal expansion of plasma cells specifically designed to produce one type of antibody geared toward a therapeutic target. They can exist in different forms, depending on the engineering method, with nomenclatures that signify whether their origin is chimeric, mouse-human recombinant (-ximab), humanized (-zumab), or fully human (-umab). Monoclonal antibodies have been successfully applied toward a broad array of illnesses, including cancer, autoimmune diseases, and infections.46 They are among the fastest growing class of therapeutic agents; as of 2022, more than 80 monoclonal antibodies were approved by the FDA for clinical use.47 Although monoclonal antibody therapy has had a profound impact within certain fields like oncology and immunology, they are relatively novel agents for the treatment of neurodegenerative diseases.
Are Monoclonal Antibodies for Treating MCI or AD Available?
Currently, there are 2 FDA-approved monoclonal antibody–based therapies aimed at targeting Aβ deposits in MCI or early AD. Aducanumab (Aduhelm), produced by Biogen, is a human immunoglobulin 1 (IgG1) that targets aggregated Aβ, such as neuritic Aβ plaques and high molecular weight Aβ oligomer forms. It is the first monoclonal antibody approved for use in AD, with initial FDA approval in June 2021 through their accelerated approval pathway.48 The accelerated approval pathway hinges on surrogate endpoint effects (cerebral amyloid as a surrogate for cognitive decline) along with high likelihood of clinical benefit; subsequent results of clinical trials that find clinical benefit are necessary to sustain approval. The approval was controversial49 due to mixed findings of clinical efficacy. In addition, prominent safety concerns included frequent, dose-dependent incidences of cerebral edema and microhemorrhage in drug-treated individuals50 (called amyloid-related imaging abnormalities [ARIAs]). After a decision by the US Centers for Medicare and Medicaid Services (CMS) to restrict coverage of Aduhelm to clinical trials,51 Biogen has focused on phase 4 confirmatory trials in individuals with early AD. The results are expected to be released by 2026.52
The other monoclonal antibody drug that has received FDA approval is lecanemab (Leqembi), an IgG1 monoclonal antibody developed by Eisai, which binds to Aβ-soluble protofibril forms. Following an 18-month, double-blind phase 3 trial among patients with MCI (with evidence of cerebral amyloid accumulation) or early AD, the therapy resulted in remarkably lower levels of amyloid in the brain and a 27% reduction in the rate of cognitive decline compared to the placebo group.53 Like aducanumab, safety concerns included a risk of treatment-induced ARIAs, with a particular propensity for these adverse events in those individuals with a homozygous ApoE ε4 genotype. Subgroup analyses of the cognitive endpoint ADAS-Cog14 found that men benefited from the treatment more than women, and that there was high variability of outcome measures due to inadequate sampling from African Americans. Leqembi was approved by the FDA in January 202354 through their accelerated approval pathway (similar to Aduhelm, based on surrogate findings of reduction of cerebral amyloid deposition and a likelihood of clinical benefit). Appropriate use recommendations for Leqembi include indications for MCI and early AD, exclusion of individuals with MRI evidence of non-AD dementia, and caution around ApoE ε4 carriers due to risk of ARIAs.55
Another promising amyloid-based monoclonal antibody in the AD pipeline is Eli Lilly’s donanemab. In a phase 3 multicenter, double-blind randomized controlled trial (RCT) of individuals with early symptomatic AD, donanemab led to significant benefit over placebo in preventing changes from baseline on a composite cognitive and functional impairment scale at 18 months.56 Donanemab has been shown to reduce the rate of tau neurofibrillary tangle accumulation in the frontal cortex and other regions53,57 and has shown similar ARIA phenomena to the other amyloid monoclonal antibodies described above. Donanemab is currently under review by the FDA.
How Safe, Tolerable, and Costly Are Monoclonal Antibodies?
Meta-analyses of various monoclonal antibody candidates for AD and MCI have revealed that there is a higher frequency of adverse events (AEs) in the experimental group compared to placebo.58 The most notable side effects from monoclonal antibody injections are vasogenic cerebral edema and cerebral microhemorrhage, first identified through MRI scans of individuals receiving bapineuzumab, one of the first studied amyloid-targeting monoclonal antibodies for AD. These cerebrovascular side effects, now called ARIAs, include ARIA-E (vasogenic edema) and ARIA-H (microhemorrhage). Notably, there is not a clear correlation between scan abnormalities and symptoms related to those findings. For example, in a lecanemab study, about 20% of people had ARIA imaging findings; however, less than 3% experienced symptoms. Still, there were 2 deaths during the clinical trial, and it is believed that both individuals had vascular conditions that predisposed them to ARIA-related complications.55
APOE4 genotype is a major risk factor for radiographic ARIA, as seen by overall rates of 32.6% (9.2% symptomatic) in APOE4 homozygotes, compared with 10.9% (1.7% symptomatic) in heterozygotes, and 5.4% (1.4% symptomatic) in noncarriers in the CLARITY AD (lecanemab) trial.55 Cerebral macrohemorrhages, though exceedingly rare overall, occurred far more frequently in individuals on anticoagulants (up to 3.6% of individuals during either the double-blinded or open-label phases of the study). While appropriate use recommendations suggest APOE4 genotyping to inform risk-benefit decision-making, it is worth remembering the high prevalence of APOE4 carriers in the general population (20%–25%) with a greater concentration of carriers among individuals with MCI and AD. Thus, informed decision-making and continuous monitoring will be essential to limit drug-related harm.55 The PCC’s office is not necessarily the ideal place to consider APOE4 screening, considering the gravity of genetic testing. It may be prudent to partner with local infusion centers, who will likely support the screening process and assist with risk-benefit conversations.
Given the high cost of manufacturing relative to chemical pharmaceutical agents, research expenditure involved in drug discovery, as well as the rights to deliver some of the first disease-modifying therapies for a field that has seen minimal strides over the past decades, monoclonal antibodies have been linked to steep costs. When a market launch was first attempted for Aduhelm in June 2021, the drug was priced at approximately $56,000 per year, based on a weight-based dosing regimen for an average weight individual (74 kg).59 After criticism of the drug cost,60 Biogen reduced the wholesale acquisition cost by 50%, equating to a yearly cost at the maintenance dose of approximately $28,200.61 This decision was also driven by the potential financial burden of the therapy on the US health care system, as a significant portion of AD and MCI patients are enrolled in Medicare. For Eisai’s Lequembi, CMS decided in July 2023 to provide broad coverage for qualified individuals enrolled in Medicare. Whether individuals not enrolled, or not yet enrolled in Medicare will receive coverage for this treatment remains uncertain at this time.
Are Other Pharmaceuticals in the AD Pipeline?
As of 2022, there were more than 100 therapeutic agents under investigation as AD disease-modifying therapies in various stages of clinical development.63 These include novel/experimental agents as well as those that are already FDA approved for other indications (such as diabetes medications, semaglutide and metformin, and cardiovascular medications, such as losartan, amlodipine, and atorvastatin). Mechanisms of action involve epigenetics, inflammation/immune modulation, metabolism/bioenergetics, neurogenesis, neurotransmitter receptors, oxidative stress, proteostasis, synaptic plasticity/neuroprotection, tau targeting, and the neurovasculature. Among phase 3 disease-modifying agents, the leading categories are those targeting oxidative stress, amyloid, synaptic plasticity/neuroprotection, and metabolism/bioenergetics. These comprise 10%–30% of the disease-modifying therapeutics in phase 3 investigations. Many are unique compounds developed by biotech companies with clinical trial data set to be announced in the next 2 years.
In addition to disease-modifying therapies, studies have examined possible cognitive enhancers and neuropsychiatric symptom attenuators. Although these treatments are not intended to alter the course of the disease, they may help to alleviate symptoms, such as agitation, which is present in up to 70% of patients with cognitive decline.64 Within these symptom-based treatments are novel compounds developed by biotech companies, as well as known substances, such as caffeine and escitalopram (an SSRI used to treat depression). Many of these studies are also expected to be completed in the next 2 years.
Of note, an important feature of studies in the AD drug pipeline is the targeted study population. Although there is significant diversity across various phase 1, 2, and 3 study compounds, most novel disease-modifying therapeutics in phase 3 involve patients diagnosed at the preclinical or prodromal/prodromal-mild (MCI) stage. This is based on the idea that early intervention may provide the greatest benefit in slowing cognitive decline. In AD, where brain atrophy is progressive and permanent, early diagnosis and intervention may be the key to preserving cognitive function as the illness advances. However, it is also worth acknowledging that these pre- and early-stage secondary prevention studies are extremely difficult given the highly variable and very slow clinical progression at early stages. In contrast, drugs that target symptoms are studied in those who are diagnosed with mild-to-moderate to severe dementia wherein therapies may confer the greatest benefit because symptoms tend to be more prominent in the later disease stages.
The landscape of AD drug development continues to shift as our understanding of AD biology progresses. Drug discovery in this field is complicated given that successful studies conducted in animal models do not yield similar results in human trials. Nonetheless, the failures of many promising therapeutic candidates over the years have contributed meaningful data leading toward more efficacious and less toxic therapies. The next wave of phase 3 clinical trial data will provide renewed hope for a field that has been rife with challenges.
What Happened to Ms B, and What Was Discussed With Her Family?
Ms B’s physician explains to Ms B and her daughter that MCI is a syndrome characterized by cognitive problems that appear on testing but do not interfere significantly with daily functioning. He explains that cognitive impairment can be caused by several things, and some cases may improve or remain stable with treatment. Potentially reversible causes include vitamin deficiencies, medication side effects, sleep disturbances, and depression. MCI would be expected to worsen with time if the underlying cause is a neurodegenerative process, of which AD is the most common. While there is no way of knowing definitively whether Ms B’s symptoms will progress, he explains that there are several things she and her family can do to further investigate her risk, reduce her risk, and prepare for the future.
Looking for potentially reversible causes and modifiable risk factors, Ms B’s physician orders basic laboratory work including a complete metabolic panel, complete blood count, lipid panel, hemoglobin A1c, thyroid-stimulating hormone, and levels of vitamin B12, folate, and vitamin D. He refers her for a non-contrast brain MRI to look for structural abnormalities and to serve as a baseline, since she has not yet had brain imaging. Given Ms B’s mild symptoms of depression and anxiety, he discusses treatment options including restarting sertraline and/or providing a psychotherapy referral. Finally, he discusses referral for neuropsychological assessment and testing. He explains that this would involve a 3- to 5-hour visit with a neuropsychologist specially trained in comprehensive evaluation of memory and cognition along with a follow-up visit to review the results. This testing can help to identify patterns of difficulty that may suggest a specific underlying condition. Additionally, the neuropsychologist might suggest ways to address or treat difficulties found on testing. Ms B becomes tearful at the mention of AD, and she asks what she can do to reduce her risk. Her physician encourages her to try and get regular exercise by doing something she enjoys (ie, walking for 30 minutes on most days), engaging in enjoyable and mentally stimulating activities, remaining as social as possible, and eating a healthy Mediterranean-style diet.
Several weeks later, Ms B returns accompanied by her daughter and signs paperwork for her daughter to receive information about her health care and to act as her proxy in the future if needed. Ms B’s laboratory tests returned within normal limits, except for a mildly elevated low-density lipoprotein level (101 mg/dL) and a borderline vitamin B12 level (270 pg/mL). Her physician recommends further laboratory testing for vitamin B12 deficiency (with methylmalonic acid [MMA] and homocysteine levels), followed by a daily oral vitamin B12 supplement. Ms B shares that she has joined a gym, and she has started walking with her neighbor twice weekly. Her physician applauds her for these positive behavioral changes. Ms B’s daughter shares that although her mother is still managing her own medications, grocery shopping, and medical appointments, she is starting to wonder if she should start helping more with finances, noting that there was a problem last year with overpayment of several bills and a delay in filing taxes. Ms B’s doctor advised that it would be a good idea to plan for this type of assistance should Ms B’s difficulties worsen, rather than wait until a crisis arises.
A few months later, Ms B and her daughter return to review the results of neuropsychological testing and brain MRI. Testing reveals a multidomain amnestic profile and MRI shows scattered cortical white matter hyperintensities expected for age and mild bilateral ventromedial temporal lobe volume loss. Ms B’s physician shares that these findings are not diagnostic, but they can be seen in AD, and he reiterates that mitigation of risk with lifestyle factors and treatment of other conditions remain of utmost importance. Ms B asks if there are any other tests that can be done to determine her risk of AD, and her daughter asks if there are medications approved for treating her current condition. The physician states that there are several biomarkers for AD that can be tested in the spinal fluid and that there may be blood tests for these biomarkers soon. There is also a brain scan that can be done with a radioactive tracer to visualize amyloid in the brain; however, these tests are not always covered by insurance policies. He explains that the decision to obtain biomarker testing is one that should be taken with care given the possibility of positive biomarkers and subsequent anxiety that may result combined with the fact that positive biomarkers do not necessarily mean that progression is inevitable. Regarding treatment, he discusses the possibility of a cognitive enhancer, donepezil, should her condition worsen, but he emphasizes the lack of evidence for benefit in MCI. For her MCI, he discusses the recent approval of a new treatment aimed at reducing harmful amyloid plaques and reducing cognitive decline in MCI with concurrent risks of dose-dependent brain swelling and/or small bleeds that may or may not cause symptoms. The doctor shares that one such drug is now covered by Medicare. He shares that there are many other medications in research trials that may provide additional options in the next few years. He offers to refer Ms B to the local university medical center where there are several ongoing clinical trials. Ms B and her daughter express interest in biomarker testing and medication for MCI, and they accept a referral to a cognitive-behavioral neurologist at the local university to learn more and inquire about participating in research.
CONCLUSION
MCI is a syndrome characterized by cognitive problems that are evident on testing but that do not significantly interfere with independence in daily life. MCI may be caused by neurodegenerative disease, of which AD is the most common. Alternatively, MCI may be caused by treatable medical conditions and may improve or remain stable with time. It is difficult to determine whether MCI is caused by AD, but there are various tests that can be done to help provide clues. Management of MCI has largely focused on cardiovascular risk reduction and lifestyle interventions. Until recently, there were no medications to modify the progression from MCI to AD to treat MCI due to AD, but there is a large amount of ongoing research in this area, and 2 monoclonal antibody medications, aducanumab and lecanumab, have been approved by the FDA for treatment of MCI in recent years, and a third, donanemab, is currently under review by the FDA.
Article Information
Published Online: November 28, 2023. https://doi.org/10.4088/PCC.23f03544
© 2023 Physicians Postgraduate Press, Inc.
Submitted: April 21, 2023; accepted July 21, 2023.
To Cite: Weinberg MS, Principe JL, Chen A, et al. Screening, assessment, and pharmacologic treatment of mild cognitive impairment and early Alzheimer’s disease: the role for monoclonal antibodies. Prim Care Companion CNS Disord. 2023;25(6):23f03544.
Author Affiliations: Department of Psychiatry, Massachusetts General Hospital, Boston (Weinberg, Chen, Stern); Department of Neurology, Massachusetts General Hospital, Boston (Weinberg, Arnold); Massachusetts General Hospital, Boston (Principe, Chen); Harvard Medical School, Boston, Massachusetts (Weinberg, Principe, Chen, Chung, Arnold, Stern); Department of Psychiatry, Brigham and Women’s Hospital, Boston, Massachusetts (Principe); McLean Hospital, Belmont, Massachusetts (Principe, Chen).
Author Information: Drs Weinberg, Principe, and Chen are co-first authors.
Corresponding Author: Theodore A. Stern, MD, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: Research supported by the following grants: NIH T32-MH112485, Alzheimer’s Association AACSF-22-970716, Harvard Catalyst NIH UL1 TR002541 to Dr Weinberg, and NIH NIA-P30AG062421 to Drs Weinberg and Arnold.
Role of the Funders/Sponsors: Sponsors served no role in the design or conduct of the study, collection, management, analysis, or interpretation of the data, nor were they involved in preparation, review, or approval of the manuscript.
Clinical Points
- Screening for MCI in a primary care setting can identify patients at risk for AD and provide the timely opportunity to educate, mitigate risk, offer potential treatment, and help patients and families plan for the future.
- Monoclonal antibodies have shown some promise for slowing cognitive decline and adverse brain changes in MCI and early AD, though risks and anticipated benefits will be important to consider with patients.
- In early AD, affective behavioral and psychological symptoms are common, and several algorithm-based treatment methods have been published.
- Geriatric psychiatrists and neurologists can be instrumental in supporting the diagnosis and treatment of neurocognitive disorders and their related symptoms.
References (64)

- Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24(4):709–711. PubMed
- Segal DL, June A, Payne M, et al. Development and initial validation of a self-report assessment tool for anxiety among older adults: the Geriatric Anxiety Scale. J Anxiety Disord. 2010;24(7):709–714. PubMed CrossRef
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. (published correction appears in J Am Geriatr Soc. 2019;67(9):1991) J Am Geriatr Soc. 2005;53(4):695–699. PubMed CrossRef
- Lind KE, Hildreth K, Lindrooth R, et al. The effect of direct cognitive assessment in the Medicare annual wellness visit on dementia diagnosis rates. Health Serv Res. 2021;56(2):193–203. PubMed CrossRef
- Chen YX, Liang N, Li XL, et al. Diagnosis and treatment for mild cognitive impairment: a systematic review of clinical practice guidelines and consensus statements. Front Neurol. 2021;12:719849. PubMed CrossRef
- Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33(2):379–388. PubMed CrossRef
- Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68(10):2207–2213. PubMed CrossRef
- Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for cognitive impairment in older adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323(8):757–763. PubMed CrossRef
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. PubMed CrossRef
- Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the memory impairment screen. Neurology. 1999;52(2):231–238. PubMed CrossRef
- Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16(3):275–293. PubMed CrossRef
- Morley JE, Tumosa N. Saint Louis University Mental Status Examination (SLUMS). APA PsycTests; 2002. [Database record]. doi: 10.1037/t27282-000.
- Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–1454. PubMed CrossRef
- Graf C. The Lawton instrumental activities of daily living scale. Am J Nurs. 2008;108(4):52–62, quiz 62–63. PubMed CrossRef
- Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. PubMed CrossRef
- Pfeffer RI, Kurosaki TT, Harrah CH Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. PubMed CrossRef
- Edemekong P, Bomgaars D, et al. Activities of daily living. In: StatPearls [Internet]. Treasure Island, Florida: StatPearls Publishing; 2023.
- Shelkey M, Wallace M. Katz index of independence in activities of daily living. J Gerontol Nurs. 1999;25(3):8–9. PubMed CrossRef
- Ward A, Tardiff S, Dye C, et al. Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: a systematic review of the literature. Dement Geriatr Cogn Disord Extra. 2013;3(1):320–332. PubMed CrossRef
- Roberts RO, Knopman DS, Mielke MM, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82(4):317–325. PubMed CrossRef
- Petersen R, Lopez O, Armstrong M, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. 2018;90(3):126–135.
- Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022;79(12):1242–1249. PubMed CrossRef
- Rajan KB, Weuve J, Barnes LL, et al. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement. 2021;17(12):1966–1975. PubMed CrossRef
- Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286–1294. PubMed CrossRef
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. PubMed CrossRef
- Liu PP, Xie Y, Meng XY, et al. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther. 2019;4(1):29. PubMed CrossRef
- Ma C, Hong F, Yang S. Amyloidosis in Alzheimer’s disease: pathogeny, etiology, and related therapeutic directions. Molecules. 2022;27(4):1210. PubMed CrossRef
- Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316(5825):750–754. PubMed CrossRef
- Rapoport M, Dawson HN, Binder LI, et al. Tau is essential to β -amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A. 2002;99(9):6364–6369. PubMed CrossRef
- Therriault J, Pascoal TA, Lussier FZ, et al. Biomarker modeling of Alzheimer’s disease using PET-based Braak staging. Nat Aging. 2022;2(6):526–535. PubMed CrossRef
- La Joie R, Visani AV, Baker SL, et al. Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med. 2020;12(524):eaau5732. PubMed CrossRef
- Largent EA, Grill JD, O’Brien K, et al. Testing for Alzheimer disease biomarkers and disclosing results across the disease continuum. Neurology. 2023;100(21):1010–1019. PubMed CrossRef
- Korczyn AD. Mixed dementia–the most common cause of dementia. Ann N Y Acad Sci. 2002;977(1):129–134. PubMed CrossRef
- Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. PubMed CrossRef
- Bucholc M, McClean PL, Bauermeister S, et al. Association of the use of hearing aids with the conversion from mild cognitive impairment to dementia and progression of dementia: a longitudinal retrospective study. Alzheimers Dement (N Y). 2021;7(1):e12122. PubMed CrossRef
- Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18(suppl):S1–S2. PubMed
- Chen A, Metzger E, Osser D. Improving pharmacologic management of behavioral and psychological symptoms of dementia (BPSD): an algorithm for real-world care. Am J Geriatr Psychiatry. 2022;30(suppl):S2. CrossRef
- McDermott CL, Gruenewald DA. Pharmacologic management of agitation in patients with dementia. Curr Geriatr Rep. 2019;8(1):1–11. PubMed CrossRef
- Gerlach LB, Kales HC. Managing behavioral and psychological symptoms of dementia. Psychiatr Clin North Am. 2018;41(1):127–139. PubMed CrossRef
- Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351(24):2509–2518. PubMed CrossRef
- FDA Drug Safety Communication. Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. FDA website. Accessed March 17, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-revised-recommendations-celexa-citalopram-hydrobromide-related
- Leong C, Alessi-Severini S, Enns MW, et al. Cerebrovascular, cardiovascular, and mortality events in new users of selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors. J Clin Psychopharmacol. 2017;37(3):332–340. PubMed CrossRef
- Tampi RR, Bhattacharya G, Marpuri P. Managing behavioral and psychological symptoms of dementia (BPSD) in the era of boxed warnings. Curr Psychiatry Rep. 2022;24(9):431–440. PubMed CrossRef
- Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960. PubMed CrossRef
- Lloyd EC, Gandhi TN, Petty LA. Monoclonal antibodies for COVID-19. JAMA. 2021;325(10):1015. PubMed CrossRef
- LiverTox: An Online Resource for Information on Drug-induced Liver Injury. NIDDK website. Accessed March 17, 2023. https://www.niddk.nih.gov/news/archive/2022/livertox-online-resource-information-drug-induced-liver-injury
- Lu RM, Hwang YC, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1. PubMed CrossRef
- Shi M, Chu F, Zhu F, et al. Impact of anti-amyloid-beta monoclonal antibodies on the pathology and clinical profile of Alzheimer’s disease: a focus on aducanumab and lecanemab. Front Aging Neurosci. 2022;14:870517. PubMed CrossRef
- Alexander GC, Knopman DS, Emerson SS, et al. Revisiting FDA approval of aducanumab. N Engl J Med. 2021;385(9):769–771. PubMed CrossRef
- Salloway S, Chalkias S, Barkhof F, et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79(1):13–21. PubMed CrossRef
- National Coverage Analysis (NCA). Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease. (CAG-00460N) - Proposed Decision Memo. CMS.gov website. Accessed March 17, 2023. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=Y&NCAId=305
- Aduhelm. ALZFORUM website. Accessed March 17, 2023. https://www.alzforum.org/therapeutics/aduhelm
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. PubMed CrossRef
- FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. FDA website. Accessed March 17, 2023. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment
- Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10(3):362–377. PubMed CrossRef
- Sims JR, Zimmer JA, Evans CD, et al; TRAILBLAZER-ALZ 2 Investigators. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023;330(6):512–527. PubMed CrossRef
- Rashad A, Rasool A, Shaheryar M, et al. Donanemab for Alzheimer’s disease: a systematic review of clinical trials. Healthcare (Basel). 2022;11(1):32. PubMed CrossRef
- Lacorte E, Ancidoni A, Zaccaria V, et al. Safety and efficacy of monoclonal antibodies for alzheimer’s disease: a systematic review and meta-analysis of published and unpublished clinical trials. J Alzheimers Dis. 2022;87(1):101–129. PubMed CrossRef
- Robinson JC. Why is aducanumab priced at $56,000 per patient? lessons for drug-pricing reform. N Engl J Med. 2021;385(22):2017–2019. PubMed CrossRef
- Ross EL, Weinberg MS, Arnold SE. Cost-effectiveness of aducanumab and donanemab for early Alzheimer disease in the US. JAMA Neurol. 2022;79(5):478–487. PubMed CrossRef
- Mafi JN, Leng M, Arbanas JC, et al. Estimated annual spending on aducanumab in the US Medicare program. JAMA Health Forum. 2022;3(1):e214495. PubMed CrossRef
- Statement: Broader Medicare Coverage of Leqembi Available Following FDA Traditional Approval | CMS. Accessed Nov 10, 2023. https://www.cms.gov/newsroom/press-releases/statement-broader-medicare-coverage-leqembi-available-following-fda-traditional-approval
- Cummings J, Lee G, Nahed P, et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement (N Y). 2022;8(1):e12295. PubMed CrossRef
- Carrarini C, Russo M, Dono F, et al. Agitation and dementia: prevention and treatment strategies in acute and chronic conditions. Front Neurol. 2021;12:644317. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!