Objective: To assess the antipsychotic efficacy and safety of a combination of olanzapine and samidorphan (OLZ/SAM).
Methods: This 4-week, phase 3, randomized, double-blind, placebo- and olanzapine-controlled study was conducted from December 2015 to June 2017 in adults with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria who were experiencing an acute exacerbation. Patients were randomized 1:1:1 to OLZ/SAM, olanzapine monotherapy, or placebo. The primary and key secondary efficacy endpoint assessed was the change in Positive and Negative Syndrome Scale (PANSS) total score and Clinical Global Impressions-Severity of Illness Scale (CGI-S) score between baseline and week 4, respectively, for OLZ/SAM versus placebo. Safety monitoring occurred throughout.
Results: 401 patients received ≥ 1 dose of study drug; 352 completed treatment. Treatment with OLZ/SAM resulted in significant improvements versus placebo in PANSS total and CGI-S scores from baseline to week 4 (least squares [LS] mean ± SE: −6.4 ± 1.8 [P < .001] and −0.38 ± 0.12 [P = .002], respectively). Olanzapine treatment resulted in similar improvements (PANSS and CGI-S LS mean ± SE of −5.3 ± 1.84 [P = .004] and −0.44 ± 0.12 [P < .001], respectively). Adverse events (AEs) occurred in 54.5%, 54.9%, and 44.8% of patients on OLZ/SAM, olanzapine, and placebo, respectively. Weight gain, somnolence, dry mouth, anxiety, and headache were the most common AEs (ie, ≥ 5%) with active treatment.
Conclusions: OLZ/SAM treatment resulted in statistically and clinically significant efficacy improvements over 4 weeks versus placebo in adults with acutely exacerbated schizophrenia. Improvements were similar to those observed with olanzapine. OLZ/SAM was well tolerated, with a safety profile similar to that of olanzapine.
Trial Registrations: ClinicalTrials.gov identifier: NCT02634346; EudraCT number: 2015-003373-15‘ ‹’ ‹
ABSTRACT
Objective: To assess the antipsychotic efficacy and safety of a combination of olanzapine and samidorphan (OLZ/SAM).
Methods: This 4-week, phase 3, randomized, double-blind, placebo- and olanzapine-controlled study was conducted from December 2015 to June 2017 in adults with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria who were experiencing an acute exacerbation. Patients were randomized 1:1:1 to OLZ/SAM, olanzapine monotherapy, or placebo. The primary and key secondary efficacy endpoint assessed was the change in Positive and Negative Syndrome Scale (PANSS) total score and Clinical Global Impressions-Severity of Illness Scale (CGI-S) score between baseline and week 4, respectively, for OLZ/SAM versus placebo. Safety monitoring occurred throughout.
Results: 401 patients received ≥ 1 dose of study drug; 352 completed treatment. Treatment with OLZ/SAM resulted in significant improvements versus placebo in PANSS total and CGI-S scores from baseline to week 4 (least squares [LS] mean ± SE: −6.4 ± 1.8 [P < .001] and −0.38 ± 0.12 [P = .002], respectively). Olanzapine treatment resulted in similar improvements (PANSS and CGI-S LS mean ± SE of −5.3 ± 1.84 [P = .004] and −0.44 ± 0.12 [P < .001], respectively). Adverse events (AEs) occurred in 54.5%, 54.9%, and 44.8% of patients on OLZ/SAM, olanzapine, and placebo, respectively. Weight gain, somnolence, dry mouth, anxiety, and headache were the most common AEs (ie, ≥ 5%) with active treatment.
Conclusions: OLZ/SAM treatment resulted in statistically and clinically significant efficacy improvements over 4 weeks versus placebo in adults with acutely exacerbated schizophrenia. Improvements were similar to those observed with olanzapine. OLZ/SAM was well tolerated, with a safety profile similar to that of olanzapine.
Trial registrations: ClinicalTrials.gov identifier: NCT02634346; EudraCT number: 2015-003373-15
J Clin Psychiatry 2020;81(2):19m12769
To cite: Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 Study. J Clin Psychiatry. 2020;81(2):19m12769.
To share: https://doi.org/10.4088/JCP.19m12769
© Copyright 2020 Physicians Postgraduate Press, Inc.
aSchool of Medicine, University of California, Irvine, California
bAltea Research, Las Vegas, Nevada
cExcell Research, Oceanside, California
dAlkermes, Inc, Waltham, Massachusetts
eAlkermes Pharma Ireland Limited, Dublin, Ireland
*Corresponding author: David McDonnell, MD, Alkermes Pharma Ireland Limited, Connaught House, 1 Burlington Rd, Dublin D04 C5Y6, Ireland ([email protected]).
Olanzapine is one of the most efficacious antipsychotic agents available for the treatment of schizophrenia.1-4 Its success is exemplified in long-term studies,2-4 in which rates of all-cause discontinuation and discontinuation due to lack of efficacy were lower for patients on olanzapine than other antipsychotics. However, significant safety concerns regarding weight gain and associated metabolic dysregulation have limited the clinical use of olanzapine.5,6 To date, there have been no approved pharmacologic interventions that fundamentally change the benefit-risk profile of olanzapine.7-9
The underlying mechanisms of antipsychotic-induced weight gain and associated metabolic derangements are not understood. Past research has implicated antipsychotic medication effects at serotonin (5-HT)-2C,10 H1,11 and M3-muscarinic12 receptors, as well as increases in leptin levels,13 among others, as potential causes. As a result, various strategies have been employed to reverse or prevent such effects. A 2010 meta-analysis9 of 32 studies investigating 15 different pharmacologic interventions found that metformin, fenfluramine, sibutramine, topiramate, and reboxetine provided statistically significantly greater weight loss versus placebo, although the risk reduction was modest and the evidence inadequate to recommend broad use of any specific intervention.
Preclinical evidence suggests a critical role for the opioid system in modulating feeding behavior and metabolism. For example, decrease in weight gain has been reported in µ-, κ-, and δ-opioid receptor knockout mice despite no differences in caloric intake in µ- and κ-opioid receptor knockout mice compared with wild-type mice.14-16 Therefore, adding an opioid antagonist to a central nervous system-active drug known to cause weight gain such as olanzapine may mitigate weight gain and associated metabolic dysregulation from olanzapine use. Samidorphan is a new molecular entity that, in vitro, binds with high affinity to human μ-, κ-, and δ-opioid receptors and acts as an antagonist at μ-opioid receptors and partial agonist at κ- and δ-opioid receptors.17,18 In vivo, samidorphan has been demonstrated to function as an opioid receptor antagonist.19 The combination of olanzapine and samidorphan (OLZ/SAM) is intended to provide the antipsychotic efficacy of olanzapine while mitigating weight gain associated with olanzapine; samidorphan does not bind to any receptors other than µ-, κ-, or δ-opioid receptors18 and, as such, would not be expected to have antipsychotic properties. In a phase 1 study in healthy volunteers, weight gain was significantly lower in those treated with OLZ/SAM than those treated with olanzapine monotherapy.20 In a subsequent phase 2 study21 in patients with schizophrenia who were clinically stable (defined as a Positive and Negative Syndrome Scale [PANSS] score ≤ 80 and a Clinical Global Impressions-Severity of Illness Scale [CGI-S] score ≤ 3 at screening), the antipsychotic efficacy of OLZ/SAM was similar to that of olanzapine; the presence of samidorphan mitigated olanzapine-associated weight gain, although a difference in weight gain was not observed until after 4 weeks of treatment.
Here, we report findings from a phase 3 study evaluating the antipsychotic efficacy and safety of OLZ/SAM compared with placebo in patients with an acute exacerbation of schizophrenia, a more severely ill patient group than that studied in phase 2. The study was performed to determine if the antipsychotic efficacy of olanzapine would be adversely affected by the addition of samidorphan. An olanzapine active control arm was also included to confirm validity of the study.

- ENLIGHTEN-1 is a phase 3 study that evaluated OLZ/SAM, a combination of olanzapine and the opioid antagonist samidorphan, versus placebo in patients with an acute schizophrenia exacerbation; an olanzapine arm was included for assay sensitivity.
- PANSS total and CGI-S score reductions with OLZ/SAM were significant versus placebo and similar to those with olanzapine versus placebo.
- If approved for use, OLZ/SAM may be an option for patients experiencing an acute exacerbation of schizophrenia; it appears to retain the antipsychotic efficacy of olanzapine with the added benefit of mitigated weight gain, which would support long-term treatment.
METHODS
This multicenter study (ClinicalTrials.gov identifier: NCT02634346; European Union Drug Regulating Authority Clinical Trials [EudraCT] Database number: 2015-003373-15) was conducted in the United States and Europe from December 2015 to June 2017 and in accordance with the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice. The study protocols, amendments, and informed consent forms were approved by an independent ethics committee/institutional review board for each site. All patients provided written informed consent before entering the study.
Study Design and Treatments
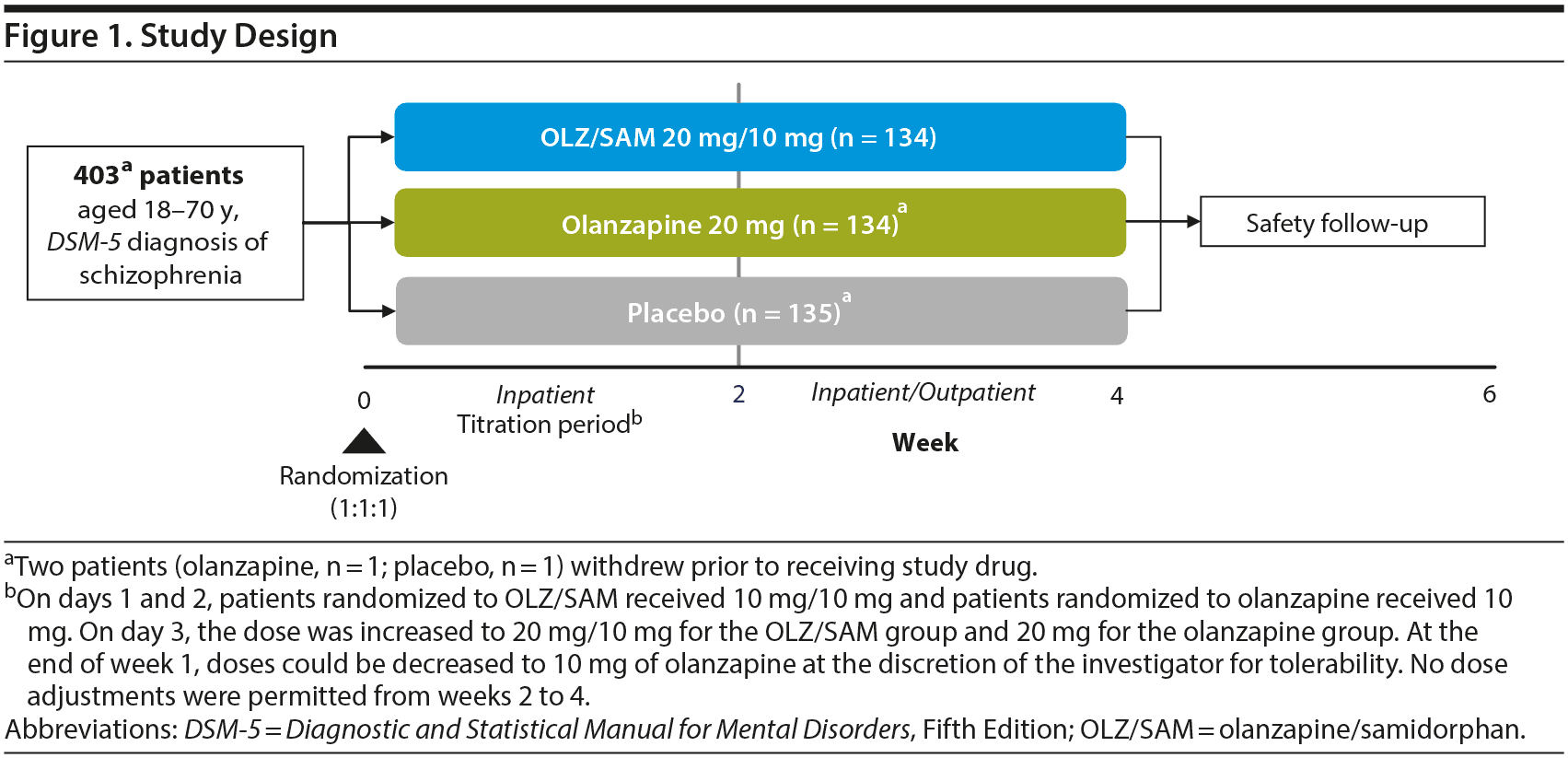
This was a 4-week, phase 3, double-blind, randomized, active- (olanzapine) and placebo-controlled study of OLZ/SAM in patients experiencing an acute exacerbation of schizophrenia. The study consisted of a screening phase of up to 10 days (Visit 1) during which prior antipsychotic treatment was discontinued, a 4-week treatment period (Visits 2−6) that included 2 weeks of inpatient treatment (dose titration permitted) followed by 2 weeks of inpatient/outpatient treatment (fixed dose), and a follow-up (Visit 7) on day 43 (Figure 1). OLZ/SAM, olanzapine, or placebo was administered as a single, coated bilayer tablet. For patients in the active treatment arms (olanzapine and OLZ/SAM), the dose of olanzapine was 10 or 20 mg (target of 20 mg). For patients in the OLZ/SAM group, the samidorphan dose was fixed at 10 mg, based on a previous phase 2 study in which 10 mg of samidorphan added to olanzapine provided significant mitigation of weight gain that was numerically greater than that observed with 5 mg of samidorphan, and comparable to that with 20 mg of samidorphan with a better safety profile.21
Patients were randomized 1:1:1 to OLZ/SAM, olanzapine, or placebo administered orally, once daily for up to 4 weeks. As the enrolled patients were acutely psychotic upon study entry and current treatments were not working, ongoing antipsychotic medications were stopped at Visit 2 (randomization), and treatment with study medication commenced immediately. There was no tapering of prior antipsychotic medication. On days 1 and 2, patients randomized to OLZ/SAM received 10 mg/10 mg, and patients randomized to olanzapine received 10 mg. On day 3, the dose was increased to 20 mg/10 mg for the OLZ/SAM group and 20 mg for the olanzapine group. At the end of week 1, doses could be decreased for tolerability to 10 mg of olanzapine at the discretion of the investigator. Thereafter, no dose adjustments were permitted from weeks 2 to 4. All clinical staff, patients, and caregivers were blinded to treatment assignment until database lock.
Patients were required to be inpatients for the first 2 weeks of the treatment period, and those who met the criteria for discharge could then complete the remaining 2 weeks on either an inpatient or outpatient basis. However, patients were encouraged to remain as inpatients for all 4 weeks.
Patients who completed the full 4-week treatment period were eligible for a 52-week, open-label, long-term safety extension study. If patients discontinued early or did not enter the long-term safety study, they were monitored for 2 weeks of safety follow-up after the last dose of treatment.
Patients
Patients included in the study were adults aged 18 to 70 years with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),22 diagnosis of schizophrenia who met criteria for an acute exacerbation or relapse of schizophrenia symptoms, a PANSS23 score ≥ 80 with a score ≥ 4 on at least 3 of the following items from the PANSS: Item 1, delusions; Item 2, conceptual disorganization; Item 3, hallucinatory behavior; and Item 6, suspiciousness/persecution, and a CGI-S24 score ≥ 4 at baseline and screening. Patients were required to have a body mass index (BMI) of 18.0 to 40.0 kg/m2 and to abide by contraception methods stipulated in the protocol. Key exclusion criteria included the presence of a clinically significant or unstable medical illness, condition, or disorder that could potentially compromise patient safety; a history of diabetes; moderate or severe alcohol or drug use disorder currently or during the 3 months prior to screening; a positive urine drug screen for opioids, amphetamine/methamphetamine, phencyclidine, or cocaine at screening; or an assessment that the patient was at risk for suicide. Previous exposure to olanzapine, mesoridazine, chlorpromazine, thioridazine, or a long-acting injectable antipsychotic medication within 6 months prior to screening (with the exception of those receiving 3-month paliperidone, which must not have been received within 12 months of screening) was also exclusionary. Patients were excluded if they initiated first antipsychotic treatment within the past 12 months, < 1 year had elapsed since the initial onset of active-phase of schizophrenia symptoms, or they received clozapine within 6 months prior to screening. Patients with a history of clozapine use for treatment-resistant schizophrenia or an inadequate response to treatment with olanzapine were also excluded. Patients taking opioid agonists within 14 days, or opioid antagonists within 60 days, prior to screening were not allowed to participate. Likewise, the use of weight-loss drugs or hypoglycemic agents at screening, or the use of statins, if initiated or the dose changed within 3 months, was exclusionary.
In general, the use of any psychotropic medications (monoamine oxidase inhibitors, oral or long-acting formulations of antipsychotic agents, nicotine replacement therapy, over-the-counter medications for weight loss, systemic steroids, topiramate or combinations thereof, antidepressants, strong inducers/inhibitors of cytochrome P450 3A4 started within 30 days, or opioid agonists started within 14 days of screening) was prohibited. Exceptions were the use of β-blockers, antihistamines, and anticholinergics for the treatment of akathisia and anticholinergics and benzodiazepines (≤ 2 mg/d of lorazepam) for the treatment of extrapyramidal symptoms.
Study Assessments
The primary endpoint was change from baseline in PANSS total score at week 4. The key secondary endpoint was change from baseline in CGI-S score at week 4. Other endpoints included change from baseline in scores on PANSS subscales (positive, negative, and general psychopathology), the proportion of PANSS responders (defined as ≥ 30% improvement from baseline in PANSS total score), and the proportion of Clinical Global Impressions-Improvement Scale (CGI-I) responders (defined as a CGI-I score of ≤ 2 [2 = much improved, 1 = very much improved]).
Subgroup analyses of the primary endpoint were performed for each of the following subgroups: sex (male, female), age (< 55 years, ≥ 55 years), race (white, black, other), and baseline PANSS total score (< 95 points, ≥ 95 points).
Safety evaluations included assessment of adverse events (AEs); ratings of extrapyramidal symptoms using the Abnormal Involuntary Movement Scale (AIMS),25 the Barnes Akathisia Rating Scale (BARS),26 and the Simpson-Angus Scale (SAS)27; the Columbia-Suicide Severity Rating Scale (C-SSRS)28,29; clinical laboratory assessments (chemistry, hematology, and urinalysis); and electrocardiograms. Height, weight, and waist circumference measurements and a full physical examination were performed at screening. Weight and waist circumference measurements and a brief physical examination were performed at Visits 2, 6, and 7.
Statistical Analysis
The efficacy population included all randomized patients who received at least 1 dose of study drug and had at least 1 postbaseline PANSS assessment. The continuous efficacy endpoints were analyzed using a mixed model with repeated measures (MMRM) with an unstructured variance-covariance matrix. The model included region (US vs non-US), visit, treatment, and the interaction of term-of-visit and treatment as categorical variables, and baseline scores as a covariate. The Kenward-Roger approximation was used to adjust the denominator degree of freedom. The least squares (LS) mean ± SE change from baseline for each treatment group was reported, as were the LS mean ± SE difference and 95% CI for the active treatment groups (OLZ/SAM and olanzapine) versus placebo. Binary efficacy endpoints were analyzed using a logistic regression model based on last-observation-carried-forward imputation for missing data. The model included the region and treatment group as factors and baseline scores as covariates. The prespecified comparison was between the placebo and OLZ/SAM groups and between the placebo and olanzapine groups; comparisons of the olanzapine and OLZ/SAM groups were performed post hoc.
Safety was assessed in all randomized patients who received at least 1 dose of the study drug. Safety and tolerability were analyzed using descriptive statistics based on observed data.
The sample size calculation was performed based on t test with the following assumptions: a 10-point improvement of PANSS total score at week 4 of OLZ/SAM relative to placebo, a standard deviation (SD) of 20, and a dropout rate of 30%. The planned sample size was 390 patients (130 per treatment group). This sample size would provide at least 90% power to show superiority of the OLZ/SAM group compared with the placebo group at a 2-sided α level of .05.
RESULTS
Patient Disposition and Baseline Characteristics
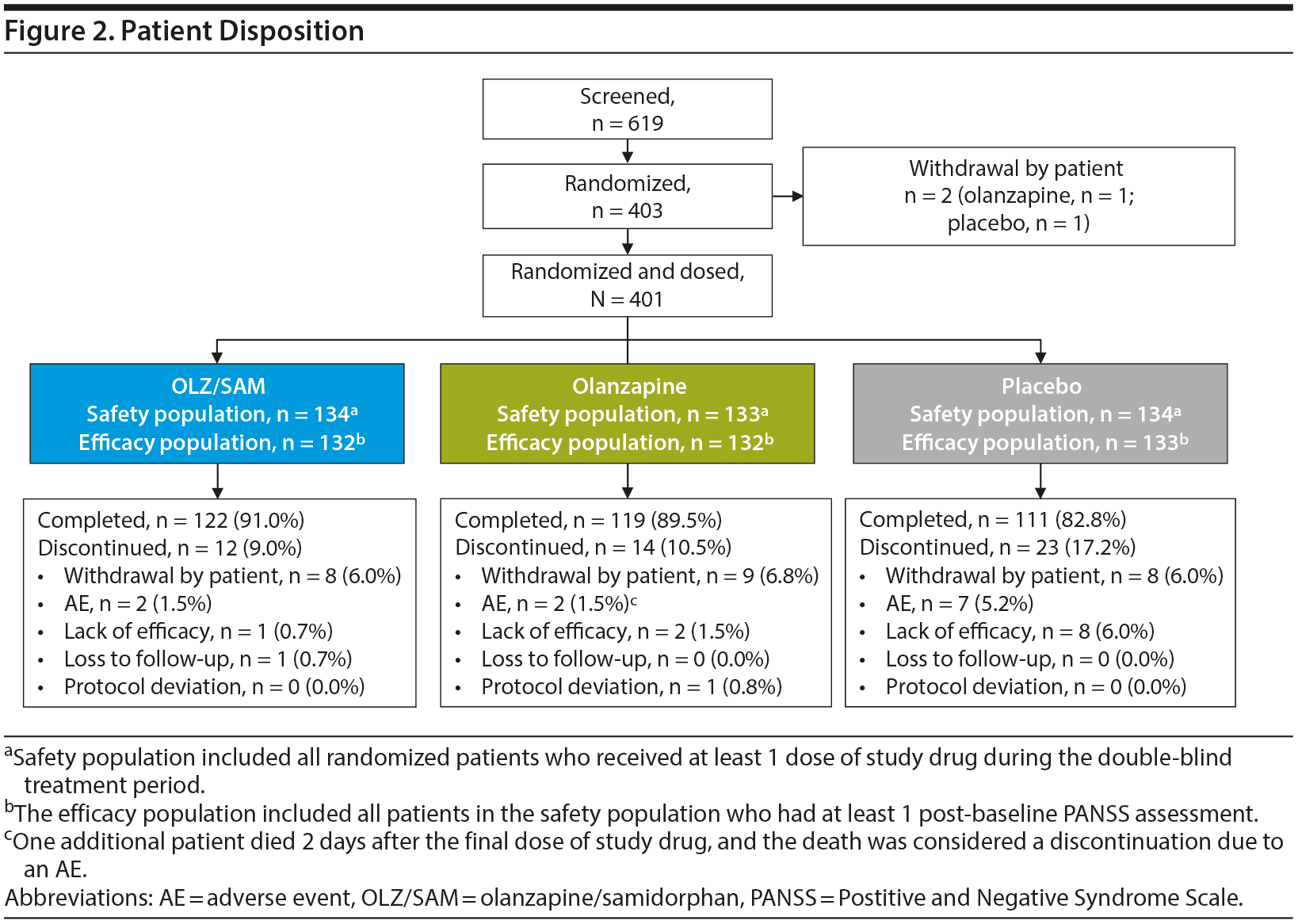
Of 403 randomized patients, 2 (1 in the olanzapine group and 1 in the placebo group) withdrew from the study prior to receiving study drug. Overall, 87.8% (n = 352) of patients completed the double-blind treatment period (91.0% [n = 122] in the OLZ/SAM group, 89.5% [n = 119] in the olanzapine group, and 82.8% [n = 111] in the placebo group); 12.2% (n = 49) of patients discontinued the double-blind treatment period early (Figure 2). The most common reasons for treatment discontinuation were withdrawal by patient (n = 25; 6.2%), AE (n = 11; 2.7%), and lack of efficacy (n = 11; 2.7%).
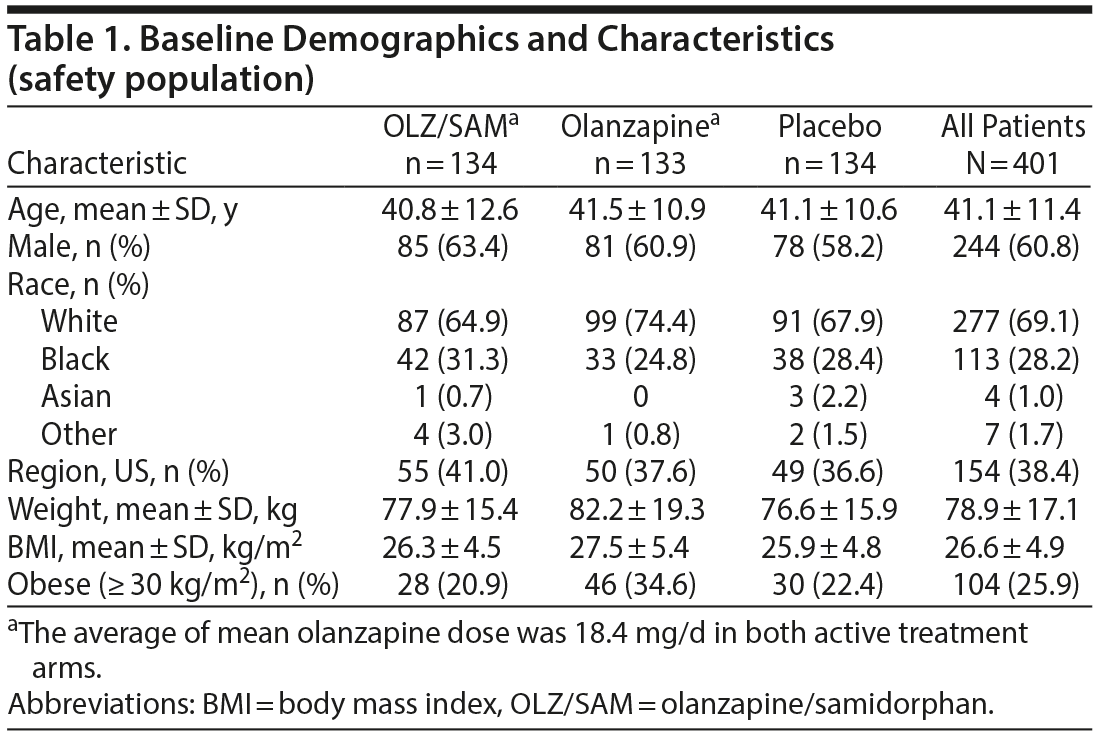
In general, baseline characteristics were similar between groups. Overall, the mean ± SD age of patients was 41.1 ± 11.4 years, 244 (60.8%) were male, 113 (28.2%) were black, and 154 (38.4%) were in the United States (Table 1). Mean baseline weight and BMI were higher in the olanzapine group (82.2 kg and 27.5 kg/m2) compared with the OLZ/SAM (77.9 kg and 26.3 kg/m2) and placebo groups (76.6 kg and 25.9 kg/m2). The OLZ/SAM group had a higher percentage of black patients (n = 42, 31.3%) than the placebo (n = 38, 28.4%) or the olanzapine groups (n = 33, 24.8%). A higher proportion of obese (BMI ≥ 30 kg/m2) patients were in the olanzapine group (n = 46, 34.6%) compared with the OLZ/SAM (n = 28, 20.9%) and placebo (n = 30, 22.4%) groups. Overall, 357 patients (89.0%) had taken at least 1 antipsychotic prior to study entry; the most common were risperidone (n = 124, 30.9%) and haloperidol (n = 122, 30.4%; Supplementary Table 1).
The mean olanzapine dose was 18.4 mg/d in both active treatment arms, and the mean modal dose of olanzapine was 19.0 mg/d for the OLZ/SAM group and 18.9 mg/d for the olanzapine group. The majority of patients in both the OLZ/SAM (n = 119, 88.8%) and olanzapine (n = 119, 89.5%) groups received olanzapine 20 mg as the final dose of study drug. Approximately 85% remained as inpatients for the full duration of the study.
Efficacy
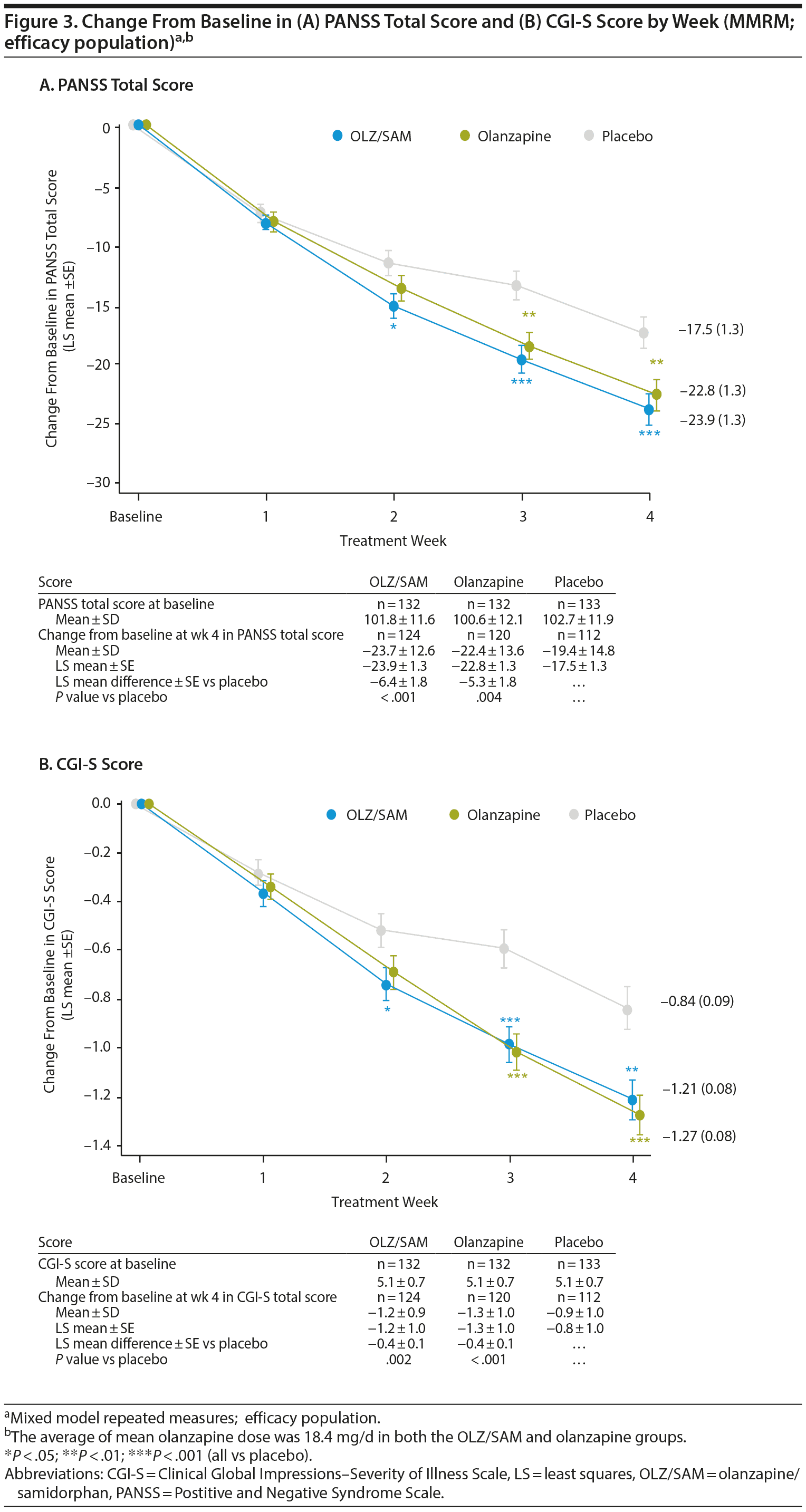
The LS mean difference ± SE versus placebo in change from baseline at week 4 in PANSS total score was −6.4 ± 1.8 (P < .001) for the OLZ/SAM group and −5.3 ± 1.8 (P = .004) for the olanzapine group (Figure 3A). A statistically significant LS mean difference from placebo in the OLZ/SAM group was observed from week 2 onward.
The LS mean difference ± SE versus placebo in change from baseline at week 4 in the OLZ/SAM and olanzapine groups was −1.9 ± 0.6 and −1.8 ± 0.6, respectively, for the PANSS positive subscale; −1.0 ± 0.5 and −0.5 ± 0.5, respectively, for the PANSS negative subscale; and −3.4 ± 0.9 and −2.8 ± 0.9, respectively, for the PANSS general psychopathology subscale (Supplementary Figure 1). The proportion of PANSS responders at week 4 was significantly greater for OLZ/SAM (n = 79, 59.8%; P < .001) and olanzapine (n = 71, 53.8%; P = .015) compared with placebo (n = 51, 38.3%; Supplementary Figure 2A).
The LS mean difference ± SE versus placebo in change from baseline at week 4 in CGI-S score was −0.38 ± 0.12 (P = .002) for the OLZ/SAM group and −0.44 ± 0.12 (P < .001) for the olanzapine group (Figure 3B). Compared with placebo (n = 44, 33.1%), the proportion of CGI-I responders at week 4 was significantly greater for OLZ/SAM (n = 76, 57.6%; P < .001) and olanzapine (n = 67, 50.8%; P = .004; Supplementary Figure 2B).
Subgroup analyses. The efficacy of OLZ/SAM versus placebo, as assessed by change from baseline at week 4 in PANSS total score, was similar to olanzapine versus placebo overall and in each subgroup of interest (age: < 55 or ≥ 55 years; sex: male or female; race: white, black, or other; and PANSS total score: < 95 vs ≥ 95; Supplementary Figure 3A). The efficacy of OLZ/SAM was also similar to olanzapine overall and in each subgroup of interest (Supplementary Figure 3B).
Safety
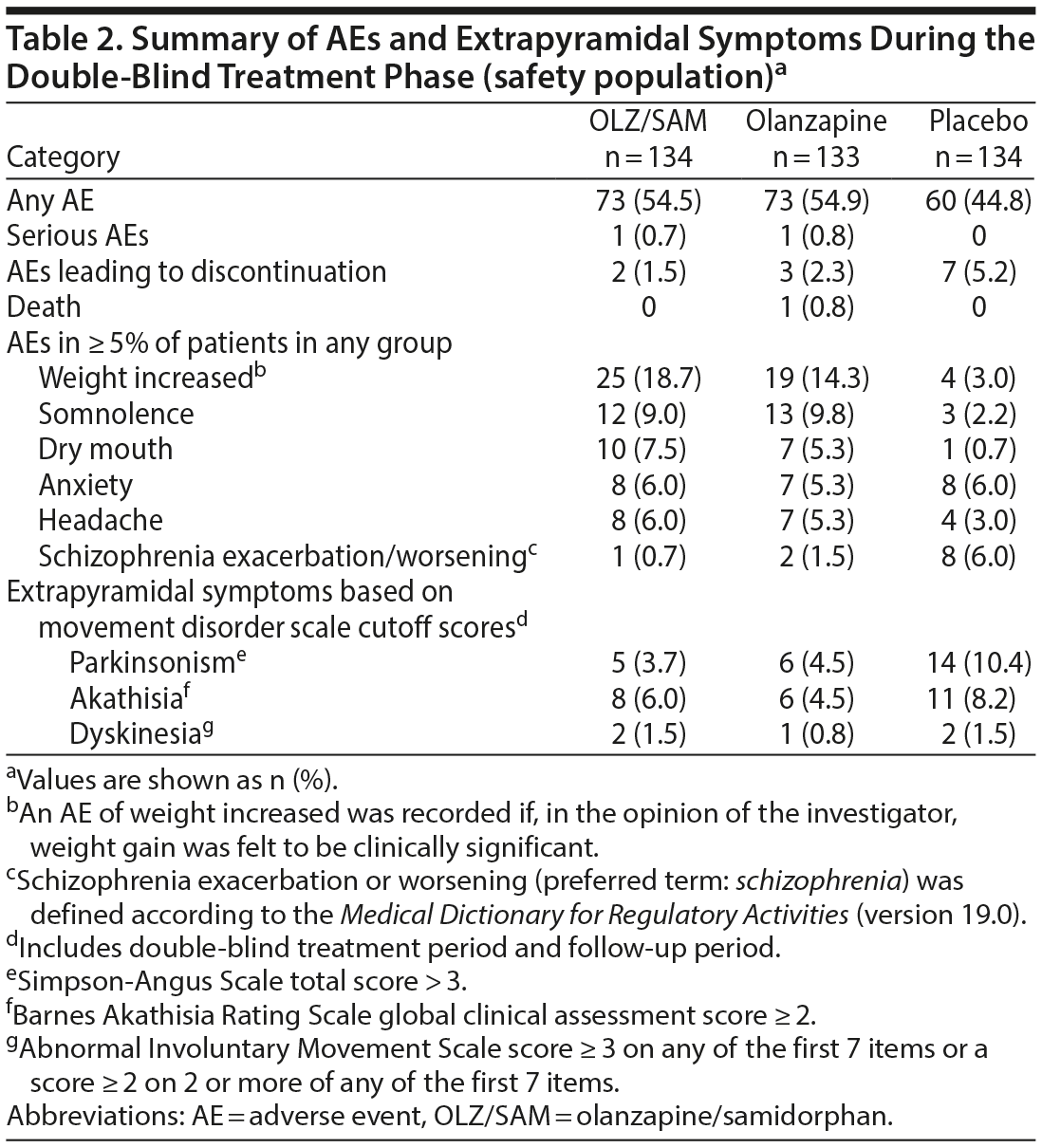
Adverse events. Overall, 206 patients (51.4%) experienced ≥ 1 AE: 73 (54.5%) treated with OLZ/SAM, 73 (54.9%) with olanzapine, and 60 (44.8%) with placebo. Most AEs were mild or moderate in severity. AEs reported in ≥ 5% of patients in any treatment group are summarized in Table 2. AEs reported in ≥ 5% of patients in the OLZ/SAM group and that occurred at a rate of at least 2-fold greater than in placebo group were weight gain, somnolence, dry mouth, and headache (Table 2). The mean ± SD change in weight from baseline to week 4 in the placebo group was 0.24 ± 2.76 kg compared with 3.02 ± 3.56 kg for OLZ/SAM and 2.38 ± 3.65 kg in the olanzapine group.
Overall, 12 patients (3.0%) discontinued treatment because of AEs. The percentage of patients who discontinued treatment owing to AEs was 1.5% (2/134) in the OLZ/SAM group, 2.3% (3/133) in the olanzapine group, and 5.2% (7/134) in the placebo group (Table 2). Of the AEs leading to study drug discontinuation, alanine aminotransferase increase (OLZ/SAM group), hyperglycemia (olanzapine group), worsening of schizophrenia (placebo group), and seizure (placebo group) were considered by the investigator to be probably or definitely related to study drug. One death was reported during the study. A patient taking olanzapine died from heroin overdose 2 days after completing study treatment; this was considered a serious AE. The patient had not expressed any suicidal ideation upon completion of the study. A different patient in the OLZ/SAM group experienced a serious AE of catatonia 2 days after discontinuing the study drug. Neither event was considered by the investigator to be related to the study drug.
Abnormal movements. Mean changes from baseline during the double-blind treatment period were small and clinically insignificant as assessed by the AIMS, BARS, and SAS, with no notable differences between treatment groups (Supplementary Table 2); dyskinesia was not reported as an AE for any patient during the study.
In the double-blind treatment and follow-up periods, rates of extrapyramidal symptoms defined by specific cutoff scores (ie, SAS total score > 3; BARS global clinical assessment score ≥ 2; AIMS score ≥ 3 on any of the first 7 items or a score of ≥ 2 on 2 or more of any of the first 7 items) are presented in Table 2. The proportion of patients who experienced parkinsonism was lower in the OLZ/SAM and olanzapine groups compared with the placebo group, and the rates of akathisia and dyskinesia were comparable across all 3 groups based on this analysis.
Columbia-Suicide Severity Rating Scale. Suicidal ideation was reported in 2 patients (1.5%) in the olanzapine group and 3 patients (2.2%) in the placebo group; no patients in the OLZ/SAM group reported suicidal ideation. No patient in any treatment group exhibited suicidal behavior or nonsuicidal self-injurious behavior at any time during the study.
DISCUSSION
Key goals in the treatment of patients with an acute exacerbation of schizophrenia are to rapidly control symptoms, such as aggression and agitation, and return the patient to the best level of functioning possible.30 In this study, treatment with OLZ/SAM, when compared with placebo, resulted in significant (P < .001) improvements in the primary endpoint, change from baseline in PANSS total score at week 4, in patients with an acute exacerbation of schizophrenia, a population with more severe schizophrenia symptoms than the population of the previous phase 2 study. Statistically significant improvements were observed as early as week 2 in the OLZ/SAM group (P = .015) compared with the placebo group and were maintained through week 4. Treatment with olanzapine also resulted in similar statistically significant improvements compared with placebo, confirming the study’s validity. The reductions in PANSS total scores for patients on active treatment were similar in magnitude to those previously reported31-33 in patients treated with olanzapine for an acute exacerbation of schizophrenia. In this study, the time course and effect size of the response for the 2 active medications, olanzapine and OLZ/SAM, were similar. These findings demonstrate that samidorphan does not have an impact on the antipsychotic efficacy of olanzapine. Post hoc analyses indicated that the efficacy of OLZ/SAM was similar to that of olanzapine.
The efficacy of OLZ/SAM was further supported by significant (P = .002) improvements compared with placebo in the key secondary endpoint, change from baseline in CGI-S score at week 4, with statistical differences from placebo observed by week 2 (P = .024). Similar findings were reported for other endpoints, including a significantly higher proportion of PANSS responders and CGI-I responders, and significant improvements in PANSS positive, negative, and general psychopathology subscale scores in the OLZ/SAM group compared with placebo. Improvements in these outcomes were similar between the OLZ/SAM and olanzapine groups at all time points. These findings are also consistent with those from prior studies33-35 examining the acute response to olanzapine in schizophrenia.
Overall, OLZ/SAM was generally well tolerated. The types and rates of AEs with OLZ/SAM were similar to those observed with olanzapine in this study and in previous studies.34,36,37 Only 2 patients discontinued treatment with OLZ/SAM due to AEs of alanine aminotransferase increase (n = 1) and schizophrenia (worsening of symptoms; n = 1). Interestingly, the rates of parkinsonism, as assessed by SAS total score > 3, were lower in patients treated with OLZ/SAM and olanzapine compared with placebo. A possible explanation for this is an anticholinergic rebound effect in patients in the placebo group as a consequence of stopping their prior antipsychotic medication.38,39
The mean increase in weight at week 4 was higher in the OLZ/SAM and olanzapine groups (3.02 and 2.38 kg, respectively) compared with placebo (0.24 kg). However, it should be noted that this 4-week study was designed to assess the antipsychotic efficacy of OLZ/SAM and not to assess the relative weight gain due to olanzapine and OLZ/SAM. In a previously reported phase 2 study21 of OLZ/SAM with a specific safety focus on weight gain, meaningful differences in weight gain with OLZ/SAM compared with olanzapine were not observed until after 4 weeks of treatment. At the end of 12 weeks, treatment with OLZ/SAM resulted in a significantly lower weight gain than treatment with olanzapine. Fortunately, another study, the 24-week, randomized, double-blind ENLIGHTEN-2 study, will provide additional important data on OLZ/SAM safety in terms of long-term weight changes and will further inform the field.
One limitation of this study was the high placebo response observed at week 4 (LS mean improvement in PANSS total score: −17.5). While a statistical difference from placebo in the primary endpoint was found for both OLZ/SAM and olanzapine, the relative effect size was small. The observed placebo effect is consistent with reported trends in placebo-controlled schizophrenia trials, including those of olanzapine.34,40 However, olanzapine has been an approved treatment for schizophrenia since 1996, and there is a large body of scientific literature and clinical evidence supporting its efficacy for the treatment of schizophrenia.36,41-45 An additional limitation of this study is the short duration of 4 weeks. The long-term efficacy and safety of OLZ/SAM remain unknown until the completion of the ongoing studies that are > 4 weeks in duration.
CONCLUSIONS
Treatment with OLZ/SAM resulted in significantly greater antipsychotic efficacy compared with placebo as assessed by PANSS and CGI-S scale scores. OLZ/SAM demonstrated efficacy similar to olanzapine monotherapy. OLZ/SAM was generally well tolerated, with a safety profile in the acute treatment setting similar to olanzapine monotherapy.
Submitted: February 5, 2019; accepted October 10, 2019.
Published online: March 3, 2020.
Potential conflicts of interest: Dr Potkin has been a consultant or participated in advisory boards for Otsuka, Sunovion, Roche, Lundbeck, FORUM, Allergan, and Alkermes; has received grants or research support from Eli Lilly, Toyama, Otsuka, FORUM, Alkermes, Eisai, and Lundbeck; and has been a member of the speakers bureau or received speaker honoraria for Otsuka, Sunovion, Novartis, Teva, Acadia, and Allergan. Dr Kunovac is the founder of Altea Research Institute and a cofounder of Excell Research and is a consultant and/or serves on advisory boards for AstraZeneca, Bristol-Myers Squibb, Pfizer, Janssen, Novartis, Otsuka, and Sunovion. Drs Silverman, Jiang, and DiPetrillo and Mr Simmons are employees of Alkermes, Inc, and may own stock/options in the company. Dr McDonnell is an employee of Alkermes Pharma Ireland Limited and may own stock/options in the company.
Funding/support: This study was sponsored by Alkermes, Inc, Waltham, Massachusetts.
Role of the sponsor: The study sponsor was involved in the design, collection, and analysis of the data and provided the study medications and placebo. Interpretation of the results was by the authors, and the decision to submit the manuscript for publication was made by the authors.
Previous presentation: Presented at the American College of Neuropsychopharmacology 56th Annual Meeting; December 5, 2017; Palm Springs, California [Abstract T214 published in Neuropsychopharmacology. 2017;42:S433-S434] ▪ Sixth Biennial Schizophrenia International Research Society Conference; April 4-8, 2018; Florence, Italy [Poster F232] ▪ 171st Annual Meeting of the American Psychiatric Association; May 5-9, 2018; New York, New York [Poster P8-169] ▪ Psych Congress 2018; October 25-28, 2018; Orlando, Florida [Poster 129] ▪ 14th Annual Neuroscience Education Institute Psychopharmacology Congress; November 7-11, 2018; Orlando, Florida [Abstract 26 published in CNS Spectrums. 2019;24(1):187-188].
Acknowledgments: The authors would like to thank Mark S. Todtenkopf, PhD, who is an employee of Alkermes and assisted in the preparation and proofreading of the manuscript; Peloton Advantage, an OPEN Health company, whose staff members provided editorial assistance; and the ALK 3831-A305 study group. Funding for editorial support was provided by Alkermes, Inc.
Supplementary material: See accompanying pages.
REFERENCES
1.Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(3):CD006654. PubMed
2.Treuer T, Anders M, Bitter I, et al. Effectiveness and tolerability of schizophrenia treatment in Central and Eastern Europe: results after 1 year from a prospective, observational study (IC-SOHO). Int J Psychiatry Clin Pract. 2006;10(2):78-90. PubMed CrossRef
3.Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. PubMed CrossRef
4.Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097. PubMed CrossRef
5.De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126. PubMed CrossRef
6.Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93. PubMed CrossRef
7.Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophr Res. 2012;140(1-3):159-168. PubMed CrossRef
8.Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403. PubMed CrossRef
9.Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530. PubMed CrossRef
10.Lord CC, Wyler SC, Wan R, et al. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J Clin Invest. 2017;127(9):3402-3406. PubMed CrossRef
11.Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs. 2011;25(12):1035-1059. PubMed CrossRef
12.Weston-Green K, Huang XF, Deng C. Second generation antipsychotic-induced type 2 diabetes: a role for the muscarinic M3 receptor. CNS Drugs. 2013;27(12):1069-1080. PubMed CrossRef
13.Potvin S, Zhornitsky S, Stip E. Antipsychotic-induced changes in blood levels of leptin in schizophrenia: a meta-analysis. Can J Psychiatry. 2015;60(suppl 2):S26-S34. PubMed
14.Czyzyk TA, Romero-Picó A, Pintar J, et al. Mice lacking δ-opioid receptors resist the development of diet-induced obesity. FASEB J. 2012;26(8):3483-3492. PubMed CrossRef
15.Czyzyk TA, Nogueiras R, Lockwood JF, et al. Kappa-opioid receptors control the metabolic response to a high-energy diet in mice. FASEB J. 2010;24(4):1151-1159. PubMed CrossRef
16.Tabarin A, Diz-Chaves Y, Carmona MdelC, et al. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene”. Diabetes. 2005;54(12):3510-3516. PubMed CrossRef
17.Wentland MP, Lou R, Lu Q, et al. Syntheses of novel high affinity ligands for opioid receptors. Bioorg Med Chem Lett. 2009;19(8):2289-2294. PubMed CrossRef
18.Bidlack JM, Knapp BI, Deaver DR, et al. In vitro pharmacological characterization of buprenorphine, samidorphan, and combinations being developed as an adjunctive treatment of major depressive disorder. J Pharmacol Exp Ther. 2018;367(2):267-281. PubMed CrossRef
19.Shram MJ, Silverman B, Ehrich E, et al. Use of remifentanil in a novel clinical paradigm to characterize onset and duration of opioid blockade by samidorphan, a potent μ-receptor antagonist. J Clin Psychopharmacol. 2015;35(3):242-249. PubMed CrossRef
20.Silverman BL, Martin W, Memisoglu A, et al. A randomized, double-blind, placebo-controlled proof of concept study to evaluate samidorphan in the prevention of olanzapine-induced weight gain in healthy volunteers. Schizophr Res. 2018;195:245-251. PubMed CrossRef
21.Martin WF, Correll CU, Weiden PJ, et al. Mitigation of olanzapine-induced weight gain with samidorphan, an opioid antagonist: a randomized double-blind phase 2 study in patients with schizophrenia. Am J Psychiatry. 2019;176(6):457-467. PubMed CrossRef
22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
23.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed CrossRef
24.Guy W. CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976:217-222.
25.Guy W. Abnormal involuntary movement scale (AIMS). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976:534-537.
26.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676. PubMed CrossRef
27.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;212:11-19. PubMed CrossRef
28.Posner K, Brent D, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS): Since Last Visit. Columbia Lighthouse Project website. https://www.cssrs.columbia.edu/wp-content/uploads/C-SSRS1-14-09-SinceLastVisit.pdf. 2009. Accessed January 28, 2019.
29.Posner K, Brent D, Lucas C, et al. Columbia-suicide severity rating scale (C-SSRS): Screening. Columbia Lighthouse Project website. https://cssrs.columbia.edu/wp-content/uploads/C-SSRS1-14-09-BaselineScreening.pdf. 2009. Accessed January 28, 2019.
30.Lehman AF, Lieberman JA, Dixon LB, et al. Practice Guideline for the Treatment of Patients with Schizophrenia. Second Edition. American Psychiatric Association website. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. 2010. Accessed: January 28, 2019.
31.Fleischhacker WW, McQuade RD, Marcus RN, et al. A double-blind, randomized comparative study of aripiprazole and olanzapine in patients with schizophrenia. Biol Psychiatry. 2009;65(6):510-517. PubMed CrossRef
32.Grootens KP, van Veelen NM, Peuskens J, et al. Ziprasidone vs olanzapine in recent-onset schizophrenia and schizoaffective disorder: results of an 8-week double-blind randomized controlled trial. Schizophr Bull. 2011;37(2):352-361. PubMed CrossRef
33.Beasley CM Jr, Hamilton SH, Crawford AM, et al. Olanzapine versus haloperidol: acute phase results of the international double-blind olanzapine trial. Eur Neuropsychopharmacol. 1997;7(2):125-137. PubMed CrossRef
34.Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111-123. PubMed CrossRef
35.Lambert M, Holzbach R, Moritz S, et al. Objective and subjective efficacy as well as tolerability of olanzapine in the acute treatment of 120 patients with schizophrenia spectrum disorders. Int Clin Psychopharmacol. 2003;18(5):251-260. PubMed
36.Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2018.
37.Kane JM, Osuntokun O, Kryzhanovskaya LA, et al. A 28-week, randomized, double-blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. J Clin Psychiatry. 2009;70(4):572-581. PubMed CrossRef
38.Davison P, Worsley A, Husband A. Drug withdrawal—the most common problems. Hosp Pharm. 2007;14(11):363-365.
39.Cerovecki A, Musil R, Klimke A, et al. Withdrawal symptoms and rebound syndromes associated with switching and discontinuing atypical antipsychotics: theoretical background and practical recommendations. CNS Drugs. 2013;27(7):545-572. PubMed CrossRef
40.Khin NA, Chen YF, Yang Y, et al. Exploratory analyses of efficacy data from schizophrenia trials in support of new drug applications submitted to the US Food and Drug Administration. J Clin Psychiatry. 2012;73(6):856-864. PubMed CrossRef
41.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed CrossRef
42.Davis JM, Chen N. The effects of olanzapine on the 5 dimensions of schizophrenia derived by factor analysis: combined results of the North American and international trials. J Clin Psychiatry. 2001;62(10):757-771. PubMed CrossRef
43.Bhana N, Foster RH, Olney R, et al. Olanzapine: an updated review of its use in the management of schizophrenia. Drugs. 2001;61(1):111-161. PubMed CrossRef
44.Leucht S, Pitschel-Walz G, Abraham D, et al. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophr Res. 1999;35(1):51-68. PubMed CrossRef
45.Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60(6):553-564. PubMed CrossRef
This PDF is free for all visitors!