- Selective serotonin reuptake inhibitors (SSRIs) inhibit metabolic pathways and can thereby increase the blood levels and hence the adverse effects of many coadministered drugs. If statin levels are increased, myopathy may result.
- Escitalopram, citalopram, and paroxetine are almost certain to be safe in cotherapy with all statins, and rosuvastatin, pitavastatin, and pravastatin are almost certain to be safe in cotherapy with all SSRIs.
- Other combinations may theoretically be associated with risks, but the magnitude of interaction is likely to be below clinical thresholds. However, cautious clinicians will use lower doses of atorvastatin, simvastatin, or lovastatin when coprescribing fluvoxamine.
ABSTRACT
Elderly patients commonly receive statin drugs for the primary or secondary prevention of cardiovascular and cerebrovascular events. Elderly patients also commonly receive antidepressant drugs, usually selective serotonin reuptake inhibitors (SSRIs), for the treatment of depression, anxiety, or other conditions. SSRIs are associated with many pharmacokinetic drug interactions related to the inhibition of the cytochrome P450 (CYP) metabolic pathways. There is concern that drugs that inhibit statin metabolism can trigger statin adverse effects, especially myopathy (which can be potentially serious, if rhabdomyolysis occurs). However, a detailed literature review of statin metabolism and of SSRI effects on CYP enzymes suggests that escitalopram, citalopram, and paroxetine are almost certain to be safe with all statins, and rosuvastatin, pitavastatin, and pravastatin are almost certain to be safe with all SSRIs. Even though other SSRI-statin combinations may theoretically be associated with risks, the magnitude of the pharmacokinetic interaction is likely to be below the threshold for clinical significance. Risk, if at all, lies in combining fluvoxamine with atorvastatin, simvastatin, or lovastatin, and even this risk can be minimized by using lower statin doses and monitoring the patient.
J Clin Psychiatry 2014;75(2):e95-e99 (doi:10.4088/JCP.13f08941)
© Copyright 2014 Physicians Postgraduate Press, Inc.
Clinical Problem
A 51-year-old man with type 2 diabetes mellitus has been receiving atorvastatin 10 mg/d for the past year for the treatment of elevated low-density lipoprotein (LDL) cholesterol levels. He has been newly diagnosed with major depressive illness. Are there statin drug interactions that may limit the choice of antidepressant drugs that he can safely receive?
Statins and Primary Prevention
Statin therapy is associated with primary prevention benefits in cardiovascular and cerebrovascular disease; the 5-year numbers needed to treat range from 49 to 155, depending on the outcomes examined.1 Recent guidelines from the American College of Cardiology and the American Heart Association encourage primary prevention with moderate- to high-intensity statin therapy in diabetic patients aged 40-75 years in whom LDL cholesterol levels are 70 mg/dL and above.2
Diabetes and Depression: Mortality Risks
When depression complicates diabetes, the course of diabetes worsens, and there is an increased risk of cardiovascular mortality3 as well as all-cause mortality.3-5 A probable explanation is that patients with depression are less likely to diet, exercise, and take necessary medicines regularly, and certain antidepressant drugs may increase appetite and weight. Other mechanisms, such as endocrine dysregulation,6 may also operate. The practical implication is that, when depression and diabetes coexist, depression should be vigorously treated, and patients should be motivated and carefully monitored for adherence to health guidance.
Diabetes and Depression: Choosing an Antidepressant
Ideally, diabetic patients should not receive antidepressants that may increase appetite and weight, because such effects could adversely impact the course of diabetes. Diabetic patients often have cardiovascular comorbidity and are at increased risk of ischemic heart disease events; ideally, therefore, they should also not receive antidepressants that raise their cardiovascular risks.
Weight gain is a well-documented adverse effect of the tricyclic antidepressants (TCAs)7-9 and mirtazapine.8,10 The TCAs are also associated with cardiovascular adverse effects such as orthostatic hypotension, slowed cardiac conduction, and increased heart rate,11 which may worsen cardiovascular outcomes.12 Adrenergic antidepressants such as venlafaxine,13 desvenlafaxine,14 reboxetine,15 and milnacipran16 can raise heart rate and/or blood pressure, but duloxetine17,18 and bupropion19,20 may be exceptions.
Selective serotonin reuptake inhibitors (SSRIs) may be ideally suited to diabetic patients. With the possible exception of paroxetine,8 these drugs are not usually associated with increase in appetite and weight; additionally, these drugs may decrease cardiovascular risks and improve clinical cardiovascular endpoints through several mechanisms.21
SSRIs, however, are commonly associated with pharmacokinetic drug interactions: many SSRIs inhibit many metabolic pathways, thereby raising the blood levels and hence the risk of adverse effects of many coadministered drugs. In the case of statins, raised blood levels can result in an increased risk of hepatic enzyme elevation and of dose-dependent myopathies that can range from troublesome myalgia, cramps, and weakness (all of which are common) to life-threatening rhabdomyolysis (which is rare).22 It is therefore important to know how statins are metabolized and whether SSRIs inhibit the enzymes that metabolize statins. At least 1 review22 on statin drug interactions lists antidepressants as agents of potential concern without addressing the subject in depth. The present article therefore examines the specific risk of metabolic drug interactions between SSRIs and statins.
Cytochrome P450 Enzyme Metabolism of Statins
Atorvastatin and rosuvastatin are the most commonly used statins. Others that have been or are being used include fluvastatin, lovastatin, pitavastatin, pravastatin, and simvastatin.2
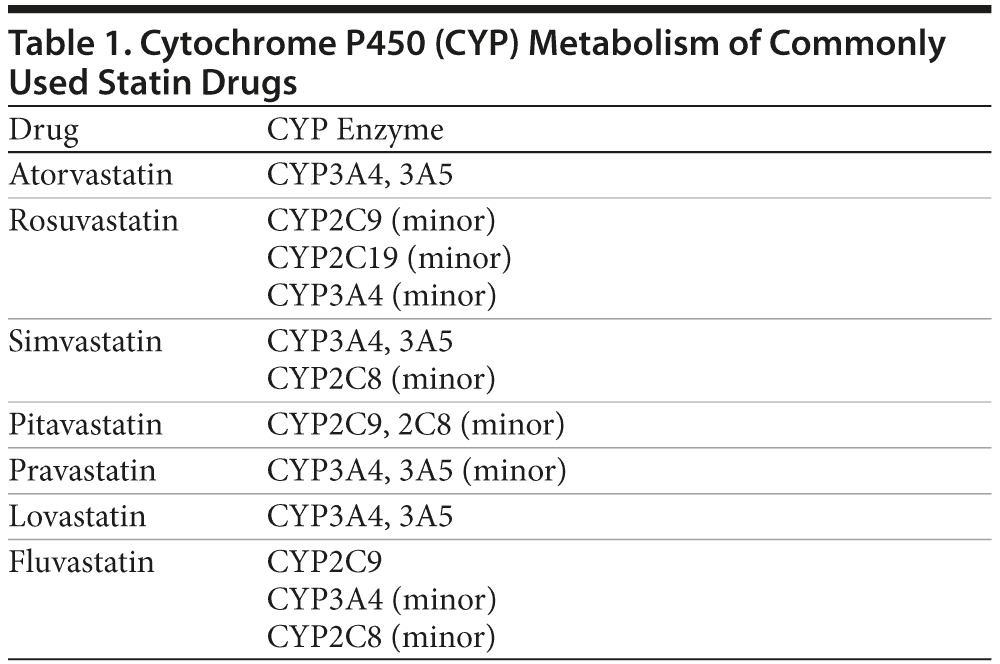
The CYP enzymes that metabolize these statins are listed in Table 1. Atorvastatin, simvastatin, and lovastatin are metabolized by CYP3A4/3A5,22,23 and simvastatin is additionally metabolized by CYP2C8.24 Fluvastatin is mostly (50%-80%) metabolized by CYP2C9,23,25 but exposure to this drug is increased less than 2-fold by CYP2C9 inhibitors.24 Fluvastatin additionally undergoes minor metabolism through CYP3A4 and 2C8.23,25 Pravastatin undergoes minor metabolism through CYP3A4/3A5, and metabolic interactions through this pathway are believed to be below clinical threshold for significance.26,27 Likewise, rosuvastatin undergoes only minor metabolism through CYP3A4, and inhibition of this pathway does not increase exposure to rosuvastatin to a clinically significant extent.28 Rosuvastatin also undergoes minor metabolism through CYP2C929 and 2C19.22 Rosuvastatin and pravastatin are mostly excreted unchanged; their plasma levels are not significantly elevated by CYP3A4 inhibitors.23,24,27
Pitavastatin is minimally metabolized by the CYP enzymes30,31; its metabolism by CYP2C9/2C8 is small and clinically insignificant.22,32 Pitavastatin is therefore minimally susceptible to metabolic drug interactions.27
CYP Enzyme Inhibition by SSRIs
A quick glance at Table 1 shows that drugs that significantly inhibit CYP3A4/5 would increase the exposure to atorvastatin, simvastatin, and lovastatin, and those that inhibit CYP2C9/8 might increase exposure to fluvastatin. Inhibition of the other CYP enzymes involved in statin metabolism is unlikely to be of clinical significance because the metabolic pathways are minor.
SSRIs and CYP3A4/5. S-fluoxetine and R-norfluoxetine inhibit CYP3A4 in vitro, and the combined fluoxetine and norfluoxetine enantiomer moiety is predicted to reduce in vivo CYP3A4 activity by about 60%.33 However, in clinical doses administered to healthy volunteers, 11 days of treatment with fluoxetine elicited no significant inhibition of CYP3A4 activity as studied using erythromycin and alprazolam as substrates.34 Fluoxetine (20-60 mg/d) did not influence the pharmacokinetics of the CYP3A4 substrate midazolam, either.35 Sertraline also inhibits CYP3A4 in vitro,36 but 8 days of treatment of healthy volunteers with clinical doses of sertraline did not significantly alter levels of the CYP3A4 substrates erythromycin and alprazolam.34
Fluvoxamine inhibited the CYP3A4 substrate tandospirone in an animal model37 but showed little in vitro effect on this enzyme.38 Twelve days of treatment with fluvoxamine (200 mg/d) increased the area under the curve of midazolam (a CYP3A4 substrate) by 66%; however, this increase was less than one-tenth of the increase produced by ketoconazole, a strong CYP3A4 inhibitor, and less than one-sixth of the increase produced by nefazodone, another strong inhibitor of this enzyme.35 In another study,39 5 days of treatment of healthy volunteers with fluvoxamine (100 mg/d) more than doubled exposure to buspirone, another CYP3A4 substrate.
Fifteen days of treatment with paroxetine (20 mg/d) did not alter the pharmacokinetics of terfenadine, a CYP3A4 substrate.40 Citalopram (20-40 mg/d) negligibly influenced the pharmacokinetics of carbamazepine and triazolam, both of which are CYP3A4 substrates.41,42 In vitro data indicate that escitalopram has negligible inhibitor effects on CYP3A4.43
No published studies were located that described the effects of the SSRIs on CYP3A5.
SSRIs and CYP2C9/8. In vitro studies found that fluvoxamine was associated with more inhibition of CYP2C9 than fluoxetine, sertraline, paroxetine, and citalopram; metabolic inhibition with the latter 4 SSRIs was small and of little significance.44,45 In a study of healthy volunteers, 5 days of treatment with fluvoxamine (75-150 mg/d) resulted in a very small (19%) reduction in the clearance of tolbutamide, a CYP2C9 substrate.46 Sertraline (200 mg/d) is also associated with a very small (16%) but statistically significant reduction in tolbutamide clearance.47 The clinical effects of citalopram and escitalopram on CYP2C9 metabolism are negligible.42
Fluoxetine does not inhibit CYP2C8.48 Fluvoxamine may be a weak inhibitor of this enzyme.49 No published studies were located that described the effects of the remaining SSRIs on CYP2C8.
SSRI-Statin Drug Interactions
The plasma concentrations of atorvastatin, simvastatin, and lovastatin are significantly increased by strong CYP3A4 inhibitors, elevating the risk of myopathies.24 However, from the previous section, it is apparent that, among the SSRIs, only fluvoxamine inhibits CYP3A4 to any appreciable extent, and even this inhibition is small to modest. Therefore, in the vast majority of patients, it is likely that all SSRIs, even those that inhibit CYP3A4, can be safely administered with atorvastatin, simvastatin, and lovastatin. This view is supported by Neuvonen et al,24 who opine that weak or moderately potent CYP3A4 inhibitors can be used cautiously with small doses of CYP3A4-dependent statins.
Clopidogrel, a CYP2C9 inhibitor, has negligible effect on the pharmacokinetics of fluvastatin50; perhaps this is because elimination by other pathways is unaffected (Table 1). Although fluvoxamine and possibly sertraline inhibit CYP2C9, the degree of inhibition is far too small to be clinically significant. Therefore, it is very unlikely that any SSRI, even those that inhibit CYP2C9, will affect the pharmacokinetics of fluvastatin or any other statin (Table 1). Neuvonen et al24 also opine that even potent CYP2C9 inhibitors do not cause clinically significant drug interactions with statins, even those statins that undergo CYP2C9 metabolism.
With regard to the statins that undergo little to no CYP metabolism and those that are metabolized through many pathways (Table 1), it is again obvious that the concurrent use of SSRIs is likely to be safe.
Might patients with inefficient CYP gene polymorphisms (that is, poor metabolizers) be more vulnerable to SSRI drug interactions? For example, median daily doses of atorvastatin, simvastatin, and lovastatin were nearly double in extensive relative to poor CYP3A4 metabolizers.51 However, the pharmacokinetic effects of an enzyme inhibitor are more apparent in intermediate to extensive metabolizers than in poor metabolizers.52 Therefore, given that the wild type of CYP genes code for intermediate to extensive metabolism status, and given that (as already discussed) SSRIs have little effect on the CYP enzymes that metabolize the statins, it is unlikely that CYP gene polymorphisms will influence the risk of SSRI-related drug interactions in patients receiving statins.
What about patients with chronic liver disease, chronic debilitating disease, and other states in which CYP enzyme status may be compromised? All of these individuals would be phenotypically poor metabolizers, and the preceding discussion would most likely apply. In any case, such patients would already be receiving only low doses of statins, if at all. Nevertheless, these special populations are easily discerned, and precautions in SSRI coprescribing are desirable.
It is worth noting that there is a several-fold interindividual variation in statin drug levels24; this is likely to drown out small effects that result from SSRI-mediated CYP enzyme inhibition. Generalizations in this section notwithstanding, some patients may be idiosyncratically predisposed to SSRI-statin pharmacokinetic interactions; however, considering the lack of published reports on the subject, it seems that the risk is theoretical rather than practical.
Competitive Inhibition
So far, this article has examined CYP enzyme inhibition as a mechanism of drug interaction between SSRIs and statins. What about competitive inhibition, in which one substrate inhibits the metabolism of another substrate because both compete for the same enzyme? The possibility was considered in a recent, detailed review on statin drug interactions.22 However, SSRIs are metabolized through multiple CYP pathways, especially CYP2D6, and neither CYP3A4 nor CYP2C9 (the major enzymes that metabolize the statins) plays a dominant role in their metabolism.53,54 Therefore, competitive inhibition is unlikely to be clinically significant. This conclusion is reinforced by the lack of published reports on SSRI-statin interactions.
Non-CYP Pharmacokinetic Pathways
Pharmacokinetic drug interactions may occur at other levels, such as at the levels of absorption and transport. For example, organic anion-transporting polypeptides (OATPs) facilitate the absorption of drugs from the intestine, and P-glycoprotein is an efflux transporter.55,56 All statins are OATP substrates, and some are P-glycoprotein substrates, as well.22 However, there is little information about the effects of SSRIs on OATPs, and, although, for example, in one study in healthy volunteers, fluvoxamine and paroxetine (but not sertraline) were associated with significant P-glycoprotein inhibition,57 there is insufficient information to evaluate the risk of a P-glycoprotein-mediated SSRI-statin drug interaction. Given the lack of published literature on SSRI-statin adverse pharmacokinetic interactions, it is likely that non-CYP pharmacokinetic pathways are also of little clinical significance.
Synthesis and Recommendations
In the light of the preceding sections, with regard to pharmacokinetic drug interactions and the risk of statin-related myopathy, escitalopram, citalopram, and paroxetine are almost certain to be safe in cotherapy with all statins, and rosuvastatin, pitavastatin, and pravastatin are almost certain to be safe in cotherapy with all SSRIs. Fluoxetine and sertraline are also likely to be safe, even when combined with atorvastatin, simvastatin, and lovastatin.
Whereas there seems to be a theoretical risk that fluvoxamine may increase the levels of atorvastatin, simvastatin, lovastatin, and perhaps fluvastatin as well, the magnitude of the interaction is likely to be below the threshold for clinical significance. Nevertheless, when fluvoxamine is coprescribed with these statins, the patient should be instructed to immediately report any unexplained muscle pain, tenderness, or weakness, especially if such symptoms are accompanied by fever, malaise, and/or dark-colored urine.22 Liver enzyme and creatine kinase levels should be obtained in such patients, and medications should be withdrawn if these levels are of clinical concern in the absence of strenuous exercise (or other predisposing causes) or if the presence of myopathy is suspected. Such precautions may be particularly warranted when patients are receiving intensive-dose statin therapy.
Non-SSRI Antidepressant Interactions With Statins
Unlike the SSRIs, nefazodone is a potent CYP3A4 inhibitor,34,35 and the combination of nefazodone with simvastatin has been associated with rhabdomyolysis in at least 4 published case reports.58-61 A possible interaction between nefazodone and pravastatin has also been reported.62
SSRI-Statin Pharmacodynamic Interactions
This article has focused on the pharmacokinetic effects of SSRIs on statin drugs. Might SSRIs and statins pharmacodynamically interact? In a data-mining study of the US Food and Drug Administration’s Adverse Event Reporting System, Tatonetti et al63 found that the coadministration of paroxetine and pravastatin was associated with a mean blood glucose increase of 19 mg/dL in nondiabetic patients (n = 135) and 48 mg/dL in diabetic patients (n = 104). Neither drug raised blood glucose in monotherapy. The interaction was limited to paroxetine and pravastatin and was not a class action between all SSRI and all statin drugs. The risk of this paroxetine-pravastatin interaction requires prospective study.
Need for Future Research
Pharmacokinetic studies are necessary to identify dose-dependent effects, if any, of different SSRIs on different statins. Prospective studies, or insurance database studies, may throw light on the relative risk of myopathies in statin-treated patients who do and do not receive SSRIs.
Afternote: Related SSRI Interactions
Two previous articles in this series also addressed SSRI drug interactions in patients receiving medications related to ischemic heart disease: aspirin and clopidogrel64 and β-blockers.65
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Taylor FC, Huffman M, Ebrahim S. Statin therapy for primary prevention of cardiovascular disease. JAMA. 2013;310(22):2451-2452. PubMed doi:10.1001/jama.2013.281348
2. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published online ahead of print November 12, 2013]. Circulation. 2013. PubMed
3. van Dooren FE, Nefs G, Schram MT, et al. Depression and risk of mortality in people with diabetes mellitus: a systematic review and meta-analysis. PLoS ONE. 2013;8(3):e57058. PubMed doi:10.1371/journal.pone.0057058
4. Hofmann M, Köhler B, Leichsenring F, et al. Depression as a risk factor for mortality in individuals with diabetes: a meta-analysis of prospective studies. PLoS ONE. 2013;8(11):e79809. PubMed doi:10.1371/journal.pone.0079809
5. Park M, Katon WJ, Wolf FM. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. Gen Hosp Psychiatry. 2013;35(3):217-225. PubMed doi:10.1016/j.genhosppsych.2013.01.006
6. Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67(suppl 1):S26-S28. PubMed doi:10.1097/01.psy.0000163456.22154.d2
7. Fava M. Weight gain and antidepressants. J Clin Psychiatry. 2000;61(suppl 11):37-41. PubMed
8. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259-1272. PubMed doi:10.4088/JCP.09r05346blu
9. Leucht C, Huhn M, Leucht S. Amitriptyline versus placebo for major depressive disorder. Cochrane Database Syst Rev. 2012;12:CD009138. PubMed
10. Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. PubMed
11. Jiang W, Davidson JR. Antidepressant therapy in patients with ischemic heart disease. Am Heart J. 2005;150(5):871-881. PubMed doi:10.1016/j.ahj.2005.01.041
12. Taylor D. Antidepressant drugs and cardiovascular pathology: a clinical overview of effectiveness and safety. Acta Psychiatr Scand. 2008;118(6):434-442. PubMed doi:10.1111/j.1600-0447.2008.01260.x
13. Feighner JP. Cardiovascular safety in depressed patients: focus on venlafaxine. J Clin Psychiatry. 1995;56(12):574-579. PubMed
14. Lieberman DZ, Massey SH. Desvenlafaxine in major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2010;4:67-82. PubMed
15. Denolle T, Pellizzoni C, Jannuzzo MG, et al. Hemodynamic effects of reboxetine in healthy male volunteers. Clin Pharmacol Ther. 1999;66(3):282-287. PubMed doi:10.1016/S0009-9236(99)70036-6
16. Trugman JM, Palmer RH, Ma Y. Milnacipran effects on 24-hour ambulatory blood pressure and heart rate in fibromyalgia patients: a randomized, placebo-controlled, dose-escalation study [published online ahead of print November 20, 2013]. Curr Med Res Opin. PubMed doi:10.1185/03007995.2013.861812
17. Wernicke J, Lledó A, Raskin J, et al. An evaluation of the cardiovascular safety profile of duloxetine: findings from 42 placebo-controlled studies. Drug Saf. 2007;30(5):437-455. PubMed doi:10.2165/00002018-200730050-00007
18. Gahimer J, Wernicke J, Yalcin I, et al. A retrospective pooled analysis of duloxetine safety in 23,983 subjects. Curr Med Res Opin. 2007;23(1):175-184. PubMed doi:10.1185/030079906X162719
19. Wenger TL, Stern WC. The cardiovascular profile of bupropion. J Clin Psychiatry. 1983;44(5 pt 2):176-182. PubMed
20. Thase ME, Haight BR, Johnson MC, et al. A randomized, double-blind, placebo-controlled study of the effect of sustained-release bupropion on blood pressure in individuals with mild untreated hypertension. J Clin Psychopharmacol. 2008;28(3):302-307. PubMed doi:10.1097/JCP.0b013e318172424e
21. Andrade C, Chethan KB, Sandarsh S. Cardiovascular mechanisms of SSRI drugs and their benefits and risks in ischemic heart disease and heart failure. Int Clin Psychopharmacol. 2013;28(3):145-155. PubMed doi:10.1097/YIC.0b013e32835d735d
22. Bellosta S, Corsini A. Statin drug interactions and related adverse reactions. Expert Opin Drug Saf. 2012;11(6):933-946. PubMed doi:10.1517/14740338.2012.712959
23. Bersot TP. Drug therapy for hypercholesterolemia and dyslipidemia. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s The Pharmacological Basic of Therapeutics. 12th ed. New York, NY: McGraw Hill Medical; 2011:877-908.
24. Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80(6):565-581. PubMed doi:10.1016/j.clpt.2006.09.003
25. Toda T, Eliasson E, Ask B, et al. Roles of different CYP enzymes in the formation of specific fluvastatin metabolites by human liver microsomes. Basic Clin Pharmacol Toxicol. 2009;105(5):327-332. PubMed doi:10.1111/j.1742-7843.2009.00453.x
26. Jacobsen W, Kirchner G, Hallensleben K, et al. Comparison of cytochrome P-450-dependent metabolism and drug interactions of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors lovastatin and pravastatin in the liver. Drug Metab Dispos. 1999;27(2):173-179. PubMed
27. Neuvonen PJ. Drug interactions with HMG-CoA reductase inhibitors (statins): the importance of CYP enzymes, transporters and pharmacogenetics. Curr Opin Investig Drugs. 2010;11(3):323-332. PubMed
28. Cooper KJ, Martin PD, Dane AL, et al. Effect of itraconazole on the pharmacokinetics of rosuvastatin. Clin Pharmacol Ther. 2003;73(4):322-329. PubMed doi:10.1016/S0009-9236(02)17633-8
29. Olsson AG, McTaggart F, Raza A. Rosuvastatin: a highly effective new HMG-CoA reductase inhibitor. Cardiovasc Drug Rev. 2002;20(4):303-328. PubMed doi:10.1111/j.1527-3466.2002.tb00099.x
30. Duggan ST. Pitavastatin: a review of its use in the management of hypercholesterolaemia or mixed dyslipidaemia. Drugs. 2012;72(4):565-584. PubMed doi:10.2165/11207180-000000000-00000
31. Barrios V, Escobar C, Zamorano JL. Searching the place of pitavastatin in the current treatment of patients with dyslipidemia. Expert Rev Cardiovasc Ther. 2013;11(12):1597-1612. PubMed doi:10.1586/14779072.2013.844546
32. Saito Y. Pitavastatin: an overview. Atheroscler suppl. 2011;12(3):271-276. PubMed doi:10.1016/S1567-5688(11)70886-8
33. Lutz JD, Vandenbrink BM, Babu KN, et al. Stereoselective inhibition of CYP2C19 and CYP3A4 by fluoxetine and its metabolite: implications for risk assessment of multiple time-dependent inhibitor systems. Drug Metab Dispos. 2013;41(12):2056-2065. PubMed doi:10.1124/dmd.113.052639
34. DeVane CL, Donovan JL, Liston HL, et al. Comparative CYP3A4 inhibitory effects of venlafaxine, fluoxetine, sertraline, and nefazodone in healthy volunteers. J Clin Psychopharmacol. 2004;24(1):4-10. PubMed doi:10.1097/01.jcp.0000104908.75206.26
35. Lam YW, Alfaro CL, Ereshefsky L, et al. Pharmacokinetic and pharmacodynamic interactions of oral midazolam with ketoconazole, fluoxetine, fluvoxamine, and nefazodone. J Clin Pharmacol. 2003;43(11):1274-1282. PubMed doi:10.1177/0091270003259216
36. Masubuchi Y, Kawaguchi Y. Time-dependent inhibition of CYP3A4 by sertraline, a selective serotonin reuptake inhibitor. Biopharm Drug Dispos. 2013;34(8):423-430. PubMed doi:10.1002/bdd.1857
37. Nishikawa H, Inoue T, Masui T, et al. Pharmacokinetic interaction between tandospirone and fluvoxamine in the rat contextual conditioned fear stress model and its functional consequence: Involvement of cytochrome P450 3A4. Psychiatry Clin Neurosci. 2008;62(5):591-596. PubMed doi:10.1111/j.1440-1819.2008.01853.x
38. Olesen OV, Linnet K. Fluvoxamine-clozapine drug interaction: inhibition in vitro of five cytochrome P450 isoforms involved in clozapine metabolism. J Clin Psychopharmacol. 2000;20(1):35-42. PubMed doi:10.1097/00004714-200002000-00007
39. Lamberg TS, Kivistö KT, Laitila J, et al. The effect of fluvoxamine on the pharmacokinetics and pharmacodynamics of buspirone. Eur J Clin Pharmacol. 1998;54(9-10):761-766. PubMed doi:10.1007/s002280050548
40. Martin DE, Zussman BD, Everitt DE, et al. Paroxetine does not affect the cardiac safety and pharmacokinetics of terfenadine in healthy adult men. J Clin Psychopharmacol. 1997;17(6):451-459. PubMed doi:10.1097/00004714-199712000-00003
41. Nolting A, Abramowitz W. Lack of interaction between citalopram and the CYP3A4 substrate triazolam. Pharmacotherapy. 2000;20(7):750-755. PubMed doi:10.1592/phco.20.9.750.35198
42. Br׸sen K, Naranjo CA. Review of pharmacokinetic and pharmacodynamic interaction studies with citalopram. Eur Neuropsychopharmacol. 2001;11(4):275-283. PubMed doi:10.1016/S0924-977X(01)00101-8
43. von Moltke LL, Greenblatt DJ, Giancarlo GM, et al. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Dispos. 2001;29(8):1102-1109. PubMed
44. Schmider J, Greenblatt DJ, von Moltke LL, et al. Inhibition of CYP2C9 by selective serotonin reuptake inhibitors in vitro: studies of phenytoin p-hydroxylation. Br J Clin Pharmacol. 1997;44(5):495-498. PubMed doi:10.1046/j.1365-2125.1997.00601.x
45. Hemeryck A, De Vriendt C, Belpaire FM. Inhibition of CYP2C9 by selective serotonin reuptake inhibitors: in vitro studies with tolbutamide and (S)-warfarin using human liver microsomes. Eur J Clin Pharmacol. 1999;54(12):947-951. PubMed doi:10.1007/s002280050580
46. Madsen H, Enggaard TP, Hansen LL, et al. Fluvoxamine inhibits the CYP2C9 catalyzed biotransformation of tolbutamide. Clin Pharmacol Ther. 2001;69(1):41-47. PubMed doi:10.1067/mcp.2001.112689
47. Tremaine LM, Wilner KD, Preskorn SH. A study of the potential effect of sertraline on the pharmacokinetics and protein binding of tolbutamide. Clin Pharmacokinet. 1997;32(suppl 1):31-36. PubMed doi:10.2165/00003088-199700321-00005
48. Polasek TM, Elliot DJ, Lewis BC, et al. Mechanism-based inactivation of human cytochrome P4502C8 by drugs in vitro. J Pharmacol Exp Ther. 2004;311(3):996-1007. PubMed doi:10.1124/jpet.104.071803
49. Pedersen RS, Damkier P, Brosen K. The effects of human CYP2C8 genotype and fluvoxamine on the pharmacokinetics of rosiglitazone in healthy subjects. Br J Clin Pharmacol. 2006;62(6):682-689. PubMed doi:10.1111/j.1365-2125.2006.02706.x
50. Ayalasomayajula SP, Vaidyanathan S, Kemp C, et al. Effect of clopidogrel on the steady-state pharmacokinetics of fluvastatin. J Clin Pharmacol. 2007;47(5):613-619. PubMed doi:10.1177/0091270006299138
51. Kitzmiller JP, Sullivan DM, Phelps MA, et al. CYP3A4/5 combined genotype analysis for predicting statin dose requirement for optimal lipid control. Drug Metabol Drug Interact. 2013;28(1):59-63. PubMed
52. Azuma J, Hasunuma T, Kubo M, et al. The relationship between clinical pharmacokinetics of aripiprazole and CYP2D6 genetic polymorphism: effects of CYP enzyme inhibition by coadministration of paroxetine or fluvoxamine. Eur J Clin Pharmacol. 2012;68(1):29-37. PubMed doi:10.1007/s00228-011-1094-4
53. Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41(2):89-295. PubMed doi:10.1080/03602530902843483
54. Andrade C. Augmenting selective serotonin reuptake inhibitors with clomipramine in obsessive-compulsive disorder: benefits and risks. J Clin Psychiatry. 2013;74(12):e1128-e1133.
55. Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158(3):693-705. PubMed doi:10.1111/j.1476-5381.2009.00430.x
56. Dolton MJ, Roufogalis BD, McLachlan AJ. Fruit juices as perpetrators of drug interactions: the role of organic anion-transporting polypeptides. Clin Pharmacol Ther. 2012;92(5):622-630. PubMed doi:10.1038/clpt.2012.159
57. Saruwatari J, Yasui-Furukori N, Niioka T, et al. Different effects of the selective serotonin reuptake inhibitors fluvoxamine, paroxetine, and sertraline on the pharmacokinetics of fexofenadine in healthy volunteers. J Clin Psychopharmacol. 2012;32(2):195-199. PubMed doi:10.1097/JCP.0b013e318248ddb9
58. Jacobson RH, Wang P, Glueck CJ. Myositis and rhabdomyolysis associated with concurrent use of simvastatin and nefazodone. JAMA. 1997;277(4):296-297. PubMed doi:10.1001/jama.277.4.296
59. Thompson M, Samuels S. Rhabdomyolysis with simvastatin and nefazodone. Am J Psychiatry. 2002;159(9):1607. PubMed doi:10.1176/appi.ajp.159.9.1607
60. Skrabal MZ, Stading JA, Monaghan MS. Rhabdomyolysis associated with simvastatin-nefazodone therapy. South Med J. 2003;96(10):1034-1035. PubMed doi:10.1097/01.SMJ.0000078621.31517.30
61. Karnik NS, Maldonado JR. Antidepressant and statin interactions: a review and case report of simvastatin and nefazodone-induced rhabdomyolysis and transaminitis. Psychosomatics. 2005;46(6):565-568. PubMed doi:10.1176/appi.psy.46.6.565
62. Alderman CP. Possible interaction between nefazodone and pravastatin. Ann Pharmacother. 1999;33(7-8):871. PubMed doi:10.1345/aph.18291
63. Tatonetti NP, Denny JC, Murphy SN, et al. Detecting drug interactions from adverse-event reports: interaction between paroxetine and pravastatin increases blood glucose levels. Clin Pharmacol Ther. 2011;90(1):133-142. PubMed doi:10.1038/clpt.2011.83
64. Andrade C. Drug interactions in the treatment of depression in patients with ischemic heart disease. J Clin Psychiatry. 2012;73(12):e1475-e1477. PubMed doi:10.4088/JCP.12f08248
65. Andrade C. Drug interactions in the treatment of depression in patients receiving β-blocker drugs. J Clin Psychiatry. 2013;74(1):e75-e78. PubMed doi:10.4088/JCP.12f08323

 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.