Objective: To evaluate the effect of 1 oral and 2 distinct long-acting injectable (LAI) formulations of the same antipsychotic on times to relapse following medication discontinuation.
Methods: Data were drawn from 3 similarly designed, multicenter, double-blind, placebo-controlled, randomized-withdrawal studies of paliperidone in adults with a schizophrenia diagnosis (according to DSM-IV criteria for ≥ 1 year before screening): once-daily extended-release oral paliperidone (ORAL paliperidone), once-monthly paliperidone palmitate (PP1M), and once-every-3-months paliperidone palmitate (PP3M). In a post hoc analysis, we compared median time to relapse across the treatment-withdrawal arms of the 3 studies using final analysis datasets. Time to relapse in the withdrawal arm of each study was examined using log-rank tests and Cox proportional hazards models.
Results: Four hundred forty-nine patients were withdrawn from 3 paliperidone formulations: 101 from ORAL paliperidone, 203 from PP1M, and 145 from PP3M. Postwithdrawal median (95% confidence interval [CI]) days to relapse were 58 days (42-114 days) for ORAL paliperidone, 172 days (134-222 days) for PP1M, and 395 days (274 days-not reached) for PP3M (P < .0001, pairwise comparisons). Relapse risk was significantly lower (P < .001) for patients who withdrew from either PP formulation relative to ORAL paliperidone and additionally for patients who withdrew from PP3M relative to PP1M.
Conclusions: Results demonstrate that 50% of patients who withdrew treatment from ORAL paliperidone, PP1M, or PP3M remained relapse free for approximately 2 months, 6 months, and 13 months, respectively. This may be relevant for risk mitigation strategies in schizophrenia, a condition in which interruptions in maintenance antipsychotic treatment are commonplace and unpredictable. LAI antipsychotic formulations may provide substantial delays over oral equivalents in times to relapse when patients discontinue therapy.
Trial Registration: ClinicalTrials.gov identifiers: NCT00086320, NCT00111189, and NCT01529515
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Does Half-Life Matter After Antipsychotic Discontinuation?
A Relapse Comparison in Schizophrenia With 3 Different Formulations of Paliperidone

ABSTRACT
Objective: To evaluate the effect of 1 oral and 2 distinct long-acting injectable (LAI) formulations of the same antipsychotic on times to relapse following medication discontinuation.
Methods: Data were drawn from 3 similarly designed, multicenter, double-blind, placebo-controlled, randomized-withdrawal studies of paliperidone in adults with a schizophrenia diagnosis (according to DSM-IV criteria for ≥ 1 year before screening): once-daily extended-release oral paliperidone (ORAL paliperidone), once-monthly paliperidone palmitate (PP1M), and once-every-3-months paliperidone palmitate (PP3M). In a post hoc analysis, we compared median time to relapse across the treatment-withdrawal arms of the 3 studies using final analysis datasets. Time to relapse in the withdrawal arm of each study was examined using log-rank tests and Cox proportional hazards models.
Results: Four hundred forty-nine patients were withdrawn from 3 paliperidone formulations: 101 from ORAL paliperidone, 203 from PP1M, and 145 from PP3M. Postwithdrawal median (95% confidence interval [CI]) days to relapse were 58 days (42-114 days) for ORAL paliperidone, 172 days (134-222 days) for PP1M, and 395 days (274 days-not reached) for PP3M (P < .0001, pairwise comparisons). Relapse risk was significantly lower (P < .001) for patients who withdrew from either PP formulation relative to ORAL paliperidone and additionally for patients who withdrew from PP3M relative to PP1M.
Conclusions: Results demonstrate that 50% of patients who withdrew treatment from ORAL paliperidone, PP1M, or PP3M remained relapse free for approximately 2 months, 6 months, and 13 months, respectively. This may be relevant for risk mitigation strategies in schizophrenia, a condition in which interruptions in maintenance antipsychotic treatment are commonplace and unpredictable. LAI antipsychotic formulations may provide substantial delays over oral equivalents in times to relapse when patients discontinue therapy.
Trial Registration: ClinicalTrials.gov identifiers: NCT00086320, NCT00111189, and NCT01529515
J Clin Psychiatry 2017;78(7):e813-e820
https://doi.org/10.4088/JCP.16m11308
© Copyright 2017 Physicians Postgraduate Press, Inc.
aUptown Research Institute, LLC, Chicago, Illinois
bJanssen Scientific Affairs, LLC, Titusville, New Jersey
cSF-CARE, Inc, San Rafael, California
dJanssen Research and Development, Titusville, New Jersey
eDr Weiden is now an employee of Alkermes, Inc, Waltham, Massachusetts
*Corresponding author: Edward Kim, MD, MBA, Therapeutic Area Leader, Schizophrenia, Janssen Scientific Affairs, LLC, 1125 Trenton Harbourton Rd, Room A32405, Titusville, NJ 08560 ([email protected]).
Preventing or delaying relapse is a major goal in the treatment of schizophrenia.1,2 Relapse is at best disruptive2; at worst, it can be lethal.3 Studies show that relapse has a multitude of negative consequences to a person’s physiologic, psychological, and social well-being.1-3 Ideally, patients with schizophrenia should receive continuous antipsychotic maintenance therapy, an approach widely recognized as an important strategy for delaying relapse.4-7 When this is not possible, symptom-targeted and intermittent antipsychotic administration strategies have been used, but are associated with unacceptable increases in relapse risk and are therefore not recommended.8,9 Psychosocial approaches to reduce relapse risk include educating patients and their caregivers about the early warning signs of relapse, maintaining open lines of communication between the patient and clinical care team, and establishing advance directives.2,10-12 Antipsychotic formulations with longer half-lives may potentially delay relapse by providing continuous exposure well beyond the point of medication discontinuation.9,13-18
While the premise that relapse may be delayed longer after discontinuing a long-acting injectable (LAI) formulation than after discontinuing its oral formulation seems intuitive, no study has examined, to our knowledge, the relationship between the half-lives of antipsychotic formulations and time to relapse following discontinuation.
The antipsychotic paliperidone is available as once-daily extended-release oral paliperidone (ORAL paliperidone),19 once-monthly LAI paliperidone palmitate (PP1M),15 and once-every-3-months LAI paliperidone palmitate (PP3M).20 The same pharmacodynamic properties of paliperidone apply across the 3 formulations, but their pharmacokinetics differ.15,19,20 Following single-dose administration of ORAL paliperidone, paliperidone concentrations gradually rise to reach peak plasma concentrations approximately 24 hours postdose.19 The half-life of ORAL paliperidone is approximately 23 hours.19 Due to their extremely low solubility in water, PP1M and PP3M dissolve slowly after intramuscular injection before being hydrolyzed to paliperidone and absorbed into the systemic circulation.15,20 Therefore, the apparent (observed) elimination half-lives of PP1M and PP3M are based on their slow release from the muscle. Paliperidone release starts as early as day 1, peaks at day 13 (PP1M) or day 30 to 33 (PP3M), and is detectable for up to 126 days (PP1M) or 18 months (PP3M).15,20 The median apparent half-life of paliperidone following single-dose administration of PP1M over a 39-mg to 234-mg range is 25 to 49 days.15 The median apparent half-life of paliperidone following PP3M administration over a 273-mg to 819-mg range is 84 to 95 days and 118 to 139 days following deltoid and gluteal injections, respectively.20
Each of the 3 paliperidone formulations has been assessed for safety and efficacy in its own long-term, double-blind, randomized withdrawal study.21-23 In these studies, relapse was defined as 1 or more of the following: psychiatric hospitalization for schizophrenia symptoms; a predefined increase in the Positive and Negative Syndrome Scale (PANSS) total score24 for 2 consecutive assessments; an increase in prespecified individual PANSS item scores for 2 consecutive assessments; clinically significant deliberate self-injury or violent behavior resulting in suicide, injury, or significant damage; or suicidal or homicidal ideation and aggressive behavior.21-23 The protocols and patient populations of each study were nearly identical, including similar inclusion and exclusion criteria, stabilization criteria, and relapse criteria.21-23 Therefore, time to relapse data from the antipsychotic withdrawal group of each study could be used to evaluate whether differences in apparent half-lives provide clinically meaningful differences in time to relapse. We tested the hypothesis that patients who discontinue 1 of the long-acting formulations of paliperidone (PP1M or PP3M) will have a longer delay to relapse than those who discontinue the oral paliperidone formulation (ORAL paliperidone), and the duration of this delay will be proportional to length of half-life, with PP3M providing a longer delay than PP1M. We explored this hypothesis by conducting a post hoc exploratory analysis that compared times to first relapse in adults with schizophrenia after double-blind discontinuation from ORAL paliperidone, PP1M, or PP3M.

- Long-acting injectable (LAI) antipsychotic formulations may provide substantial benefits over oral equivalents in times to relapse when patients discontinue therapy.
- The LAI with the longest known half-life, once-every-3-months paliperidone palmitate, confers the most enduring relapse prevention and may represent a buffer against medication interruptions, providing clinicians and caregivers with an extended opportunity to ensure continued follow-up and treatment continuity.
METHODS
Analysis groups consisted of subjects who successfully completed the open-label stabilization phase of each study and were randomized to the placebo arm (and were therefore withdrawn from 1 of the 3 paliperidone formulations) for the double-blind relapse-prevention phase. Patients in each of the 3 placebo arms would have had therapeutic levels of paliperidone until randomized withdrawal.
Designs of the 3 Trials
This analysis used data from similarly designed, randomized, double-blind, placebo-controlled, relapse-prevention studies with ORAL paliperidone,21 PP1M,22 and PP3M.23 Each was a manufacturer-sponsored registration trial to support the long-term use of each formulation.21-23 Collectively, the studies spanned 10 years, with the ORAL paliperidone study conducted from 2004 to 2005, PP1M from 2005 to 2007, and PP3M from 2012 to 2014.21-23 Each study was approved by the local ethics committee, written informed consent was obtained, and the studies were registered at ClinicalTrials.gov (identifiers: NCT00086320, NCT00111189, and NCT01529515, respectively).
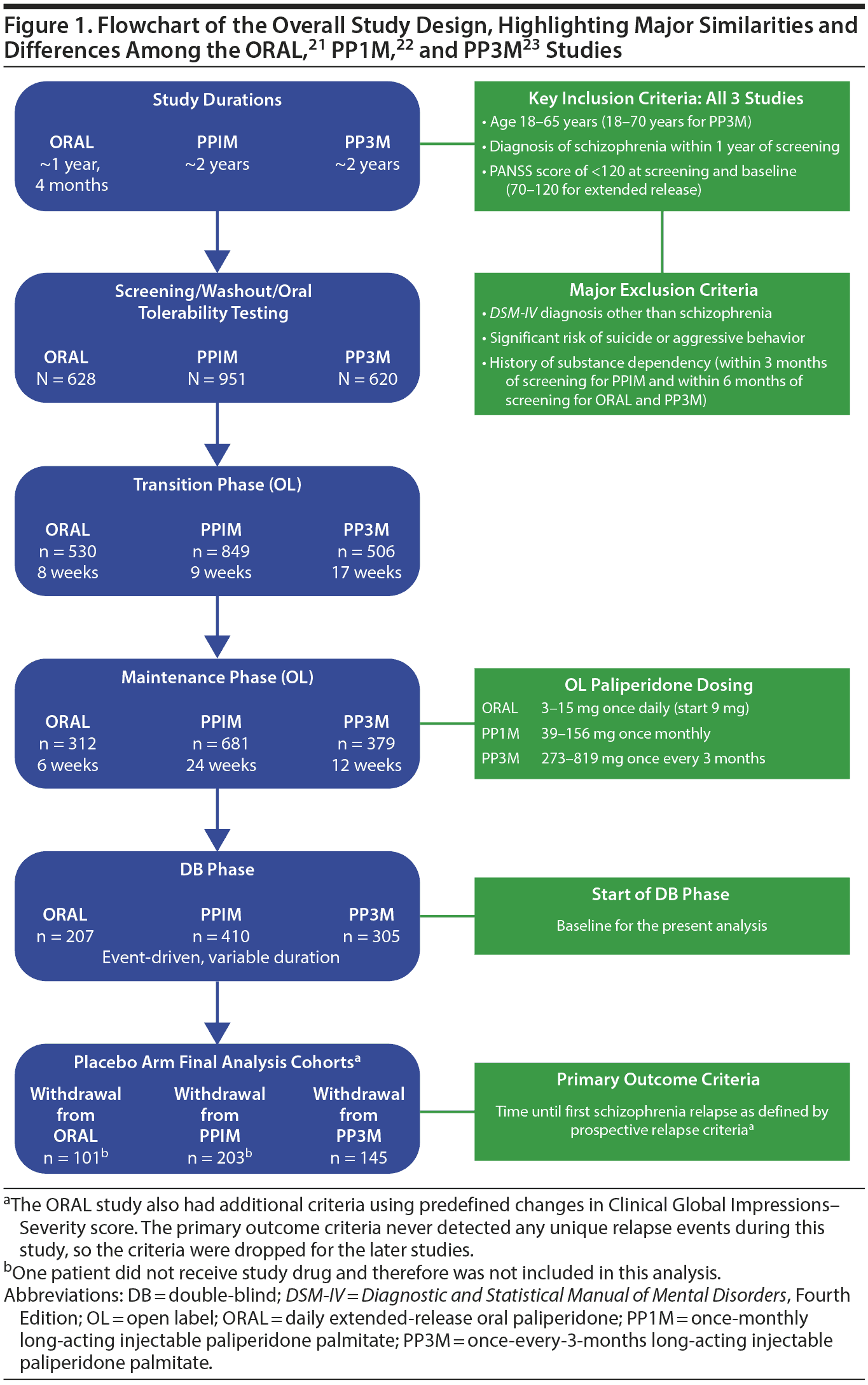
Shared elements of the overall study design and modifications and differences are shown in Figure 1. Each study had a screening phase, after which patients entered an open-label stabilization period ranging from 8 to 17 weeks (8 weeks for ORAL paliperidone, 9 weeks for PP1M, and 17 weeks for PP3M). Detailed information about the range of paliperidone dosing regimens used for each of the studies is described in Supplementary eTables 1-3 of eAppendix 1.
Stabilization criteria in each paliperidone study consisted of establishing a stable study drug dose with acute-symptom control, defined as a PANSS total score below a predetermined threshold (≤ 70 for the ORAL paliperidone study, ≤ 75 for PP1M, and < 70 for PP3M); PANSS scores of ≤ 4 (moderate or less) on selected individual items (P1 [delusions], P2 [conceptual disorganization], P3 [hallucinatory behavior], P6 [suspiciousness/persecution], P7 [hostility], G8 [uncooperativeness], and, for PP1M and PP3M only, G14 [poor impulse control]), in addition to a Clinical Global Impressions-Severity (CGI-S)25 score of ≤ 4 (moderately ill or better) for ORAL paliperidone only.21-23
Patients who met stabilization criteria were randomly assigned to continue treatment with the active paliperidone medication or were withdrawn from active paliperidone to placebo under double-blind conditions, continuing the same dosage schedule used at the end of the respective stabilization phase (daily for ORAL paliperidone, every 4 weeks for PP1M, and every 12 weeks for PP3M). The interval between the last dose of antipsychotic medication and initiation of placebo after randomization was based on the normal dosing schedule for each formulation studied: 1 day for ORAL paliperidone, 1 month for PP1M, and 3 months for PP3M. Patients remained in the double-blind phase until they relapsed or withdrew from the study, or until the study was terminated.21-23
Key Inclusion/Exclusion Criteria
Supplementary eTable 4 shows full inclusion and exclusion criteria for the 3 trials; Figure 1 presents the major criteria. Briefly, men and women aged 18-65 years (≤ 70 years in the PP3M trial) were eligible if they had a schizophrenia diagnosis according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria for ≥ 1 year before screening.21-23 Original DSM-IV criteria were used in the ORAL paliperidone and PP1M studies,21,22 and DSM-IV Text Revision criteria were used in the PP3M study.23 Criteria for a schizophrenia diagnosis were the same in the original DSM-IV and Text Revision editions. A total PANSS score < 120 at screening and baseline was another common criterion.21-23
All studies shared standard exclusion criteria such as DSM-IV diagnosis other than schizophrenia and other standard medical or psychiatric exclusion criteria (see eAppendix 1). There were some differences between studies in history of long-acting formulations. Patients were also excluded if they used a 4-week depot antipsychotic within 28 days (PP1M study)22 or within 120 days (ORAL paliperidone study)21 of screening. In the PP3M study, symptomatically stable patients could transition from another LAI antipsychotic to PP1M before transitioning to PP3M if there was a clinical reason to switch medications.23
Study End Points
The primary outcome measure for all studies was time until first schizophrenia relapse, as defined by Csernansky et al26 (Figure 2). Patients were considered to have relapsed if they met 1 or more of the following: psychiatric hospitalization for schizophrenia symptoms; a predefined increase in the PANSS total score for 2 consecutive assessments; an increase in prespecified individual PANSS item scores for 2 consecutive assessments; clinically significant deliberate self-injury or violent behavior resulting in suicide, injury, or significant damage; or suicidal or homicidal ideation and aggressive behavior.21-23
Relevant secondary efficacy measures were changes from double-blind baseline to end point in the PANSS total and factor, CGI-S, and Personal and Social Performance (PSP) scale scores.21-23 Safety measures and results for each study were reported previously21-23 and were not included in the analysis.
During each study, an independent data-monitoring committee performed a preplanned interim analysis after a predefined number of relapse events (ORAL paliperidone, no. = 43; PP1M, no. = 68; and PP3M, no. = 42). The study was terminated early if efficacy was established at this interim analysis at a prespecified level of significance (.01, .0106, and .0101 for ORAL paliperidone, PP1M, and PP3M, respectively). A final analysis evaluating all events that occurred by study termination was conducted as a supporting analysis in each study.21-23
Primary Outcome Measure for Post Hoc Analysis
Time to first relapse after antipsychotic discontinuation was explored in the 3 cohorts of patients who were symptomatically stable and received a paliperidone formulation during the open-label transition and maintenance phases. During the double-blind phase of their respective studies, active drug was discontinued and patients were assigned to placebo.
Statistical Methods
Each study was terminated early for efficacy following an interim analysis. Because the interim analysis demonstrated a statistically significant difference in favor of study drugs compared with placebo, with regard to the time to relapse, the independent data-monitoring committee recommended stopping the trial for efficacy in all 3 studies.
The final data analysis, which included data points subsequent to the interim analysis data cutoff and cumulative up to the date of study completion, was considered the final database use for this study. The double-blind intent-to-treat population, including all randomly assigned patients who received ≥ 1 dose of double-blind study drug, was used for this analysis. Only those patients randomly assigned to placebo were included. Demographic and baseline characteristics were summarized using descriptive statistics for the double-blind phase of each study and were compared using analysis of variance or χ2 tests for continuous and categorical variables, respectively, to identify potential confounders.
The cumulative distribution function of time to relapse was estimated using the Kaplan-Meier method, and time to relapse among studies was evaluated using a log-rank test. Differences in relapse risk among trials were evaluated using Cox proportional hazards models. Estimates of hazard ratios and 95% confidence intervals (CIs) among studies were provided. Patient characteristics that differed (P < .2) between the groups at baseline were included as covariates in the analysis to increase statistical power and to examine the influence of these baseline differences on analysis results. The impact of baseline prognostic factors and parametric Cox regression models were evaluated using differences in log-likelihoods. Model fits and diagnostics were examined for violation of the assumption of proportional hazards, influential data points, and nonlinearity. Reasons for relapse were summarized. No adjustment was made for multiplicity.
RESULTS
Patient Characteristics
This post hoc analysis includes data from 101, 203, and 145 patients randomly assigned to the double-blind placebo arms of the ORAL paliperidone, PP1M, and PP3M studies, respectively. Baseline demographic and disease characteristics were generally well-balanced across studies (Supplementary eTable 5). Patients were predominantly white (60%-66%) with mean ages ranging from 37.5 to 39.4 years. In each group, schizophrenia diagnosis occurred in the mid-to-late 20s. As indicated by mean ± SD total PANSS scores, symptom severity appeared comparable in the ORAL paliperidone (53.4 ± 10.6), PP1M (53.1 ± 11.9), and PP3M (54.2 ± 9.3) placebo arms at randomization (P = .642) and was consistent with symptomatic stabilization.21-23 Small but statistically significant differences were observed across arms for gender, race, mean baseline PSP scale scores, and number of prior hospitalizations; these differences were not considered clinically meaningful.
Baseline demographics and clinical characteristics of all patients who entered the double-blind phase and were randomly assigned to placebo are shown in Supplementary eTable 6.
Time to First Relapse After Initiation of Placebo
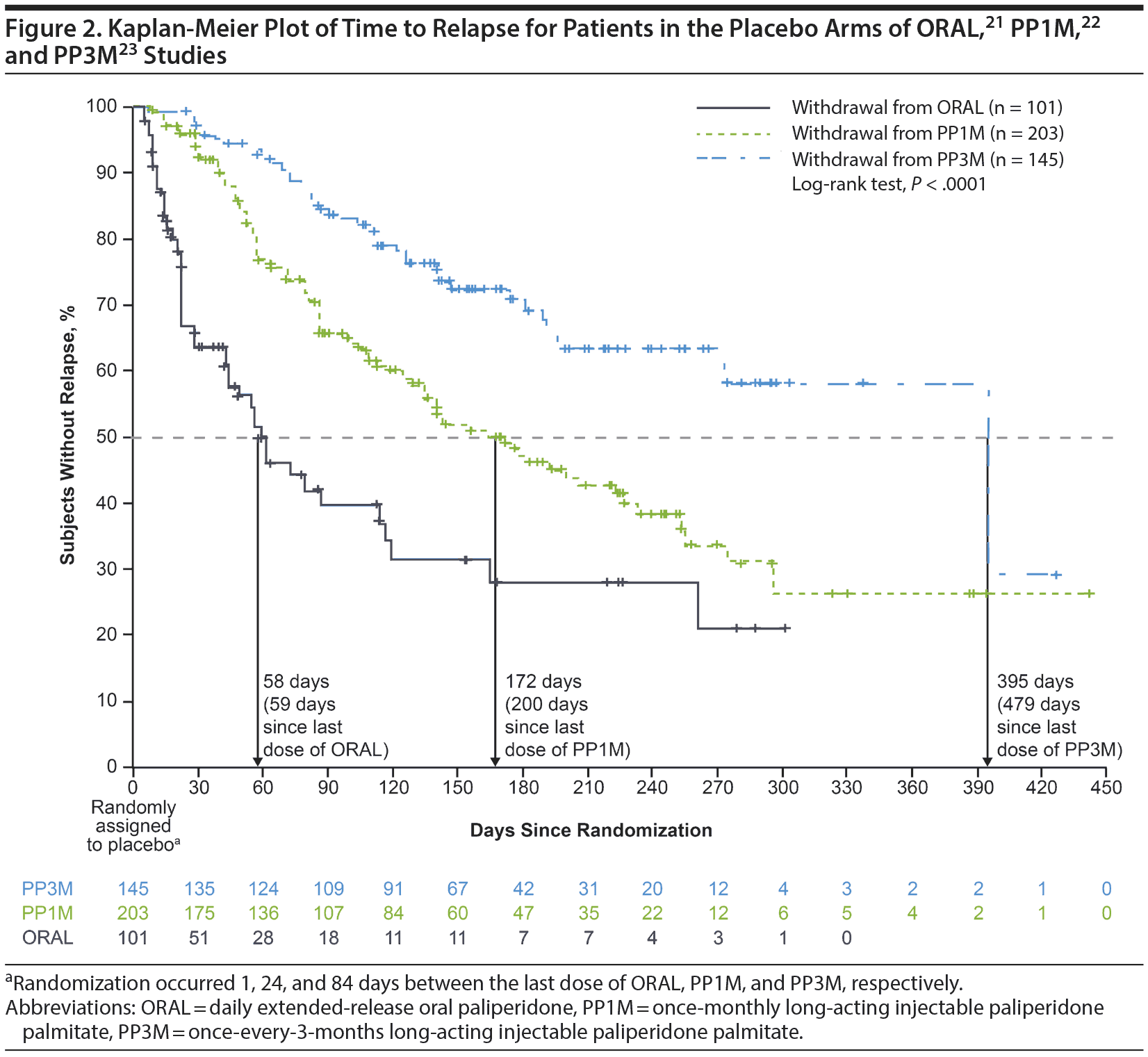
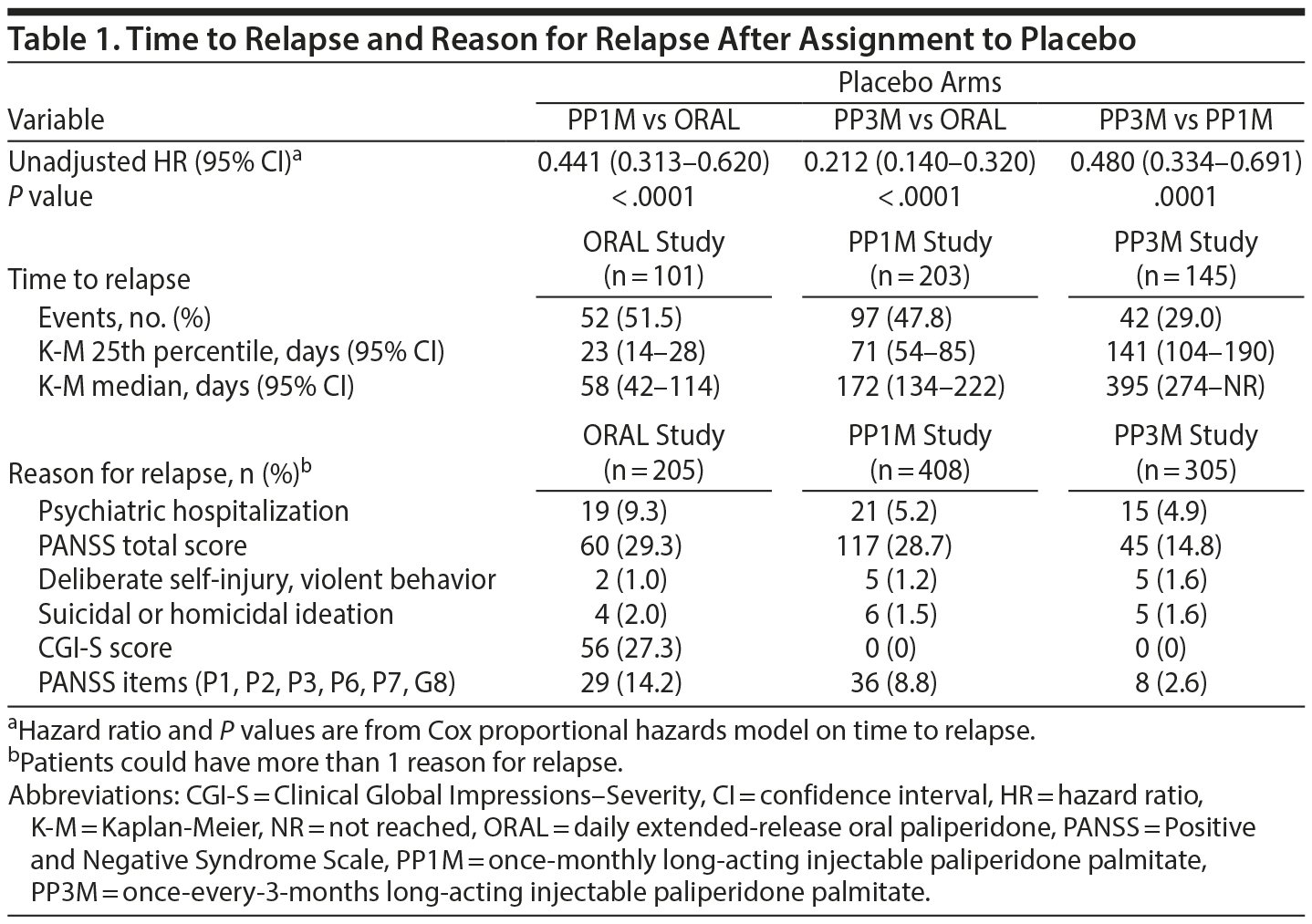
In the placebo arms of the respective studies, median time from double-blind baseline to relapse differed significantly in the final analysis set: 58 days (95% CI, 42-114 days) for ORAL paliperidone, 172 days (95% CI, 134-222 days) for PP1M, and 395 days (95% CI, 274 days-not reached) for PP3M (P < .0001, pairwise comparisons; Figure 2, Table 1). These data indicate that withdrawal from either PP1M or PP3M was associated with delayed time to relapse relative to that of ORAL paliperidone. Further comparison shows that patients in the PP3M withdrawal group remained stable for longer than those in the PP1M withdrawal group. Relapse risk was 56% lower for patients discontinuing PP1M than for those discontinuing ORAL paliperidone (P < .001), 79% lower for patients discontinuing PP3M than for those discontinuing ORAL paliperidone (P < .001), and 52% lower for patients discontinuing PP3M than for those discontinuing PP1M (P < .001) (Figure 2, Table 1).
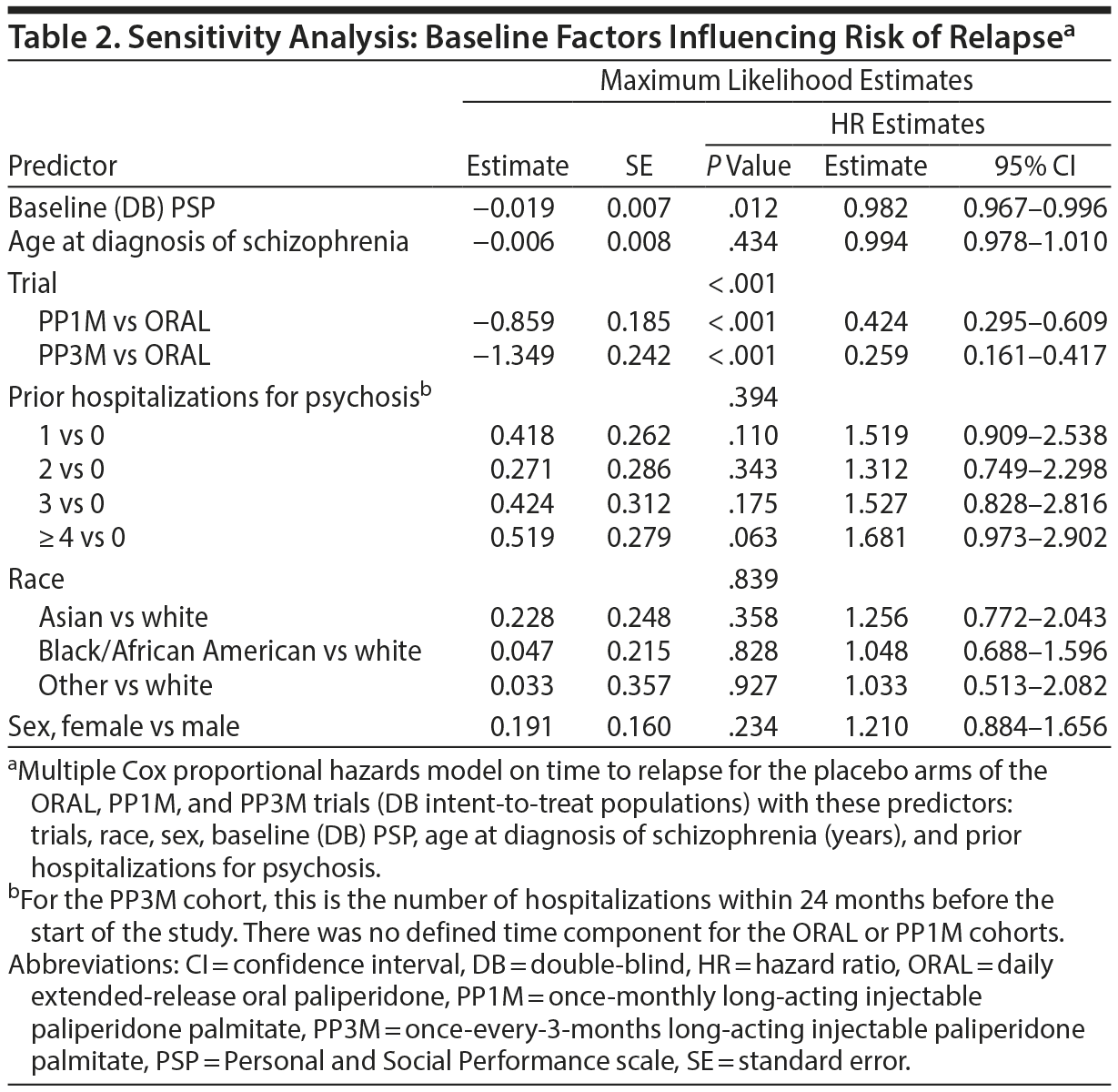
Sensitivity analyses and tests of model assumptions indicated that these results were robust. Baseline (prerandomization) PSP scores and number of prior hospitalizations for psychosis differentially affected risk of relapse (Table 2), indicating that these variables could potentially confound the interpretation of the data from time-to-event analysis for the overall study population. Cox proportional hazards models using these factors as covariates yielded similar results, further demonstrating the robustness of findings (Table 1, Table 2, and Supplementary eTable 7). Model fits and diagnostics were examined for violation of the assumption of proportional hazards, influential data points, and nonlinearity. Multicollinearity among the predictors was also assessed. The assumptions of constant hazard ratio among the groups were confirmed (Supplementary eTable 7, Supplementary eFigure 1).
Reason for Relapse
Symptom exacerbation as reflected by increase in PANSS total score was the most common reason for relapse in each trial (Table 1). Increases in PANSS total scores were the main reason for relapse in 29.3%, 28.7%, and 14.8% of patients, respectively, in the ORAL paliperidone, PP1M, and PP3M studies. Other reasons for relapse varied across trials (Table 1).
DISCUSSION
To our knowledge, this is the first analysis comparing the effects of 3 distinct LAI antipsychotic formulations on the risk and timing of relapse following antipsychotic discontinuation. This post hoc analysis, which included data from 3 similarly designed studies,21-23 compared times to first relapse for adults with schizophrenia who were withdrawn to placebo after double-blind treatment with ORAL paliperidone, PP1M, or PP3M. Postwithdrawal median (95% CI) relapse times were 58 days (42-114 days) for ORAL paliperidone, 172 days (134-222 days) for PP1M, and 395 days (274 days-not reached) for PP3M.
Because individual patients may have different relapse trajectories, these times to relapse should be considered estimates rather than precise predictions. The relapse risk (hazard ratio) was 2.27-fold higher for patients discontinuing ORAL paliperidone than for those discontinuing PP1M, 4.71-fold higher for patients discontinuing ORAL paliperidone than for those discontinuing PP3M, and 2.08-fold higher for patients discontinuing PP1M than for those discontinuing PP3M (Table 1). These relative risk reductions are conservative in that they assess only time since randomization to placebo in the double-blind phase and do not include time since the last dose of medication in the maintenance phase.
Our findings are consistent with the expectation that longer half-lives are associated with longer periods of relapse-free clinical stability following discontinuation. Both the PP1M and PP3M formulations were associated with a longer time to relapse than the oral formulation. These results are in agreement with interim analyses of the individual studies, in which median times from double-blind baseline to relapse were 62, 163, and 274 days, respectively, with ORAL paliperidone,21 PP1M,22 and PP3M.23 Patients randomized to the PP3M placebo arm also had a longer median time to relapse than those randomized to the PP1M placebo arm, again consistent with the hypothesis that longer half-life is associated with longer time to relapse after discontinuation, even among LAI formulations.13
Presently, there is no established or consensus definition of relapse for schizophrenia. Historically, hospitalization was the hallmark of relapse,27,28 but over the past 10 to 20 years, the definition of relapse has evolved. The definition of relapse used in the 3 randomized controlled paliperidone studies, which served as the basis for this post hoc analysis, was based on regulatory guidelines and is more rigorous than definitions used a few decades ago.21-23 Relapse-prevention studies are now designed to detect early signs of impending relapse (eg, suicidal behavior and ideation, increased PANSS item scores), so that at-risk subjects are immediately discontinued from the study and optimal treatment interventions are instituted. Differences in the definition of relapse used in older studies preclude comparison to the present findings. A strength of our analysis is that all 3 paliperidone studies used the same definition of relapse, allowing the comparison of relapse data across studies.
Continuous exposure to antipsychotic medication is key to effective long-term schizophrenia treatment because it provides sustained symptom control and optimizes clinical and psychosocial outcomes.4,5,12,29 Nevertheless, patients with schizophrenia often have difficulty maintaining consistent medication adherence,27,30-32 increasing relapse risk and its negative consequences.27,32 Given the prevalence and seriousness of relapse in schizophrenia, relapse risk mitigation may be of benefit when medication discontinuation cannot be prevented.2,12 One risk-mitigation approach is asking patients to continue antipsychotic medication with a gradual down-titration schedule rather than abrupt medication discontinuation,2,33 thereby enabling patients to retain medication as part of their treatment plan while still benefiting from a reduced dose and closely monitoring for emerging relapse. It also preserves the therapeutic relationship between health care provider and patient and provides immediate access to crisis services after medication cessation, making it easier for patients to seek help during early stages of relapse.2,11 While a gradual dose reduction is possible with oral therapy, patients who decide to stop oral medication often do so without informing their treatment team. On the other hand, those treated with LAIs will continue to have slowly diminishing levels of medication until they can be persuaded to resume antipsychotic therapy.
This analysis has several limitations. First, this was a post hoc analysis of data from 3 separate studies. Although the patient populations and study designs were nearly identical, the studies were not designed to assess time to relapse after withdrawal from active treatment. Second, although the studies had similar designs, they differed in the duration of paliperidone exposure during the open-label lead-in phases, length of follow-up during the double-blind phases, and timing of interim analyses. The stabilization phases were different lengths in the 3 studies and evaluated different equivalent dose ranges of paliperidone. Most notably, the range of PP1M doses evaluated was slightly lower than that of the other paliperidone formulations. However, sensitivity analyses controlling for observed relapse risk factors only modestly reduced the magnitude of differences observed in our primary analysis.
In conclusion, results of this post hoc analysis demonstrate that 50% of patients who withdrew treatment from ORAL paliperidone, PP1M, or PP3M remained relapse free for approximately 2 months, 6 months, and 13 months, respectively. This observation may be relevant for risk mitigation strategies in schizophrenia, a condition in which interruptions in maintenance antipsychotic treatment are commonplace and unpredictable. Of the 3 formulations evaluated, PP3M conferred the most enduring relapse prevention and may represent a buffer against medication interruptions, providing clinicians and caregivers with an extended opportunity to ensure continued follow-up and treatment continuity.
Submitted: October 27, 2016; accepted March 22, 2017.
Online first: June 20, 2017.
Potential conflicts of interest: At the time of this analysis, Dr Weiden was an employee of Uptown Research Institute, LLC, but is now a full-time employee of Alkermes, Inc. Prior to his employment with Alkermes, he was on the speaker bureaus of Alkermes, Forum, Janssen, Lundbeck, Otsuka, and Sunovion; was a consultant for Allergan, Alkermes, Delpor, Forum, Janssen, Lundbeck, Otsuka, Novartis, Sunovion, Teva, and Vanda; received research funding for clinical trials (as investigator or sub-investigator with Uptown Research Institute, LLC) from Allergan, Alkermes, Boehringer-Ingelheim, Forum, Intracellular, Janssen, Neurocrine, Otsuka, Reckitt Benckiser, and Takeda; and is a Delpor stockholder. His financial conflicts of interest had ended and his work on conceptualizing, writing, and revising this manuscript was completed prior to his employment with Alkermes (June 2016). Dr Kim is an employee of Janssen Scientific Affairs, LLC, and a Johnson & Johnson stockholder. Dr Bermak was a principal investigator on the R092670-PSY-3012 study (NCT01529515), has received research funding for this and other Janssen clinical trials, and has received honoraria for his participation in the Janssen speaker bureau. Drs Turkoz and Gopal are employees of Janssen Research and Development, LLC, and are Johnson & Johnson stockholders. Dr Berwaerts is a former employee of Janssen Research and Development, LLC.
Funding/support: This study was funded by Janssen Scientific Affairs, LLC.
Role of the sponsor: This study was funded by Janssen Scientific Affairs, LLC, which was responsible for the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. Writing and editorial assistance was also funded by Janssen Scientific Affairs, LCC.
Acknowledgments: The authors thank Matthew Grzywacz, PhD, Lynn Brown, PhD, and Marguerite York, PhD (ApotheCom, Yardley, Pennsylvania), for their writing and editorial assistance, which was funded by Janssen.
Additional information: Janssen makes its trial data available through the Yale Open Data Access (YODA) project. Requests can be made at: http://yoda.yale.edu.
Supplementary material: See accompanying pages.
REFERENCES
1. Kane JM. Treatment strategies to prevent relapse and encourage remission. J Clin Psychiatry. 2007;68(suppl 14):27-30. PubMed
2. Kane JM. Improving patient outcomes in schizophrenia: achieving remission, preventing relapse, and measuring success. J Clin Psychiatry. 2013;74(9):e18. PubMed doi:10.4088/JCP.12117tx1c
3. Kennedy JL, Altar CA, Taylor DL, et al. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29(2):63-76. PubMed doi:10.1097/YIC.0b013e32836508e6
4. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103. PubMed doi:10.1093/schbul/sbp130
5. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93. PubMed doi:10.1093/schbul/sbp116
6. Schooler NR. Maintenance medication for schizophrenia: strategies for dose reduction. Schizophr Bull. 1991;17(2):311-324. PubMed doi:10.1093/schbul/17.2.311
7. Schooler NR, Keith SJ, Severe JB, et al. Relapse and rehospitalization during maintenance treatment of schizophrenia: the effects of dose reduction and family treatment. Arch Gen Psychiatry. 1997;54(5):453-463. PubMed doi:10.1001/archpsyc.1997.01830170079011
8. Wistedt B, Wiles D, J׸rgensen A. A depot neuroleptic withdrawal study neurological effects. Psychopharmacology (Berl). 1983;80(2):101-105. PubMed doi:10.1007/BF00427950
9. De Hert M, Sermon J, Geerts P, et al. The use of continuous treatment versus placebo or intermittent treatment strategies in stabilized patients with schizophrenia: a systematic review and meta-analysis of randomized controlled trials with first- and second-generation antipsychotics. CNS Drugs. 2015;29(8):637-658. PubMed doi:10.1007/s40263-015-0269-4
10. Hamann J, Mischo C, Langer B, et al. Physicians’ and patients’ involvement in relapse prevention with antipsychotics in schizophrenia. Psychiatr Serv. 2005;56(11):1448-1450. PubMed doi:10.1176/appi.ps.56.11.1448
11. Sariah AE, Outwater AH, Malima KI. Risk and protective factors for relapse among individuals with schizophrenia: a qualitative study in Dar es Salaam, Tanzania. BMC Psychiatry. 2014;14:240. PubMed doi:10.1186/s12888-014-0240-9
12. Kane JM, Leucht S, Carpenter D, et al; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64(suppl 12):5-19. PubMed
13. Zhornitsky S, Stip E. Oral versus long-acting injectable antipsychotics in the treatment of schizophrenia and special populations at risk for treatment nonadherence: a systematic review. Schizophr Res Treatment. 2012;2012:407171. PubMed doi:10.1155/2012/407171
14. Abilify (aripiprazole) [package insert]. Tokyo, Japan: Ostuka Pharmaceutical Co Ltd; 2017.
15. Invega Sustenna (paliperidone palmitate) extended-release injectable suspension, for intramuscular use [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2017.
16. Risperdal Consta (risperidone) long-acting injection [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2016.
17. Grundmann M, Kacirova I, Urinovska R. Therapeutic drug monitoring of atypical antipsychotic drugs. Acta Pharm. 2014;64(4):387-401. PubMed doi:10.2478/acph-2014-0036
18. Zyprexa Relprevv (olanzapine) for extended release injectable suspension [package insert]. Indianapolis, IN: Eli Lilly and Company; 2017.
19. Invega (paliperidone) extended-release tablets [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2017.
20. Invega Trinza (paliperidone palmitate) extended-release injectable suspension, for intramuscular use [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc; 2017.
21. Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2007;27(1):6-14. PubMed doi:10.1097/JCP.0b013e31802dda4a
22. Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2-3):107-117. PubMed doi:10.1016/j.schres.2009.10.026
23. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. PubMed doi:10.1001/jamapsychiatry.2015.0241
24. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
25. Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
26. Csernansky JG, Mahmoud R, Brenner R; Risperidone-USA-79 Study Group. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346(1):16-22. PubMed doi:10.1056/NEJMoa002028
27. Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull. 1995;21(3):419-429. PubMed doi:10.1093/schbul/21.3.419
28. Falloon IR, Marshall GN, Boyd JL, et al. Relapse in schizophrenia: a review of the concept and its definitions. Psychol Med. 1983;13(3):469-477. PubMed doi:10.1017/S0033291700047899
29. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063-2071. PubMed doi:10.1016/S0140-6736(12)60239-6
30. Mojtabai R, Lavelle J, Gibson PJ, et al. Gaps in use of antipsychotics after discharge by first-admission patients with schizophrenia, 1989 to 1996. Psychiatr Serv. 2002;53(3):337-339. PubMed doi:10.1176/appi.ps.53.3.337
31. Valenstein M, Blow FC, Copeland LA, et al. Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophr Bull. 2004;30(2):255-264. PubMed doi:10.1093/oxfordjournals.schbul.a007076
32. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891. PubMed doi:10.1176/appi.ps.55.8.886
33. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56. PubMed
This PDF is free for all visitors!