LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2024;26(2):23f03614
Author affiliations are listed at the end of this article.
Have you ever wondered what transcranial magnetic stimulation (TMS) is and when it is indicated? Have you been uncertain about whether it is safe and as effective (or more effective) than other treatments for mood disorders? Have you been curious about the absolute and relative contraindications for administration of TMS? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Ms M, a 45-year-old woman with a history of recurrent major depressive disorder (MDD) without psychotic features, posttraumatic stress disorder (PTSD), 3 psychiatric hospitalizations, and 1 suicide attempt, but no significant medical history, was referred by the inpatient psychiatric service for a consultation on the use of transcranial magnetic stimulation (TMS) in the context of worsening depression and suicidal ideation. Prior to her current psychiatric hospitalization, Ms M accumulated medications for a premeditated plan for suicide; fortunately, before checking into a hotel room to carry out her plan, she aborted the suicide attempt and reached out to her psychiatrist for assistance.
Sadly, Ms M’s young daughter passed away 5 years ago due to a rare disease. Since then, Ms M struggled with symptoms of depression around the anniversary of her child’s death.
Ms M had failed more than 4 antidepressant medication trials (with augmentation strategies) as well as a course of cognitive-behavioral therapy (CBT). Although electroconvulsive therapy (ECT) was recommended by the inpatient psychiatry team, Ms M declined; however, she was open to having a TMS consultation. Ms M’s depression was confirmed by validated questionnaires. Ms M had no contraindications to undergoing TMS, ie, she had no history of seizures and had no brain stimulators, pacemakers, or other ferromagnetic foreign bodies implanted in her body.
The TMS procedure was reviewed with Ms M, and the benefits and potential risks of the treatment were discussed with her (including the risk of seizures, headaches, scalp sensitivity, spasms of the facial muscles, lightheadedness, and hearing loss due to the noise of the treatment). Ms M understood and accepted those risks.
DISCUSSION
What Is TMS?
Transcranial magnetic stimulation is a noninvasive neurotherapeutic procedure that utilizes a strong electromagnetic field to change brain activity in a targeted fashion to treat psychiatric illnesses.1,2 The TMS magnetic device is designed to deliver electromagnetic pulses that traverse the scalp and skull to either diffusely or focally change the brain’s neuronal activity. While single-pulse stimulation is being used for obtaining the proper dosage, the treatment utilizes repetitive TMS (rTMS), in which hundreds of pulses are delivered at various frequencies over time. Therapeutic TMS is a nonpharmacologic and generally well-tolerated treatment; its risks are mitigated through safety and dosing protocols. No anesthesia is required, and patients can drive themselves to and from the treatment center.
What Does the Administration of TMS Entail?
The initial evaluation often involves a consultation with a psychiatrist or neuropsychiatrist who takes a comprehensive history (including a history of medication trials [with their duration, dosing, and timing]) and reviews relevant data. In addition, the consultant reviews the indication for therapeutic TMS and discusses its side effects and contraindications. Treatment logistics of TMS (eg, transportation, leave accommodations) are also reviewed, as patients are expected to present to the TMS center daily for treatment for several weeks. Currently, most insurance companies require at least 4 failed antidepressant trials (from drugs in at least 2 different pharmacologic categories) as well as a course of CBT before approving coverage of TMS, although TMS can sometimes be clinically indicated earlier. There are occasional insurances that approve TMS after 2 failed antidepressant trials.
What Is Therapeutic TMS Used For?
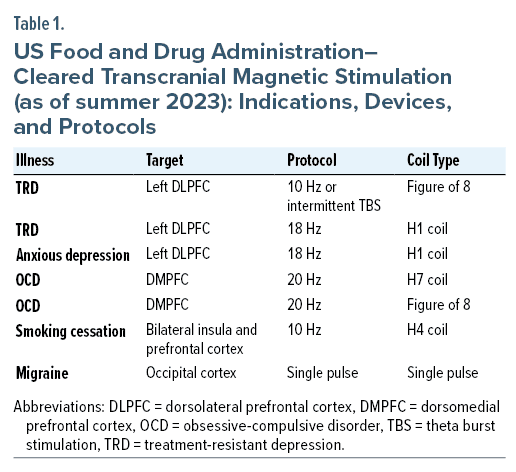
Multiple devices and approaches have been used to treat a variety of neuropsychiatric conditions, including treatment-resistant depression (TRD), anxious depression (deep TMS), obsessive-compulsive disorder (OCD), nicotine use disorder (for smoking cessation with deep TMS), and migraine headache (using single-pulse TMS). Practitioners often use TMS as an off-label, adjunctive treatment for generalized anxiety disorder and PTSD when it is comorbid with TRD. In addition, recent investigations of substance use disorders with cravings for opioids and alcohol have been encouraging. Currently, the US Food and Drug Administration (FDA) has approved therapeutic TMS as a treatment for MDD, OCD, migraine headache, and smoking cessation; efforts are underway to expand its indications (Table 1).3–8
How Does TMS Work?
Psychiatric illnesses are thought to arise from dysfunction of neurocircuits (functionally and structurally connected pathways that represent and generate mental and body functions).9 For nearly 40 years, investigators have been optimizing noninvasive TMS neuromodulation (ie, TMS magnetic pulses that can be delivered in multiple frequencies and patterns) to help reverse neuropsychiatric disease states.
Currently, common targets for TMS in the treatment of TRD include the left dorsolateral prefrontal cortex (DLPFC) and the right DLPFC. Targets for OCD treatment include the dorsomedial prefrontal cortex (DMPFC) (Table 1). Other targets and protocols are under investigation and being used off-label. On occasion, when TRD is resistant to left DLPFC treatment, a right-side protocol can be added to create a bilateral treatment.10
Where Is TMS Administered?
TMS is usually administered in outpatient settings, although it can be administered in inpatient settings. When treatment is administered, the patient sits in a comfortable chair, and the practitioner places a magnetic coil on the primary motor cortex (located on the frontal lobe) to measure the “motor threshold” (MT). The practitioner then sends electromagnetic pulses into the brain until the contralateral hand begins to twitch. The amount of energy required to make the contralateral fingers twitch is the MT, which is different for everyone; moreover, the MT varies depending on use of medications and substances, as well as a patient’s sleep characteristics. It is worth mentioning that changes in these characteristics can lead to changes in the MT and risk a miscalibration of the patients’ cortical excitability, which subsequently can potentially lead to the risk of overdosing (seizures in an extreme form) or the risk of underdosing that can impact efficacy.
Once the MT is determined, the magnetic coil is positioned on the target. The patient then receives the treatment as a series of pulses that are applied to their head. Up to 120% of the resting MT is administered. The treatment does not involve use of any anesthesia or sedation, and the patient remains awake and alert during the treatment.
How Effective Is TMS?
TMS is a safe, effective, and well-tolerated noninvasive neuromodulation treatment for the management of MDD in adults. In a systematic review and meta-analysis of unilateral and bilateral TMS randomized sham-controlled trials conducted over the last 2 decades,11 the pooled remission and response rates for unilateral TMS were 16.0% and 25.1% (as compared to 5.7% and 11.0% for sham treatment, respectively), while the pooled remission and response rates for bilateral TMS were 16.6% and 25.4% (as compared to 2.0% and 6.8% for sham treatment, respectively). The conclusion of the investigators11 was that TMS had a moderate antidepressant effect for the acute treatment of patients with unipolar TRD. Naturalistic, multicenter therapeutic TMS studies have generally shown higher response rates of 41%–56% and remission rates of 26%–28% in real-world practice.12 An increase in the total number of sessions, pulses, and intensity, while maintaining safety, may have improved response rates in naturalistic studies.
Theta burst stimulation (TBS) is a TMS paradigm whereby pulses are applied in bursts of 3, at a frequency of 50 Hz, and with an inter-burst interval of 200 ms (5 Hz). The major advantage of TBS protocols over conventional TMS approaches is the reduced treatment time. Voigt and colleagues,13 based on meta-analysis and systematic review of 10 randomized controlled trials (RCTs) (N = 667), concluded that TBS is superior to sham treatment, and the response rates for TBS versus conventional TMS were not statistically different (RR = 1.02; 95% CI, 0.85–1.23; P = .80; I2 = 0%).
What Are the Side Effects of TMS?
The paucity of systemic side effects is the main advantage of therapeutic TMS as compared to more invasive treatments for the management of MDD. According to a systematic review and meta-analysis14 that included 53 randomized sham-controlled trials (N = 3,273), there was no increased risk of dropout. The findings suggested that therapeutic TMS may significantly increase the risk of nonserious (predominantly mild and transient) adverse events including headaches, discomfort, and pain at the stimulation site.14 Rare but serious adverse events associated with TMS treatment include hearing loss, treatment-emergent mania (TEM), and the induction of seizures. Although a small proportion of patients have had a transient reduction in hearing after TMS, and a permanent decrease in hearing has been observed in one individual who did not wear hearing protection while being treated with an H-coil, most studies found that no change in hearing was apparent after TMS when hearing protection devices were used.2,15 Therefore, approved hearing protection should be used, and prompt referral provided for the auditory assessment of all individuals who complain of hearing loss, tinnitus, or aural fullness during TMS.15
According to a review of 10 RCTs,16 the pooled rate of TEM was 0.84% for the active TMS treatment group compared to 0.73% for the sham group (not statistically significant), with TEM more commonly diagnosed in those with a history of bipolar disorder. In a study17 that examined the seizure rates across various TMS devices in naturalistic clinical settings, members of the Clinical TMS Society were surveyed about seizures in their practices. Among 134 members who responded, a total of 18 seizures were reported after 586,656 TMS sessions in 25,526 patients across all device manufacturers. The overall seizure rate was 0.31 (95% CI, 0.18–0.48) per 10,000 sessions and 0.71 (95% CI, 0.42–1.11) per 1,000 patients. However, the H-coil seizure rate of 5.56 per 1,000 patients (95% CI, 2.77–9.95) was significantly higher (P < .001) than a combined seizure rate of 0.14 per 1,000 patients (95% CI, 0.01–0.51) in figure-8 coil devices.17 The risk of having a seizure depends on the frequency, intensity, and interval between 2 trains of TMS, with a higher risk in those who are sleep deprived, who use alcohol or proconvulsant medications, or who have preexisting neurological disorders. Therefore, the potential of seizure induction with TMS requires that careful attention be paid to patient selection and preparedness for this adverse event.15
Who Should Not Receive TMS?
The presence of ferromagnetic hardware that is in close contact with the discharging coil (eg, cochlear implants) is the only absolute contraindication to TMS due to the risk of triggering malfunctioning of such implanted devices. Relative contraindications include conditions in which there is an increased or uncertain risk of inducing an epileptic seizure (such as a personal history of epilepsy) and traumatic, vascular, tumoral, or infectious lesion of the brain. A history of intracranial ferromagnetic metal implants is typically exclusionary.2,15 Therefore, a standard safety screening questionnaire should be used for all patients who are seeking TMS to determine the risk:benefit ratio of this procedure.2,15
When Should Other Treatments Be Selected?
It is important for clinicians to understand the comparative efficacy of TMS and other treatments to decide on the best next-step interventions for the management of TRD. Ren and colleagues18 examined 9 RCTs that directly compared TMS and ECT for MDD (N = 425). They reported that ECT was superior to high-frequency TMS in terms of response (64.4% vs 48.7%, RR = 1.41, P = .03) and remission (52.9% vs. 33.6%, RR = 1.38, P = .006), while discontinuation rates were similar between the 2 treatments (8.3% vs 9.4%, RR = 1.11, P = .80). Both TMS and ECT were reasonably well-tolerated; however, specific cognitive domains (such as visual memory and verbal fluency) were more impaired in patients who received ECT. Furthermore, ECT was superior in those with psychotic depression, while (high frequency) TMS was as effective as ECT in patients with nonpsychotic depression.18 Therefore, TMS is an effective option for management of nonpsychotic depression; however, ECT should take precedence in the management of patients with MDD and psychosis, catatonic features, or active thoughts of suicide due to its superior efficacy.
To date, there have been no head-to-head published studies in patients with MDD that have compared TMS with medication strategies (including augmentation or switching). The ASCERTAIN TRD study, which is currently underway in 10 university hospitals across the United States, aims to compare the outcomes of treatment interventions, including TMS, augmentation with atypical antipsychotic (aripiprazole), and switching to another antidepressant (venlafaxine) for management of patients with TRD who have not responded to 2 adequate antidepressant trials. Similarly, no comparative studies have been conducted regarding the use of TMS and ketamine in the management of TRD. In a retrospective naturalistic study, Mikellides and colleagues19 compared the acute antidepressant efficacy of intramuscular (IM) ketamine and TMS for the management of TRD (N = 24). Twelve patients received IM ketamine (twice weekly for 8 sessions), and 12 patients received 30 sessions of intermittent TBS at the left DLPFC. The authors19 reported that both treatments were equally effective regarding pre- to post-depressive and anxiety symptoms.
More research with larger sample sizes is needed to compare the effects of TMS and ketamine and to establish a more effective intervention if medication strategies alone are ineffective for management of TRD. There is also a paucity of evidence comparing TMS and vagus nerve stimulation (VNS) for the management of TRD; however, for acute treatment, TMS is preferred, as VNS is invasive and has been shown to be effective for long-term management of TRD. Finally, deep brain stimulation (DBS), which remains experimental for the management of TRD, is thought of as the treatment of “last resort,” and it should be considered only after the conventional treatments and noninvasive neuromodulation have been tried and failed.
What Is the Efficacy of Accelerated TMS?
Accelerated TMS treatment consists of ≥ 2 TMS treatments conducted daily over the course of several days or weeks.20 While many accelerated TMS schedules have been used in research studies, currently only SNT (Stanford neuromodulation therapy) accelerated TMS neuromodulation has been approved by the FDA. The SNT protocol consists of 10 daily intermittent TBS treatments over 5 days, using specialized neuronavigational equipment to deliver the treatment to a functional magnetic resonance imaging (fMRI)–derived cortical target in the left DLPFC.21 Treatments are delivered with a 50-minute interval between treatments. The SNT protocol may be associated with an accelerated treatment response as compared to once-daily treatment, since it is completed during a shorter period (5 days vs 4–8 weeks). In the open-label SNT protocol trial (21 participants), 86.4% of patients met criteria for remission by the end of the acute course, and in the RCT SNT (29 participants: active [N = 14], sham [N = 15]), 78.6% of patients in the active group met remission criteria in 1 of the 5 follow-up assessments across 4 weeks.21,22 While the results of the SNT studies are encouraging, the limited number of participants should be considered in the interpretation of the results. A recent review of 23 depression studies in which accelerated TMS was used found an average response rate and remission rate of 42.4% and 28.4%, respectively.20 Better outcomes were associated with delivery of more treatment sessions per day, a higher number of pulses, and a higher number of sessions. More research is needed to determine the efficacy and durability of accelerated TMS protocols.
Does Extension of an Acute Course of TMS Treatment Work in Nonresponders?
A standard course of TMS treatment consists of 36 treatments delivered 5 days per week for 6 weeks, followed by a taper or maintenance period during which treatments are given 1–3 times per week for a few weeks.12,23 This treatment schedule was established by clinical trials that used specific endpoints, and not by dose response studies that determined the optimal number of treatments. One retrospective naturalistic study found that by extending the acute course up to 72 treatments in patients not meeting response criteria by session 36, the response and remission rates were 53.6% and 32.1%, respectively.24 A larger multisite observational study25 that used registry data from more than 7,000 patients (738 with > 36 treatments) found that depression severity scores significantly improved with an extended course. When comparing outcomes from session 36 to the endpoint (median number of sessions = 41), response rates increased from 48.9% to 59.9%, and remission rates increased from 20.3% to 27.2%.25 Thus, evidence suggests that subgroups of patients may follow different response trajectories and that longer treatment courses likely lead to superior clinical outcomes.
How Long Does the Benefit of TMS Last?
TMS is usually administered 5 days a week for several weeks (usually 5–8 weeks). The treatment length varies based on the protocol used, the clinical response, and availability of the patient to receive the treatment. Most clinical protocols aim at completion of 36 treatments. The treatment could be performed faster (with about 10 treatments each day for 5 days) using the accelerated protocol.
The durability of the TMS treatment response is an important consideration when advising patients about the long-term prognosis, response preservation strategies, and re-treatment outcomes. The largest multisite naturalistic, observational study that evaluated TMS response durability monitored 120 acute TMS treatment responders and remitters for 12 months following their acute treatment.26 Nearly two-thirds (62.5%) of patients had a sustained response during the observation period and showed better durability outcomes in those who benefited the most from the acute treatment. The study26 concluded that the clinical benefits of TMS last for at least 1 year. Almost half (49.2%) of the TMS responders and remitters received at least 1 TMS treatment during the observational period, making it difficult to determine the role that additional TMS treatments had on the results. To address the question of how additional TMS sessions following the acute course affected response durability, a meta-analysis27 of 18 TMS durability studies (involving 732 patients) compared the response durability in studies that did and did not use additional TMS treatments to preserve treatment response (preservation TMS). Overall, the combined sustained response rates at 3 months, 6 months, and 12 months following acute treatment were 66.5%, 52.9%, and 46.3%, respectively. Significantly higher sustained response rates were observed in studies that used planned or symptom-triggered preservation TMS strategies compared to studies without preservation TMS (n = 8) at the 3-month (76.2% vs 56.1%, respectively) and 6-month (61.1% vs 38.5%) follow-up time points. These results suggest that preservation TMS strategies may improve TMS response durability.
While evidence suggests that preservation TMS improves response durability, many different preservation TMS strategies have been reported; however, consensus recommendations to guide physicians on when and how to use preservation TMS are lacking. Proactive preservation TMS approaches employ regularly scheduled treatments following the acute course to avoid symptom exacerbation, while reactive approaches withhold additional treatments until a predefined symptom threshold has been crossed.28 The number of and interval between treatments also vary among preservation approaches. Some studies have delivered 1 treatment at fixed or gradually increasing intervals,29,30 while other studies31,32 have used a clustered treatment strategy whereby several treatments are given over a few days (eg, 5 treatments over 2 to 3 days) with fixed or progressively longer intervals between treatments. It should be noted that the majority of preservation data comes from uncontrolled studies. More research is needed to determine the optimal preservation TMS strategy. Despite the lack of consensus guidelines, preservation TMS is commonly used in clinical practice, although it is not widely covered by insurance plans.
Is the Cost of TMS Covered By All Insurance Plans?
Most insurance companies cover TMS that is used for MDD if other criteria are met. An increasing number of insurance companies cover TMS for OCD. Insurance practices in different states have different policies; therefore, authorization for the treatment should be obtained locally. Many TMS centers seek authorization from the patient’s insurance company before starting the treatment. During the initial consultation, the TMS team should assess whether the criteria for coverage are being met; if so, they will typically apply to the insurance company for prior authorization.
What Happened to Ms M?
Ms M completed a course of 36 TMS treatments over the left DLPFC. She received part of her treatment course while she was still on the inpatient service and then received the remainder after hospital discharge. She reported feeling much better after receiving TMS (ie, her negative thoughts, mood, energy, and motivation improved significantly). Her score on the 16-item Quick Inventory of Depressive Symptomatology33 improved from 30 to 3, and her score on the Patient Health Questionnaire34 improved from 25 to 2, indicating that her depression had remitted. In addition, Ms M had no significant side effects from the treatment. Although TMS is generally considered to be a relatively durable treatment, a follow-up appointment was scheduled before the next anniversary of her child’s death so that another course of TMS could be considered.
Clinical Points
- Transcranial magnetic stimulation (TMS) is a safe, effective, and well-tolerated noninvasive neurotherapeutic procedure that utilizes a strong electromagnetic field to change brain activity in a targeted fashion to treat a variety of psychiatric illnesses.
- Currently, the US Food and Drug Administration has approved therapeutic TMS as a treatment for major depressive disorder, obsessive-compulsive disorder, migraine headache, and smoking cessation; efforts are underway to expand its indications.
- TMS may increase the risk of mild and transient headaches, discomfort, and pain at the stimulation site. Rare, but serious, adverse events include hearing loss, treatment-emergent mania, and the induction of seizures.
- The only absolute contraindication to TMS is the presence of metallic hardware that is in close contact with the discharging coil (eg, cochlear implants) due to the risk of triggering malfunctioning of such implanted devices. Relative contraindications include conditions in which there is an increased or uncertain risk of inducing an epileptic seizure and traumatic, vascular, tumoral, or infectious lesion of the brain. A history of intracranial ferromagnetic metal implants is typically exclusionary.
- TMS is usually administered in outpatient settings 5 days a week for several weeks (usually 5–8 weeks). Most clinical protocols aim to complete 36 treatments. Treatments can be performed faster (with about 10 treatments each day for 5 days) using an accelerated TMS protocol.
Article Information
Published Online: March 12, 2024. https://doi.org/10.4088/PCC.23f03614
© 2024 Physicians Postgraduate Press, Inc.
Submitted: July 27, 2023; accepted: November 7, 2023.
To Cite: Razafsha M, Barbour TA, Chopra A, et al. Transcranial magnetic stimulation in primary care: indications, risks, and outcomes. Prim Care Companion CNS Disord. 2024;26(2):23f03614.
Author Affiliations: Division of Neuropsychiatry, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Razafsha, Barbour, Kritzer); Transcranial Magnetic Stimulation Service, Psychiatric Neurotherapeutics Program, McLean Hospital, Harvard Medical School, Belmont, Massachusetts (Razafsha); Transcranial Magnetic Stimulation Clinical Service, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Barbour, Kritzer); Depression Clinical and Research Program, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Chopra); Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts (Stern); Harvard Medical School, Boston, Massachusetts (Stern).
Corresponding Author: Mahdi Razafsha, MD, 115 Mill St, Mail Stop 132, Belmont MA 02478-1064 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
References (34)

- Fox MD, Buckner RL, White MP, et al. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72(7):595–603. PubMed CrossRef
- Rossi S, Antal A, Bestmann S, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol. 2021;132(1):269–306. PubMed CrossRef
- Pell GS, Harmelech T, Zibman S, et al. Efficacy of deep TMS with the H1 coil for anxious depression. J Clin Med. 2022;11(4):1015. PubMed CrossRef
- Petrosino NJ, Cosmo C, Berlow YA, et al. Transcranial magnetic stimulation for posttraumatic stress disorder. Ther Adv Psychopharmacol. 2021;11:20451253211049921. PubMed CrossRef
- Lan L, Zhang X, Li X, et al. The efficacy of transcranial magnetic stimulation on migraine: a meta-analysis of randomized controlled trails. J Headache Pain. 2017;18(1):86. PubMed CrossRef
- Ward HB, Mosquera MJ, Suzuki J, et al. A systematic review of noninvasive brain stimulation for opioid use disorder. Neuromodulation. 2020;23(3):301–311. PubMed CrossRef
- Philip NS, Sorensen DO, McCalley DM, et al. Noninvasive brain stimulation for alcohol use disorders: state of the art and future directions. Neurotherapeutics. 2020;17(1):116–126. PubMed CrossRef
- Zangen A, Moshe H, Martinez D, et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry. 2021;20(3):397–404. PubMed CrossRef
- Camprodon JA, Pascual-Leone A. Multimodal applications of transcranial magnetic stimulation for circuit-based psychiatry. JAMA Psychiatry. 2016;73(4):407–408. PubMed CrossRef
- Somani A, Kar SK. Efficacy of repetitive transcranial magnetic stimulation in treatment-resistant depression: the evidence thus far. Gen Psychiatr. 2019;32(4):e100074. PubMed CrossRef
- Sehatzadeh S, Daskalakis ZJ, Yap B, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression: a meta-analysis of randomized controlled trials over 2 decades. J Psychiatry Neurosci. 2019;44(3):151–163. PubMed CrossRef
- Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587–596. PubMed CrossRef
- Voigt JD, Leuchter AF, Carpenter LL. Theta burst stimulation for the acute treatment of major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):330. PubMed CrossRef
- Wang WL, Wang SY, Hung HY, et al. Safety of transcranial magnetic stimulation in unipolar depression: a systematic review and meta-analysis of randomized-controlled trials. J Affect Disord. 2022;301:400–425. PubMed CrossRef
- Rossi S, Hallett M, Rossini PM, et al; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. PubMed CrossRef
- Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119–130. PubMed CrossRef
- Taylor JJ, Newberger NG, Stern AP, et al. Seizure risk with repetitive TMS: survey results from over a half-million treatment sessions. Brain Stimul. 2021;14(4):965–973. PubMed CrossRef
- Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181–189. PubMed CrossRef
- Mikellides G, Michael P, Psalta L, et al. A retrospective naturalistic study comparing the efficacy of ketamine and repetitive transcranial magnetic stimulation for treatment-resistant depression. Front Psychiatry. 2022;12:784830. PubMed CrossRef
- Caulfield KA, Fleischmann HH, George MS, et al. A transdiagnostic review of safety, efficacy, and parameter space in accelerated transcranial magnetic stimulation. J Psychiatr Res. 2022;152:384–396. PubMed CrossRef
- Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. 2020;177(8):716–726. PubMed CrossRef
- Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132–141. PubMed CrossRef
- O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–1216. PubMed CrossRef
- Razafsha M, Barbour T, Uribe S, et al. Extension of transcranial magnetic stimulation treatment for depression in nonresponders: results of a naturalistic study. J Psychiatr Res. 2023;158:314–318. PubMed CrossRef
- Hutton T, Carpenter L, Pages K, et al. Dosing transcranial magnetic stimulation: efficacy in major depressive disorder as a function of number of treatment sessions [abstract P1.154]. Brain Stimul. 2023;16(1):269–270. CrossRef
- Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394–1401. PubMed CrossRef
- Senova S, Cotovio G, Pascual-Leone A, et al. Durability of antidepressant response to repetitive transcranial magnetic stimulation: systematic review and meta-analysis. Brain Stimul. 2019;12(1):119–128. PubMed CrossRef
- Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79–88. PubMed CrossRef
- Benadhira R, Thomas F, Bouaziz N, et al. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD). Psychiatry Res. 2017;258:226–233. PubMed CrossRef
- Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251–257. PubMed CrossRef
- Pridmore S, May T. Relapse prevention (RP) TMS. Brain Stimul. 2018;11(6):1391–1392. PubMed CrossRef
- Wang HN, Wang XX, Zhang RG, et al. Clustered repetitive transcranial magnetic stimulation for the prevention of depressive relapse/recurrence: a randomized controlled trial. Transl Psychiatry. 2017;7(12):1292. PubMed CrossRef
- Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. PubMed CrossRef
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!