Objective: The aim of this study was to examine the effectiveness of ramelteon and suvorexant for delirium prevention in real-world practice. It explored whether ramelteon and/or suvorexant would affect delirium prevention among both patients at risk for but without delirium (patients at risk) and those with delirium the night before a consultation.
Methods: This multicenter, prospective, observational study was conducted by trained psychiatrists at consultation-liaison psychiatric services from October 1, 2017, to October 7, 2018. Patients who were aged 65 years or older and hospitalized because of acute diseases or elective surgery, had risk factors for delirium, and had insomnia or delirium on the night before the consultation were prescribed ramelteon and/or suvorexant. The decision to take medication was left to the discretion of each patient. The primary outcome was incidence of delirium based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, during the first 7 days.
Results: Among 526 patients at risk, those taking ramelteon and/or suvorexant developed delirium significantly less frequently than those who did not, after control for the effects of risk factors on the estimate of an independent association between the effects of ramelteon and/or suvorexant and the outcome of developing delirium (15.7% vs 24.0%; odds ratio [OR] = 0.48;, 95% CI, 0.29-0.80; P = .005). Similar results were found among 422 patients with delirium (39.9% vs 66.3%; OR = 0.36; 95% CI, 0.22-0.59; P < .0001).
Conclusions: Ramelteon and suvorexant appear to be effective for delirium prevention in real-world practice.

ABSTRACT
Objective: The aim of this study was to examine the effectiveness of ramelteon and suvorexant for delirium prevention in real-world practice. It explored whether ramelteon and/or suvorexant would affect delirium prevention among both patients at risk for but without delirium (patients at risk) and those with delirium the night before a consultation.
Methods: This multicenter, prospective, observational study was conducted by trained psychiatrists at consultation-liaison psychiatric services from October 1, 2017, to October 7, 2018. Patients who were aged 65 years or older and hospitalized because of acute diseases or elective surgery, had risk factors for delirium, and had insomnia or delirium on the night before the consultation were prescribed ramelteon and/or suvorexant. The decision to take medication was left to the discretion of each patient. The primary outcome was incidence of delirium based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, during the first 7 days.
Results: Among 526 patients at risk, those taking ramelteon and/or suvorexant developed delirium significantly less frequently than those who did not, after control for the effects of risk factors on the estimate of an independent association between the effects of ramelteon and/or suvorexant and the outcome of developing delirium (15.7% vs 24.0%; odds ratio [OR] = 0.48;, 95% CI, 0.29-0.80; P = .005). Similar results were found among 422 patients with delirium (39.9% vs 66.3%; OR = 0.36; 95% CI, 0.22-0.59; P < .0001).
Conclusions: Ramelteon and suvorexant appear to be effective for delirium prevention in real-world practice.
J Clin Psychiatry 2020;81(1):19m12865
To cite: Hatta K, Kishi Y, Wada K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry. 2020;81(1):19m12865.
To share: https://doi.org/10.4088/JCP.19m12865
© Copyright 2019 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Juntendo University Nerima Hospital, Tokyo, Japan
bDepartment of Psychiatry, Nippon Medical School Musashikosugi Hospital, Kawasaki, Japan
cDepartment of Psychiatry, Hiroshima City Hospital, Hiroshima, Japan
dDepartment of Psychiatry, Tokyo Medical and Dental University, Tokyo, Japan
eDepartment of Psychiatry, Tokushima Prefectural Central Hospital, Tokushima, Japan
fDepartment of Psycho-oncology, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Tokyo, Japan
gDepartment of Psychiatry, Fukuyama City Hospital, Fukuyama, Japan
hDepartment of Psychiatry, Kurashiki Central Hospital, Kurashiki, Japan
iDepartment of Psychiatry and Neurosciences, Hiroshima University Hospital, Hiroshima, Japan
jDepartment of Psychiatry, Kurume University School of Medicine, Kurume, Japan
kDepartment of Environmental and Preventive Medicine, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan
*Corresponding author: Kotaro Hatta, MD, PhD, Department of Psychiatry, Juntendo University Nerima Hospital, Takanodai 3-1-10, Nerima-ku, Tokyo 177-8521, Japan ([email protected]).
The fundamental concept of delirium involves altered consciousness and fluctuations caused by the direct physiologic consequence of another medical condition, substance intoxication or withdrawal, or exposure to a toxin; delirium can also be the result of multiple etiologies.1,2 Although delirium has traditionally been considered transient, a growing body of literature suggests that this is not necessarily true. Delirium has been shown to be independently associated with an increased risk of death.3 Furthermore, delirium reportedly increases the risk of incident dementia and is associated with worsening dementia severity, deterioration in global functioning score, and loss of 1 point or more per year on the Mini-Mental State Examination (95% CI, 0.11-1.89) compared with those with no history of delirium.4 These associations are very likely due to the vulnerability to delirium caused by preclinical neuropathologic processes and to the worsening of dementia associated with an increased likelihood of delirium. Therefore, the importance of delirium prevention has been increasingly recognized. Evidence shows that multicomponent, nonpharmacologic delirium prevention interventions are effective in reducing delirium incidence and preventing falls, with trends toward decreasing length of stay in the hospital and nonsignificant reductions in mortality.5,6 Meanwhile, evidence related to the effectiveness of antipsychotic prophylaxis in patients at high risk for delirium remains contradictory.7-11 In our previous prospective observational study,12 we found that extrapyramidal symptoms occurred in 5.6% of 2,453 patients receiving antipsychotics to treat delirium. Such a relatively high rate of extrapyramidal symptoms may lead to hesitation about the prophylactic use of antipsychotics.
Recently, clinicians and researchers have begun paying attention to pharmacologic interventions for sleep-wake cycle disturbances, which are common clinical features of delirium and plausible contributing factors toward a pathophysiology of delirium given that hospitalized patients experience multiple sleep alterations, including sleep loss, sleep fragmentation, and sleep-wake cycle disorganization. In addition, some emerging literature13,14 has suggested that the function of the period clock gene may be disturbed in delirium. Additionally, diurnal sleep/melatonin dysregulation and orexin neurotransmission deserve further mention because of their key role in sleep-wake cycle regulation.15 Melatonin has a variety of physiologic functions, such as regulating circadian rhythms, vasomotor responses, sleep, retinal neuromodulation, and inflammatory and immune processes and scavenging oxidative stress.16 Nocturnal secretion, a major characteristic of melatonin, promotes sleep with nocturnal hypotensive, vasodilating, and hypothermic actions. However, the nocturnal secretion of melatonin decreases with age.17 This finding may have clinical implications, such that hospitalized older patients may be more vulnerable to circadian-related sleep-wake disturbances, and hence to delirium, because of their much lower peak of melatonin secretion.
Orexin is an alerting neuropeptide, and orexin signaling is necessary for normal circadian regulation of consolidated wakefulness. Accordingly, as compared with melatonin, orexin is predominantly secreted during the daytime in humans.18 Interestingly, patients with moderate-to-severe Alzheimer’s disease have been reported to have higher mean orexin levels in cerebrospinal fluid and significantly impaired nocturnal sleep compared with patients with mild Alzheimer’s disease and controls.19 Furthermore, significantly higher levels of orexin have been found in the brains of rats with acute pancreatitis compared with healthy controls.20 As dementia and inflammation are risk factors for delirium, it is possible that patients with delirium have increased orexin levels, which would account for the resultant sleep-wake disturbances.21 Therefore, we hypothesized that supplying a melatonin receptor agonist and an orexin receptor antagonist in the evening could prevent delirium subsequent to improving sleep-wake cycle disturbances. To our knowledge, 3 randomized controlled trials (RCTs)22-24 to date have reported the effects of melatonin on delirium prevention to some extent. In addition, 2 RCTs25,26 have shown that ramelteon, a more potent melatonin receptor agonist than melatonin, has preventive effects on delirium. Moreover, 2 RCTs27,28 have shown that suvorexant, a potent and selective orexin antagonist, affects delirium prevention. Due to the few known side effects and the preventive effects of this melatonin receptor agonist and orexin receptor antagonist on delirium, their use to treat patients at risk for delirium is increasing.
The aim of the present study was to examine the effectiveness of ramelteon and suvorexant on delirium prevention in real-world practice. First, we hypothesized that ramelteon and/or suvorexant would help prevent delirium among patients at risk for but without delirium on the night before the consultation. Second, we hypothesized that ramelteon and/or suvorexant would help prevent delirium even among patients with delirium on the night before the consultation.

- Despite some success regarding the preventive effects of ramelteon and suvorexant on delirium in randomized, placebo-controlled trials, no strong evidence regarding the effectiveness of these medications in real-world practice has been reported.
- For patients at risk for delirium and those with delirium on the night before a consultation, ramelteon and suvorexant are viable considerations for delirium prevention.
METHODS
Study Design
This multicenter, prospective, observational study proceeded over a 1-year period (from October 1, 2017, to October 7, 2018). All study protocols were approved by the institutional review board of Juntendo University Nerima Hospital and each relevant institutional review board. The approved protocol did not require informed consent from the patients, as the protocol did not differ from ordinary practice and because the data remained anonymous and were analyzed in aggregate. Instead, we posted a notice in each hospital providing a means for patients to opt out.
Setting
This study was conducted by trained psychiatrists as consultation-liaison psychiatric services in 9 general hospitals located all over Japan. The period of enrollment was 1 year (from October 1, 2017, to September 30, 2018), and that of exposure or follow-up was 7 days.
Participants
Eligible patients were aged 65 years or older and were hospitalized because of acute disease or elective surgery, with the following risk factors for delirium: dementia/mild cognitive impairment (MCI), current hip fracture, severe illness (that is, a clinical condition that is deteriorating or at risk of deterioration),29 or a history of delirium and with insomnia or delirium on the night before registration. Patients who could not take medicine orally, who took antipsychotic drugs, or who refused to allow their data to be used were excluded. All patients received multicomponent, nonpharmacologic delirium prevention interventions by nurses.29 Patients who still had altered arousal at the time of consultation were excluded. On the night before the consultation, all patients had the hyperactive subtype of delirium, as the patients with the hypoactive or mixed subtype did not become alert at the time of consultation.
All patients were introduced into consultation-liaison psychiatric services by the physician or nurse in charge, assessed by trained psychiatrists, and prescribed ramelteon (8 mg/d) and/or suvorexant (15 mg/d). The package inserts of both drugs indicate that administration should be within 30 minutes of going to bed. Therefore, hospitalized patients who require these drugs are suggested to take them at around 9:00 pm. However, melatonin secretion begins to increase in the early evening, and the lag in elevated plasma melatonin levels from sunset to nighttime increases with age.17 Therefore, some consultation-liaison psychiatrists instruct hospitalized older patients prescribed ramelteon to take the drug at around 7:00 pm. Patients who took ramelteon at 7:00 pm and did not feel sleepy by around 9:00 pm were suggested to take suvorexant. The decision to take the medications was left to the discretion of each patient, so several patterns of administration for both drugs were observed.
Variables and Measurement
The patients’ demographic and clinical characteristics, including age; sex; presence or absence of dementia/MCI; previous delirium; admission diagnosis; emergency hospitalization; duration until the beginning of the intervention after hospitalization; habitual use of alcohol; habitual use of benzodiazepine receptor agonists, opioids, and corticosteroids; discontinuation of habitual benzodiazepine receptor agonists at the beginning of the intervention; serum levels of C-reactive protein at the beginning and end of the intervention; and adverse events, were recorded. Also, sleep-wake cycle disturbances were evaluated according to item 1 of the Delirium Rating Scale-Revised-98, from 0 (not present) to 3 (severe disruption) at both the beginning and end of the intervention (reference 30 and P. T. Trzepacz, MD; J. R. Maldonado, MD; J. Kean, PhD; et al, unpublished manuscript, 2010).
The primary outcome was the incidence of delirium during the first 7 days after the administration of ramelteon and/or suvorexant. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),2 criteria were used to diagnose delirium. All delirium evaluations were performed by trained psychiatrists.
Statistical Analyses
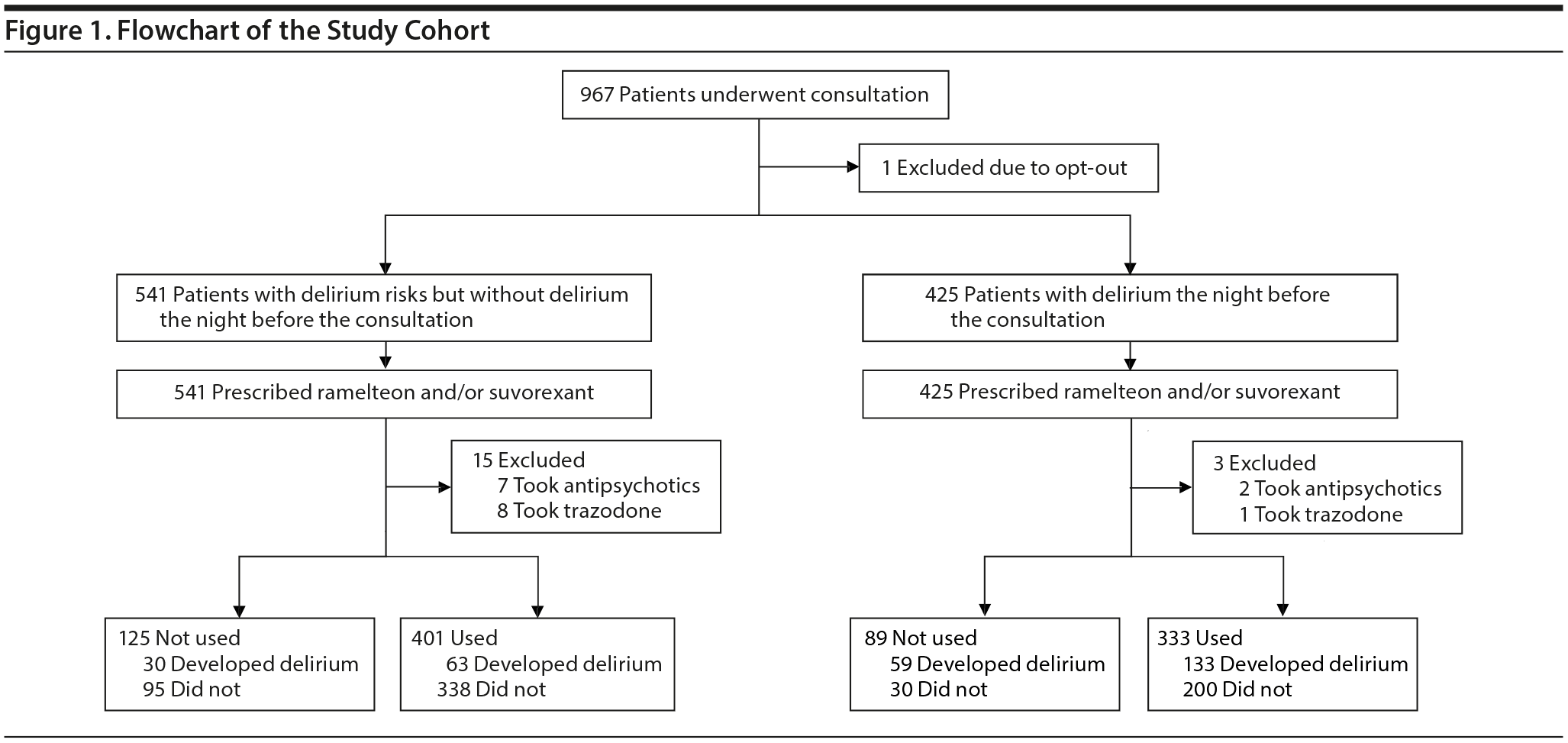
Patients were divided into 2 groups: those at risk for but without delirium (patients at risk) and those with delirium (patients with delirium) on the night before the 7-day observational period. As the decision to take ramelteon and/or suvorexant was left to the discretion of each patient, each group included patients taking and not taking ramelteon and/or suvorexant (Figure 1). For patients who were discharged before the end of the 7-day observational period, data during hospitalization were utilized.
To examine whether ramelteon and/or suvorexant would help prevent delirium in patients at risk for but without delirium on the night before registration, we compared patients taking and not taking ramelteon and/or suvorexant. Next, to examine whether ramelteon and/or suvorexant would help prevent delirium even in patients with delirium on the night before registration, we compared patients taking and not taking ramelteon and/or suvorexant.
Data were collected on standardized forms, and statistical analyses were performed using SPSS (version 25-J; IBM Japan; Tokyo, Japan). Differences between categorical variables in the patients’ demographics and clinical characteristics were calculated using the Fisher exact test. Differences between sequential variables were calculated using unpaired t tests (with Welch correction if applicable). If data were not sampled from Gaussian distributions, a nonparametric test (Mann-Whitney test) was used. We constructed multivariate logistic regression models to control for risk factors in estimating independent associations between the effects of ramelteon and/or suvorexant and the outcome of delirium as an exploratory analysis. Kaplan-Meier curves were used to estimate the probability of developing delirium at 7 days. All statistical tests were 2-tailed. Differences were considered statistically significant at P < .05.
RESULTS
Overall, 967 patients (517 [53.5%] men; mean [SD] age = 79.6 [9.1] years) at high risk for delirium were consulted between October 1, 2017, and September 30, 2018, among whom 541 were at risk for but without delirium and 425 had delirium on the night before the consultation. One patient who chose to opt out was excluded (Figure 1).
Patients at Risk for but Without Delirium on the Night Before the Consultation
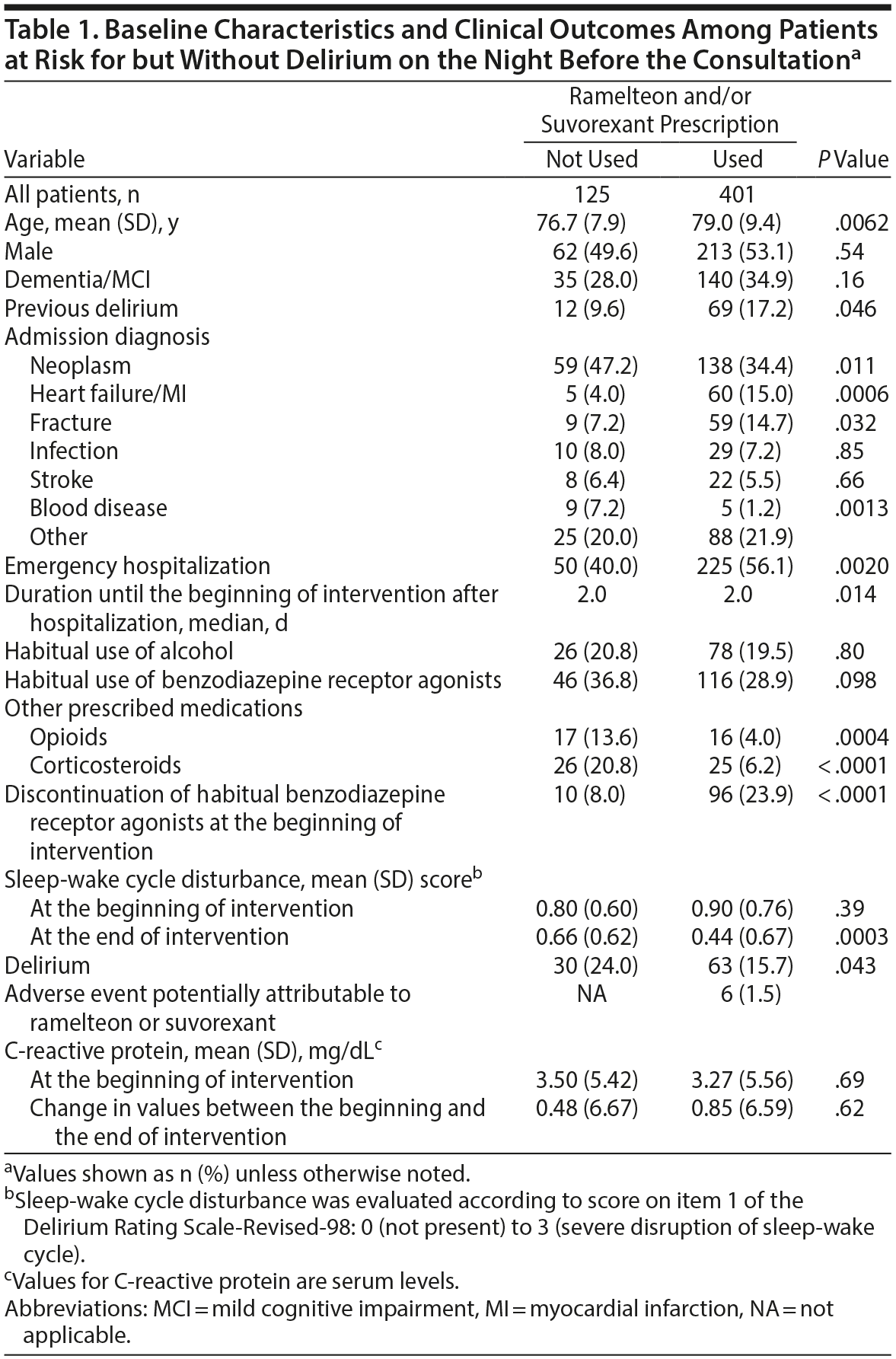
Among 541 patients at risk for but without delirium on the night before the consultation, 15 were excluded because they were currently taking antipsychotics (n = 7) or trazodone (n = 8) despite the prescription of ramelteon and/or suvorexant. In total, 401 patients chose to take ramelteon and/or suvorexant; the remaining 125 chose not to take these drugs at their discretion because of subjective ease of sleep or a dislike of sleeping tablets. Significant differences were observed at the beginning of intervention between the two groups in age, duration until the beginning of the intervention after hospitalization, history of delirium, some admission diagnoses, emergency hospitalization, use of opioids and corticosteroids, and discontinuation of habitual benzodiazepine receptor agonists (Table 1).
Patients who took ramelteon and/or suvorexant developed delirium significantly less frequently than those who did not (15.7% vs 24.0%, respectively; relative risk [RR] = 1.53; 95% CI, 1.04-2.25, P = .043). As significant differences between groups were observed for several variables, we constructed multivariate logistic regression models to control for the effects of such risk factors on the estimate of an independent association between ramelteon and/or suvorexant effects and the outcome of developing delirium. Even after control for the effects of these risk factors, ramelteon and/or suvorexant was still significantly associated with a lower incidence of delirium (odds ratio [OR] = 0.48; 95% CI, 0.29-0.80; P = .005). Interestingly, although no significant differences in sleep-wake cycle disturbance scores were observed between groups at the beginning of the intervention, significant improvements were seen at the end of the intervention in patients taking ramelteon and/or suvorexant (P = .0003; Table 1).
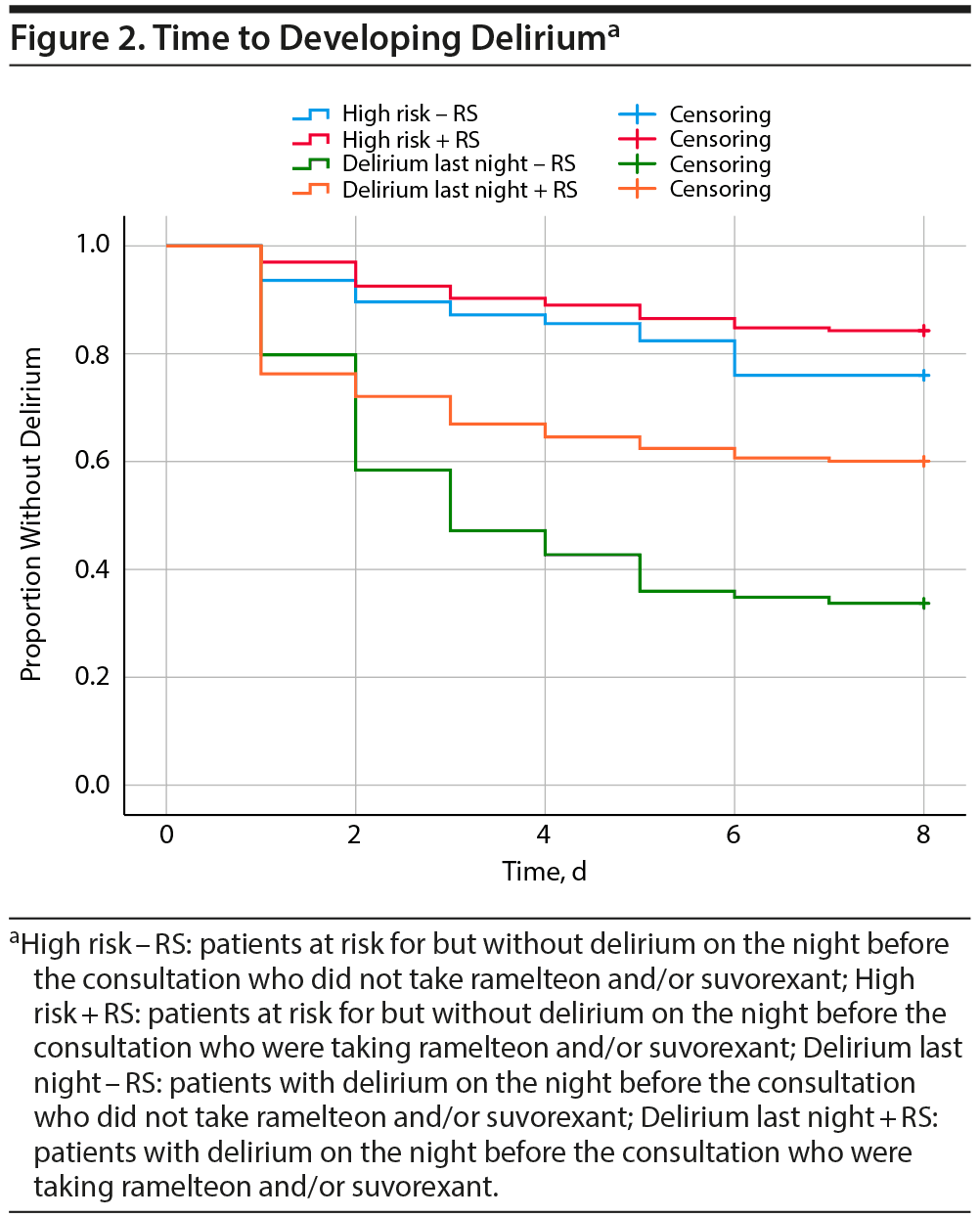
Among 526 patients at risk for but without delirium on the night before the consultation, Kaplan-Meier estimates of the interval to the development of delirium were 7.24 days (95% CI, 7.06-7.43 days) and 6.90 days (95% CI, 6.52-7.29 days) for those taking and not taking ramelteon and/or suvorexant, respectively (Figure 2). A comparison using the log rank test showed that delirium developed significantly less frequently in patients taking ramelteon and/or suvorexant compared with those who were not (χ2 = 4.43, P = .035).
No serious adverse events were observed, but 6 patients (1.5%) showed sleepiness as an adverse event potentially attributable to ramelteon or suvorexant.
Among the 401 patients taking ramelteon and/or suvorexant, the details regarding prescription patterns and the incidence of delirium, shown as n/total n (%), were as follows: ramelteon at 7:00 pm, 4/54 (7.4%); suvorexant at 7:00 pm, 2/9 (22.2%); ramelteon at 9:00 pm, 24/117 (20.5%); suvorexant at 9:00 pm, 15/110 (13.6%); ramelteon at 7:00 pm and suvorexant at 9:00 pm, 17/119 (14.3%); and suvorexant at 7:00 pm and ramelteon at 9:00 pm, 1/2 (50%).
Patients With Delirium on the Night Before the Consultation
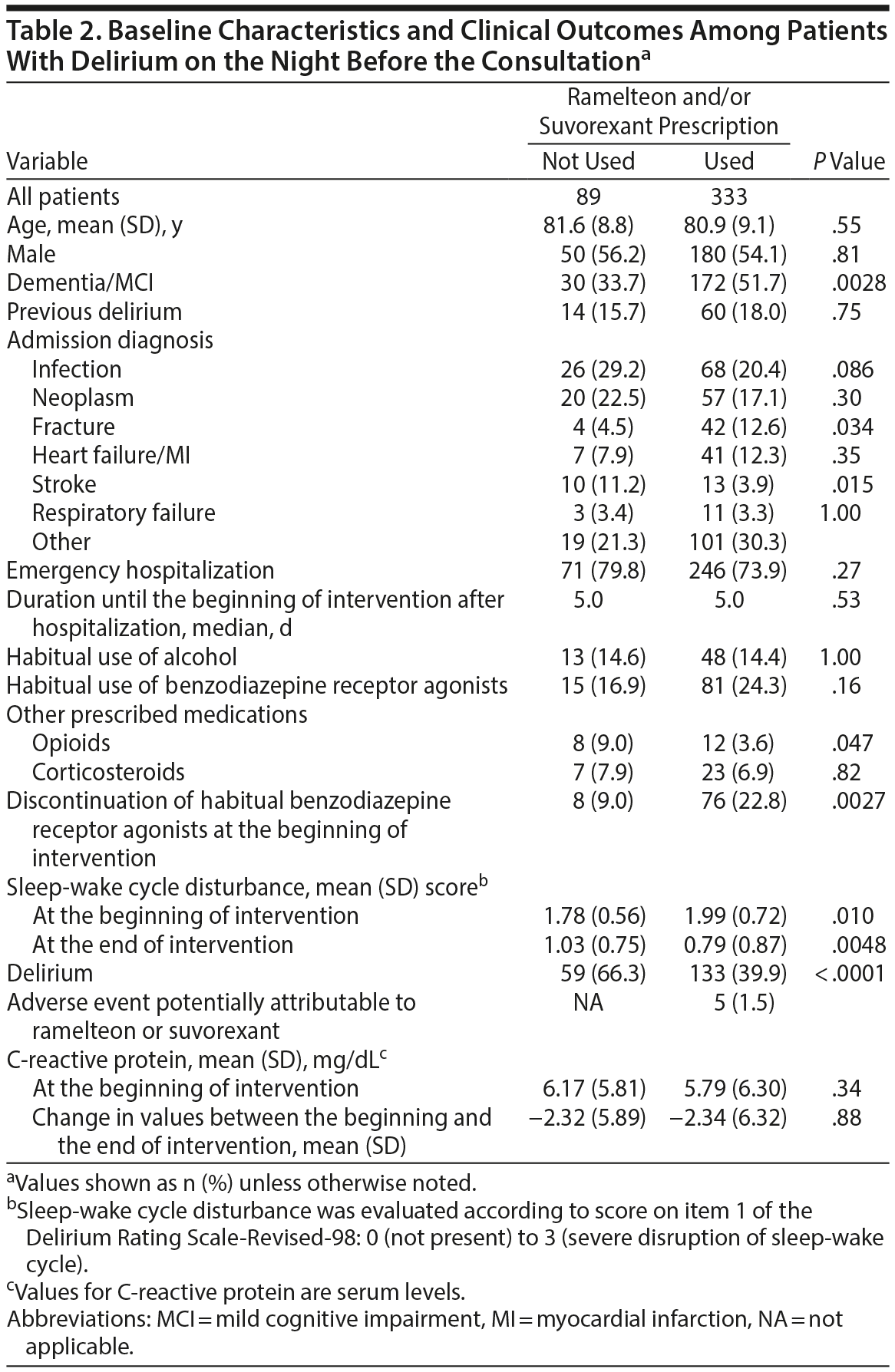
Among the 425 patients with delirium on the night before the consultation, 3 were excluded from the analysis because they were taking antipsychotics (n = 2) or trazodone (n = 1) despite being prescribed ramelteon and/or suvorexant. Overall, 333 patients chose to take ramelteon and/or suvorexant at their own discretion, but the remaining 89 did not for reasons such as subjective ease of sleep or a dislike of sleeping tablets. Significant differences were observed between the groups in rates of dementia, some admission diagnoses, use of opioids, and discontinuation of habitual benzodiazepine receptor agonists at the beginning of the intervention (Table 2).
Patients taking ramelteon and/or suvorexant developed delirium significantly less frequently than those who did not (39.9% vs 66.3%; RR = 1.66; 95% CI, 1.36-2.02; P < .0001). As significant differences were observed between groups in several variables, we constructed multivariate logistic regression models to control for the effects of such risk factors on the estimate of an independent association between the effects of ramelteon/suvorexant and the outcome of developing delirium. Even after control for the effects of these risk factors, an association remained between ramelteon and/or suvorexant and a lower incidence of delirium (OR = 0.36; 95% CI, 0.22-0.59; P < .0001). Interestingly, although the sleep-wake cycle disturbance score at the beginning of the intervention was significantly worse in patients taking ramelteon and/or suvorexant than that in those who were not (P = .010; Table 2), the sleep-wake cycle disturbance score in patients taking ramelteon and/or suvorexant improved significantly compared with that in patients who were not at the end of the intervention (P = .0048; Table 2).
Among the 422 patients with delirium on the night before the consultation, Kaplan-Meier estimates of the interval to the development of delirium were 5.63 days (95% CI, 5.30-5.96 days) and 4.33 days (95% CI, 3.73-4.92 days) for those taking and not taking ramelteon and/or suvorexant, respectively (Figure 2). A comparison using the log rank test showed that patients taking ramelteon and/or suvorexant developed delirium significantly less frequently than those who were not (χ2 = 17.94, P < .0001).
No serious adverse events were observed, but 5 patients (1.5%) showed sleepiness as an adverse event potentially attributable to ramelteon or suvorexant.
Among the 333 patients taking ramelteon and/or suvorexant, the details regarding prescription patterns and the incidence of delirium were as follows: ramelteon at 7:00 pm, 8/56 (14.3%); suvorexant at 7:00 pm, 3/11 (27.3%); ramelteon at 9:00 pm, 56/105 (53.3%); suvorexant at 9:00 pm, 29/69 (42.0%); ramelteon at 7:00 pm and suvorexant at 9:00 pm, 34/85 (40.0%); and suvorexant at 7:00 pm and ramelteon at 9:00 pm, 3/7 (42.9%). The prescription patterns depended on the discretion of the attending psychiatrist.
DISCUSSION
Effects of Ramelteon and/or Suvorexant on Delirium Prevention in Patients at High Risk
In the present study, the incidence of delirium in patients who did not take ramelteon and/or suvorexant despite being at high risk for but without delirium on the night before the consultation was 24.0%. This rate is consistent with a previous report,31 in which the incidence during hospitalization was 10%-82% in general medical and geriatric wards, intensive care units, and postoperative and palliative care settings. Remarkably, patients taking ramelteon and/or suvorexant developed delirium less frequently than those who were not. This finding from large-scale real-world practice is consistent with our previous RCTs involving ramelteon and suvorexant,25-28 which strengthens the evidence of the effects of ramelteon and/or suvorexant on delirium prevention.
The present study also showed that sleep-wake cycle disturbance scores improved significantly at the end of the intervention in patients taking ramelteon and/or suvorexant compared with those who were not. Our previous RCTs found no statistically significant differences in improved sleep-wake cycle disturbance between the ramelteon and/or suvorexant and placebo groups25,27; however, this lack of significant differences may have been because of the relatively small sample size in comparison with the present study, which had a large sample size. This finding suggests that improvements in sleep-wake cycle disturbance through the administration of ramelteon and/or suvorexant are a contributing factor for delirium prevention.
Effects of Ramelteon and/or Suvorexant on Delirium Prevention in Patients With Delirium on the Night Before the Consultation
The incidence of delirium in patients who did not take ramelteon and/or suvorexant despite having delirium on the night before the consultation was 66.3%. To our knowledge, no previous study has reported a similar finding in which two-thirds of patients with delirium on the previous night developed delirium again during a subsequent 7-day period despite receiving multicomponent, nonpharmacologic delirium prevention interventions.
Although antipsychotics have been used in the past, we use ramelteon and/or suvorexant as a first-line treatment to prevent next-night delirium in patients with delirium on the previous night. In the present study, patients taking ramelteon and/or suvorexant developed delirium significantly less frequently than those who were not. This finding is meaningful because it could lead to a decrease in the prescription of antipsychotics to older people. Our prospective observational study12 involving 2,453 patients receiving antipsychotics for delirium showed a rate of extrapyramidal symptoms of 5.6%, whereas only 1.5% of patients receiving ramelteon and/or suvorexant showed sleepiness. Therefore, ramelteon and/or suvorexant appears to offer an advantage over antipsychotics in terms of safety.
The sleep-wake cycle disturbance score improved significantly in patients taking ramelteon and/or suvorexant compared with those who were not at the end of the intervention. This finding strengthens the evidence of the effects of ramelteon and/or suvorexant on delirium prevention and improved sleep-wake cycle disturbance.
Clinical Significance of Ramelteon and/or Suvorexant on Delirium Prevention
As the incidences of delirium in patients taking and not taking ramelteon and/or suvorexant were 15.7% and 24.0%, respectively, the risk of delirium was about 8% lower among those at risk for but without delirium on the night before the consultation who were taking compared with not taking ramelteon and/or suvorexant. The difference was statistically significant, but small, mainly because of the relatively low incidence of delirium in the patients who did not take either drug. By contrast, the incidence of delirium in patients who had delirium on the night before the consultation and did not take either drug was high (66.3%), meaning that the risk of delirium was about 26% lower with ramelteon and/or suvorexant use compared with nonuse; therefore, ramelteon and suvorexant appear to be effective for delirium prevention among patients at risk for delirium and especially among those with delirium on the night before the consultation. This finding could lead to a decrease in the use of antipsychotics to treat delirium and warrant replication in similar large-scale studies.
Another interesting finding involved the effects of the timing of the drug administration. The incidence of delirium in patients taking ramelteon at 7:00 pm was much lower than that in patients taking ramelteon at 9:00 pm among those at risk for but without delirium on the night before the consultation (n/total n [%] = 4/54 [7.4%] vs 24/117 [20.5%]) and among those with delirium on the night before the consultation (n/total n [%] = 8/56 [14.3%] vs 56/105 [53.3%]). These strong effects of administration time emphasize the need for both placebo-controlled trials and the development of best practices regarding the use of sleep medication for preventing delirium.
Finally, it is worth discussing the difference between delirium and “sundowning” (ie, agitation later in the day in patients with dementia). In this study, patients with delirium on the night before but not at the time of the consultation were included. Although their disturbances had lasted only a few hours, they could have reappeared as a result of fluctuations in severity during the course of a day, as described by the DSM-5.2 Indeed, 66.3% of patients who did not take ramelteon and/or suvorexant developed delirium within 1 week (Table 2). All patients were hospitalized because of acute physical illness or surgery, so the existence of pathophysiologic changes associated with delirium, such as acute inflammation or hypoxia, was evident; this is one of the diagnostic criteria for delirium in the DSM-5. Furthermore, only patients with disturbances in attention, awareness, and cognition that could not be better explained by another preexisting, established, or evolving neurocognitive disorder as defined by the DSM-52 were included in this study.
By contrast, sundowning has been observed to represent the second most common type of disruptive behavior after wandering in institutionalized patients with dementia and has frequently been described as “endemic” in nursing homes hosting cognitively impaired older patients.32 At the same time, it has also been commonly described among community-dwelling individuals with dementia illnesses.33 In contrast to delirium, however, no standardized diagnostic criteria have been formulated for sundowning because of the lack of consensus surrounding its definition.34
Therefore, delirium is well established and appears in the DSM-5, whereas sundowning remains undefined and a subject of controversy, mainly because of its limited evaluation in homes and nursing homes. This ambiguity surrounding sundowning could be explained by the existence of some type of short-lasting delirium in the evening that overlaps sundowning or by the fact that some types of sundowning may be considered a subtype of delirium. A systematic review35 reports that sundowning/agitated behavior improves with melatonin treatment in patients with dementia. The results of the present study suggest that the administration of ramelteon and/or suvorexant could also help with such conditions.
Strengths and Limitations
A strength of the present study is that it was a full survey of nearly 1,000 eligible patients, which reflects real-world practice. At the same time, this study was observational in nature and is therefore subject to some potential limitations. First, as mentioned above, several differences were noted in the demographic and clinical characteristics of the patients taking and not taking ramelteon and/or suvorexant at their own discretion, such as subjective ease of sleep or a dislike of sleeping tablets. In addition, the patients with delirium on the night before the consultation could have experienced subtle aberrations in their decision-making ability that affected their capacity to consent to taking the study drugs. This point may generally be a potential concern in inpatients at risk for delirium, but such patients with a clear consciousness who do not pose an immediate danger to themselves or others cannot be compelled to take medication.
Unexpectedly, patients at risk for but without delirium on the night before the consultation who chose not to take the medications were not necessarily more cognitively impaired than those who did (dementia/MCI: not used 28.0% vs used 26.1%; P = .16; Table 1). In addition, fewer cognitively impaired than not cognitively impaired patients with delirium on the night before the consultation chose not to take the medications (dementia/MCI: not used 33.7% vs used 51.7%; P = .0028; Table 2). Therefore, whether to accept the recommendation from physicians or nurses to take the medications may have depended on the personality of each patient and not on the presence of MCI. To mitigate this limitation, we constructed multivariate logistic regression models.
Second, the individuals who assessed the patients’ state and symptoms and devised their therapeutic plans were not independent. However, the psychiatrists who participated in this study were all well-trained and always discussed each patient’s condition with his or her consultation-liaison team members.
The present findings are considered to be generalizable to patients at risk for or with delirium on the night before a consultation associated with medical or surgical diseases but not to patients in a delirious state.
Submitted: April 9, 2019; accepted August 1, 2019.
Published online: December 17, 2019.
Potential conflicts of interest: Dr Hatta has received lecture honoraria from Dainippon-Sumitomo, Janssen, Meiji Seika, Merck Sharp & Dohme (MSD), Otsuka, Takeda, and Tanabe-Mitsubishi and has served as a consultant for Dainippon-Sumitomo, MSD, and Meiji Seika within the past 3 years. Dr Kishi has received lecture honoraria from MSD, Eisai, Mochida, Eli Lilly, Daiichi-Sankyo, Otsuka, Tanabe-Mitsubishi, Pfizer, Shionogi, and Dainippon-Sumitomo and has served as a consultant for MSD, Meiji-Seika, and Tsumura. Dr Wada has received honoraria for Eisai, Meiji Seika, MSD, Otsuka, Pfizer, Dainippon-Sumitomo, Tanabe-Mitsubishi, Daiichi-Sankyo, and Janssen. Dr Takeuchi has received lecture honoraria from Otsuka, GlaxoSmithKline, MSD, Dainippon-Sumitomo, Janssen, Eisai, Eli Lilly, Daiichi-Sankyo, Shionogi, and Tanabe-Mitsubishi. Dr Suda has received lecture honoraria from Yoshitomi. Dr Taira has received lecture honoraria from MSD, Eisai, Meiji Seika, Otsuka, and Pfizer. Dr Tsuchida has received lecture honoraria from MSD, Shionogi, and Janssen. Dr Ohmori has received lecture honoraria from MSD, Takeda, Otsuka, Daiichi-Sankyo, and Janssen. Dr Akizuki has received lecture honoraria from Otsuka, Shionogi, and Kyowa Hakko Kirin. Dr Usui has received lecture honoraria from Shionogi, Eli Lilly, Eisai, Asahi Kasei, MSD, Nipro, and Pfizer and has served as a consultant for Pfizer. She also has received research grants from Eisai and Pfizer. Dr Kurata has received lecture honoraria from Shionogi. Dr Horikawa has received lecture honoraria from Otsuka, Eli Lilly, Janssen, Mylan, and MSD. Dr Ito has received lecture honoraria from Yoshitomi and Meiji Seika. Dr Muto has received lecture honoraria from MSD. Dr Uchimura has received lecture honoraria from MSD, Eli Lilly, Eisai, Takeda, Tanabe-Mitsubishi, Meiji Seika, and Otsuka. Drs Hashimoto, Nishio, Nakanishi, Eguchi, and Nakamura declare that they have no conflicts of interest.
Funding/support: This work was supported by Japan Society for the Promotion of Science (JSPS KAKENHI Grant-in-Aid for Scientific Research [C]: 17K10342).
Role of the sponsor: The sponsor of the study had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
1.European Delirium Association; American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 2014;12(1):141. PubMed CrossRef
2.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
3.Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. PubMed CrossRef
4.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135(pt 9):2809-2816. PubMed CrossRef
5.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. PubMed CrossRef
6.Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological, multicomponent interventions be used? a systematic review and meta-analysis of the literature. Age Ageing. 2015;44(2):196-204. PubMed CrossRef
7.Teslyar P, Stock VM, Wilk CM, et al. Prophylaxis with antipsychotic medication reduces the risk of post-operative delirium in elderly patients: a meta-analysis. Psychosomatics. 2013;54(2):124-131. PubMed CrossRef
8.Serafim RB, Bozza FA, Soares M, et al. Pharmacologic prevention and treatment of delirium in intensive care patients: a systematic review. J Crit Care. 2015;30(4):799-807. PubMed CrossRef
9.Neufeld KJ, Yue J, Robinson TN, et al. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-714. PubMed CrossRef
10.Schrijver EJ, de Graaf K, de Vries OJ, et al. Efficacy and safety of haloperidol for in-hospital delirium prevention and treatment: a systematic review of current evidence. Eur J Intern Med. 2016;27:14-23. PubMed CrossRef
11.Santos E, Cardoso D, Neves H, et al. Effectiveness of haloperidol prophylaxis in critically ill patients with a high risk of delirium: a systematic review. JBI Database Syst Rev Implement Reports. 2017;15(5):1440-1472. PubMed CrossRef
12.Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253-262. PubMed CrossRef
13.Azama T, Yano M, Oishi K, et al. Altered expression profiles of clock genes hPer1 and hPer2 in peripheral blood mononuclear cells of cancer patients undergoing surgery. Life Sci. 2007;80(12):1100-1108. PubMed CrossRef
14.Tamiya H, Ogawa S, Ouchi Y, et al. Rigid cooperation of Per1 and Per2 proteins. Sci Rep. 2016;6(1):32769. PubMed CrossRef
15.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222. PubMed CrossRef
16.Pandi-Perumal SR, BaHammam AS, Brown GM, et al. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res. 2013;23(3):267-300. PubMed CrossRef
17.Nair NP, Hariharasubramanian N, Pilapil C, et al. Plasma melatonin—an index of brain aging in humans? Biol Psychiatry. 1986;21(2):141-150. PubMed CrossRef
18.Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29(1):70-87. PubMed CrossRef
19.Liguori C, Romigi A, Nuccetelli M, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71(12):1498-1505. PubMed CrossRef
20.Hamasaki MY, Barbeiro HV, Barbeiro DF, et al. Neuropeptides in the brain defense against distant organ damage. J Neuroimmunol. 2016;290:33-35. PubMed CrossRef
21.Krystal AD, Benca RM, Kilduff TS. Understanding the sleep-wake cycle: sleep, insomnia, and the orexin system. J Clin Psychiatry. 2013;74(suppl 1):3-20. PubMed CrossRef
22.Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth. 2010;4(3):169-173. PubMed CrossRef
23.Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687-694. PubMed CrossRef
24.de Jonghe A, van Munster BC, Goslings JC, et al; Amsterdam Delirium Study Group. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. 2014;186(14):E547-E556. PubMed CrossRef
25.Hatta K, Kishi Y, Wada K, et al; DELIRIA-J Group. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71(4):397-403. PubMed CrossRef
26.Nishikimi M, Numaguchi A, Takahashi K, et al. Effect of administration of ramelteon, a aelatonin receptor agonist, on the duration of stay in the ICU: a single-center randomized placebo-controlled trial. Crit Care Med. 2018;46(7):1099-1105. PubMed CrossRef
27.Hatta K, Kishi Y, Wada K, et al; DELIRIA-J Group. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78(8):e970-e979. PubMed CrossRef
28.Azuma K, Takaesu Y, Soeda H, et al. Ability of suvorexant to prevent delirium in patients in the intensive care unit: a randomized controlled trial. Acute Med Surg. 2018;5(4):362-368. PubMed CrossRef
29.Young J, Murthy L, Westby M, et al; Guideline Development Group. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. PubMed CrossRef
30.Kato M, Kishi Y, Okuyama T, et al. Japanese version of the Delirium Rating Scale, Revised-98 (DRS-R98-J): reliability and validity. Psychosomatics. 2010;51(5):425-431. PubMed
31.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. PubMed CrossRef
32.Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287. PubMed CrossRef
33.Gallagher-Thompson D, Brooks JO 3rd, Bliwise D, et al. The relations among caregiver stress, “sundowning” symptoms, and cognitive decline in Alzheimer’s disease. J Am Geriatr Soc. 1992;40(8):807-810. PubMed CrossRef
34.Canevelli M, Valletta M, Trebbastoni A, et al. Sundowning in dementia: clinical relevance, pathophysiological determinants, and therapeutic approaches. Front Med (Lausanne). 2016;3:73. PubMed CrossRef
35.de Jonghe A, Korevaar JC, van Munster BC, et al. Effectiveness of melatonin treatment on circadian rhythm disturbances in dementia, are there implications for delirium? a systematic review. Int J Geriatr Psychiatry. 2010;25(12):1201-1208. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at [email protected], or Gary W. Small, MD, at [email protected].
This PDF is free for all visitors!