Objective: To review the literature on the metabolic side effects of aripiprazole. Three cases of aripiprazole-induced hypertriglyceridemia are also presented.
Data Sources: A search was conducted of English-language articles and abstracts (meta-analyses, randomized controlled trials, clinical trials, naturalistic open-label trials, reviews, and case reports) published up to August 31, 2014, in electronic databases (PubMed, MEDLINE).
Study Selection: Free-text and MeSH search keywords included aripiprazole, cholesterol, triglyceride, lipid profile, hyperlipidemia, and hypercholesterolemia and their differing terminations and combinations. The search was supplemented by a manual review of reference lists from the identified publications. Pediatric studies were excluded.
Data Extraction: Twenty-two articles were found and 3 aspects of the metabolic side effects of aripiprazole were reviewed: (1) the prevalence of the metabolic syndrome in mentally ill patients prior to any antipsychotic use to highlight the initial predisposition of this group of patients to develop the metabolic syndrome, (2) the prevalence of metabolic changes depending on the choice of antipsychotic (aripiprazole compared to other antipsychotics), and (3) metabolic changes reported after switching from an antipsychotic to aripiprazole.
Results: Patients with mental disorders are at high risk for developing dyslipidemia, diabetes, and the full criteria of the metabolic syndrome. Antipsychotic use exacerbates this risk, thus increasing the mortality in this population. Nevertheless, it seems that the risk for these side effects varies with each antipsychotic. Although by and large the literature supports the supposition that aripiprazole causes less metabolic effects than other antipsychotics, we report 3 cases of serious aripiprazole-related dyslipidemia in young subjects.
Conclusion: On the basis of these 3 cases, aripiprazole can cause hypertriglyceridemia. Triglyceride levels should be carefully monitored in patients with mental disorders taking aripiprazole.
Aripiprazole-Induced Hyperlipidemia:
An Update

ABSTRACT
Objective: To review the literature on the metabolic side effects of aripiprazole. Three cases of aripiprazole-induced hypertriglyceridemia are also presented.
Data Sources: A search was conducted of English-language articles and abstracts (meta-analyses, randomized controlled trials, clinical trials, naturalistic open-label trials, reviews, and case reports) published up to August 31, 2014, in electronic databases (PubMed, MEDLINE).
Study Selection: Free-text and MeSH search keywords included aripiprazole, cholesterol, triglyceride, lipid profile, hyperlipidemia, and hypercholesterolemia and their differing terminations and combinations. The search was supplemented by a manual review of reference lists from the identified publications. Pediatric studies were excluded.
Data Extraction: Twenty-two articles were found and 3 aspects of the metabolic side effects of aripiprazole were reviewed: (1) the prevalence of the metabolic syndrome in mentally ill patients prior to any antipsychotic use to highlight the initial predisposition of this group of patients to develop the metabolic syndrome, (2) the prevalence of metabolic changes depending on the choice of antipsychotic (aripiprazole compared to other antipsychotics), and (3) metabolic changes reported after switching from an antipsychotic to aripiprazole.
Results: Patients with mental disorders are at high risk for developing dyslipidemia, diabetes, and the full criteria of the metabolic syndrome. Antipsychotic use exacerbates this risk, thus increasing the mortality in this population. Nevertheless, it seems that the risk for these side effects varies with each antipsychotic. Although by and large the literature supports the supposition that aripiprazole causes less metabolic effects than other antipsychotics, we report 3 cases of serious aripiprazole-related dyslipidemia in young subjects.
Conclusion: On the basis of these 3 cases, aripiprazole can cause hypertriglyceridemia. Triglyceride levels should be carefully monitored in patients with mental disorders taking aripiprazole.
Prim Care Companion CNS Disord 2016;18(4):doi:10.4088/PCC.16r01958
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Lebanese University, Beirut, Lebanon
*Corresponding author: Caroline Tarraf, MD, Department of Psychiatry, Lebanese University, Beirut 961, Lebanon ([email protected]).
Patients with schizophrenia or bipolar disorder often have an increased risk of developing dyslipidemia, diabetes, hypertension, and coronary heart disease.1,2 Moreover, the metabolic adverse effects related to the use of antipsychotic medications, which include weight gain, dyslipidemia, and impaired glucose metabolism, can exacerbate cardiovascular morbidity and mortality in these patients, placing them at a higher mortality risk when on neuroleptic therapy.1 Nevertheless, it seems that the risk for these side effects varies within the palette of second-generation or novel antipsychotics.1
Aripiprazole is an antipsychotic medication that improves both the positive and negative symptoms of schizophrenia, presumably because of its partial agonist rather than antagonist activity at the dopamine D2 receptors.3 Aripiprazole is also a partial agonist at the serotonin 5-hydroxytryptamine 1A (5-HT1A) receptors, which results in an anxiolytic effect, in addition to its 5-HT2A serotonin receptor antagonist action, which alleviates the depressive symptoms and cognitive dysfunction in patients with schizophrenia.3,4 The impact of aripiprazole on lipid levels is reported in randomized clinical trials.5 Aripiprazole has been found to be a safe drug with a lower likelihood of inducing metabolic abnormalities than other second-generation antipsychotics.5 In a 26-week trial, Pigott et al4 evaluated changes in fasting total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol levels and triglycerides in patients with chronic, stable schizophrenia (N = 310). Minimal changes were recorded in mean HDL cholesterol (+2.0 mg/dL with aripiprazole and +0.89 mg/dL with placebo) and LDL cholesterol levels (−5.1 mg/dL with aripiprazole and −2.9 mg/dL with placebo).4 Furthermore, there was a significant mean decrease in total cholesterol and triglyceride levels with aripiprazole compared to placebo (−2.7 mg/dL and −2.9 mg/dL, respectively).4 Yet, despite these findings, we have experienced with 3 of our patients an elevation of their triglyceride levels while taking aripiprazole.
In this article, we review the literature on the metabolic side effects of aripiprazole. Three cases of aripiprazole-induced hypertriglyceridemia are also presented.
METHOD
We conducted a comprehensive search of English-language articles and abstracts (meta-analyses, randomized controlled trials, clinical trials, naturalistic open-label trials, reviews, and case reports) published up to August 31, 2014, in electronic databases (PubMed, MEDLINE) using both free-text and MeSH search keywords aripiprazole, cholesterol, triglyceride, lipid profile, hyperlipidemia, and hypercholesterolemia and their differing terminations and combinations. The search was supplemented by a manual review of reference lists from the identified publications. Pediatric studies were excluded.
Twenty-two articles were found,1-22 and 3 aspects of the metabolic side effects of aripiprazole were reviewed: (1) the prevalence of the metabolic syndrome in mentally ill patients prior to any antipsychotic use to highlight the initial predisposition of this group of patients to develop the metabolic syndrome, (2) the prevalence of metabolic changes depending on the choice of antipsychotic (aripiprazole compared to other antipsychotics), and (3) metabolic changes reported after switching from an antipsychotic to aripiprazole.

- Aripiprazole has been considered to be a safe drug with regard to metabolic side effects; however, the literature shows conflicting results.
- Monitoring of triglyceride levels and other criteria of the metabolic syndrome in patients taking aripiprazole should be considered.
RESULTS
Metabolic Syndrome in Untreated Mentally Ill Patients
Patients with severe mental illness have higher rates of cardiovascular disease and diabetes compared to the general population,6 so practitioners must be more cautious about the metabolic side effects while prescribing an antipsychotic. Indeed, the metabolic syndrome, defined by the National Cholesterol Education Program (NCEP)23 as the presence of 3 or more of the following 5 risk factors: obesity with a waist circumference > 102 cm in men or > 88 cm in women, hypertriglyceridemia of > 150 mg/dL, HDL cholesterol levels < 40 mg/dL in men and < 50 mg/dL in women, hypertension of > 130/85 mm Hg, and a fasting blood sugar level > 110 mg/dL, was found to be a key risk factor for the development of these 2 conditions.7 Thus, the prevalence of the metabolic syndrome in mentally ill patients and whether it is related to the psychiatric illness characteristics or to the secondary effects of treatment with antipsychotics is the subject of various studies.8-11
Reddy et al8 conducted a literature review of studies evaluating the prevalence of the metabolic syndrome in drug-naive schizophrenic patients. Findings from 12 studies meeting the inclusion criteria revealed that the rates of metabolic syndrome in antipsychotic-naive and first-episode schizophrenic patients did not significantly differ from controls (average prevalence of 10.8%).8 However, severe mood disorders and psychotic disorders may predispose to physiologic changes that will lead to an increase in the prevalence of the metabolic syndrome before the prescription of any antipsychotic agent.6
Different mechanisms have been proposed for the observed metabolic dysregulation in schizophrenic patients. The most commonly proposed mechanism is the hypothalamic-pituitary-adrenal axis dysfunction: stress-induced adrenocorticotropic release that leads to chronic hypercortisolemia, which is responsible for the central obesity and lipid and glycemic derangements that constitute the metabolic syndrome.9 It is also plausible that behavioral consequences of schizophrenia contribute to these changes, such as smoking, unhealthy diet, and exercise patterns common in this population.10 Furthermore, Ryan et al9 measured various fatness and fat distribution parameters (by computed tomography scanning and anthropometry) and the plasma cortisol level in 19 drug-naive and drug-free schizophrenic patients in comparison to a matched group of healthy controls. Patients with schizophrenia were found to consume less fiber and more saturated fat and to have over 3 times more visceral fat compared to the body mass index-matched control group,11 which places them at a higher risk of developing a metabolic syndrome.
Metabolic Profiles of Different Antipsychotics
McEvoy and colleagues7 used baseline data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial (patients with schizophrenia on different antipsychotics) to assess metabolic syndrome prevalence on the basis of NCEP criteria and using a fasting glucose threshold of 100 mg/dL (American Heart Association [AHA]24). Of 1,460 CATIE baseline subjects, 689 met the analysis criteria. Metabolic syndrome prevalence was 40.9% (n = 686) and 42.7% (n = 687), respectively, using the NCEP- and AHA-derived criteria. In females, it was 51.6% and 54.2%, respectively, using the NCEP and AHA criteria compared to 36.0% (P = .0002) and 36.6% (P = .0003), respectively, for males.7 Hence, the metabolic syndrome is more prevalent among patients with schizophrenia taking antipsychotics, particularly females, than the general population. However, evidence from published data12 indicates that second-generation antipsychotic agents differ in their effects on weight and blood glucose and lipid levels.
While clozapine and olanzapine are associated with an increased risk of elevation in plasma triglyceride levels, diabetes mellitus, and weight gain, other studies12 with a smaller effect size suggest limited, if any, increased risk for treatment-induced diabetes mellitus and dyslipidemia during risperidone and quetiapine treatment, and available data suggest that aripiprazole and ziprasidone treatments are not associated with an increased risk of dyslipidemia. Olfson et al13 assessed the risk of developing hyperlipidemia after treatment with antipsychotics (aripiprazole, clozapine, olanzapine, quetiapine, risperidone, ziprasidone, or typical antipsychotics) among 4,725 treated adults. The analysis showed that treatment with clozapine (odds ratio [OR] = 1.8), olanzapine (1.6), quetiapine (1.5), risperidone (1.5), ziprasidone,1,4 or typical antipsychotics (1.3), but not aripiprazole (OR = 1.2), was associated with a significant increase in the incidence of hyperlipidemia (P < .05).13 Moreover, an open-label 12-week study14 among 42 patients in which drug-naive, first-episode patients with schizophrenia received aripiprazole indicates that triglyceride and cholesterol levels did not significantly vary when taking aripiprazole: the mean total cholesterol level at baseline was 180.6 mg/dL and at endpoint was 172.1 mg/dL, the mean LDL cholesterol level at baseline was 90.2 mg/dL and at endpoint was 86.5 mg/dL, and the mean triglyceride level at baseline was 107.3 mg/dL and at endpoint was 114.3 mg/dL.14
Furthermore, the CATIE study consisted of 3 phases, and aripiprazole was only studied in the third phase.15 Patients who discontinued antipsychotic treatment in phase 2 were eligible for phase 3, which was an open-label trial conducted to assess the efficacy, safety, and tolerability of 9 flexibly dosed antipsychotic treatment options in 270 adults (aged 18-65 years, mean ± SD age of 40.5 ± 11.0 years) with a DSM-IV diagnosis of schizophrenia. Treatment options included monotherapy with oral aripiprazole (approved by the US Food and Drug administration in November 2002, added only to phase 3 of this study and after the enrollment of 65% of the patients), clozapine, olanzapine, perphenazine, quetiapine, risperidone, ziprasidone, long-acting injectable fluphenazine decanoate, or a combination of any 2 of these medications.16 The respective mean changes in the cholesterol levels from baseline for aripiprazole, clozapine, olanzapine, perphenazine, quetiapine, risperidone, ziprasidone, and long-acting injectable fluphenazine decanoate were −0.6, 0.0,15.9, 13.3, 4.0, 6.6, −16.1, and 8.3 mg/dL. Conversely, the mean changes in the triglyceride levels were −6.9, 55.6, 27.5, 28.2, 13.9, 24.6, −24.8, and −23.9 mg/dL, respectively.16 Hence, aripiprazole and ziprasidone were found to be associated, contrary to other antipsychotics, with a decrease from the baseline levels of cholesterol and triglycerides.
Metabolic Side Effects of Aripiprazole
McQuade et al5 studied 317 patients with schizophrenia in acute relapse after randomization to treatment with aripiprazole or olanzapine. At week 26, the mean changes in fasting triglyceride levels were +79.4 mg/dL with olanzapine and +6.5 mg/dL with aripiprazole (P < .05). The change in HDL cholesterol from baseline also favored aripiprazole (+3.61 mg/dL for aripiprazole vs −3.39 mg/dL for olanzapine).5 Moreover, the difference in the incidence of new-onset dyslipidemia was significant between the 2 groups: total cholesterol > 200 mg/dL in 47% of olanzapine group patients versus 17% in the aripiprazole group, LDL cholesterol > 130 mg/dL in 38% of olanzapine group patients versus 19% in the aripiprazole group, and triglycerides > 150 mg/dL in 50% of olanzapine group patients versus 18% in the aripiprazole group.5
Another 26-week, randomized, naturalistic, open-label study17 was conducted in Europe to assess the effectiveness of aripiprazole versus standard of care (which included the atypical antipsychotic agents olanzapine, quetiapine, and risperidone) among 555 patients with schizophrenia. In the aripiprazole group compared to the standard of care group, a clinically significant total cholesterol level was found in 52.9% versus 70.2% of the patients, respectively; a relevant LDL cholesterol level was found in 39.1% versus 60.0%; and a relevant triglyceride level was found in 47.8% versus 59.7%. No clinically significant differences in fasting HDL cholesterol level were observed between treatment groups.17 Furthermore, a 26-week randomized, double-blind, placebo-controlled, multicenter study3 enrolled patients with bipolar I disorder (manic or mixed episodes) stabilized on aripiprazole. The results indicate no difference in the change from baseline of the metabolic parameters (fasting HDL and LDL cholesterol levels) between the aripiprazole group and the placebo group.3 Fornal et al18 conducted a post hoc analysis on the lipid profile of the same sample and reported no significant difference in lipid profile between the aripiprazole-randomized versus placebo-randomized groups.
In contrast, in a letter to the editor, Tolliver et al19 reported 3 cases of reversible hypertriglyceridemia among patients with schizophrenia, schizoaffective disorder, or bipolar disorder and comorbid substance use disorders (alcohol, cocaine, and cannabis). Twenty patients were assigned to an 8-week open-label clinical trial in which a minimum of 1 dose of aripiprazole was given (mean dose = 17 mg/dL). A clinically significant increase in triglyceride level was observed in 3 cases (+107%, +54%, and +225% over baseline; mean = +256.6 mg/dL) in addition to an increase in the random cholesterol level in each of these cases (+40%, +12%, and +31% over baseline; mean = +45.0 mg/dL). However, triglyceride levels declined within 2 weeks after aripiprazole discontinuation.19 There is a single study20 that assessed the long-term safety and tolerability of aripiprazole augmentation to antidepressant therapy in patients with major depressive disorder. No clinically significant mean changes from baseline in fasting cholesterol, high- and low-density lipoprotein cholesterol, or triglyceride levels were observed when aripiprazole was used as an add-on in this population.
Antipsychotic Switch to Aripiprazole and Lipid Metabolism
An analysis by De Hert et al21 suggests that when amisulpride, clozapine, olanzapine, quetiapine, risperidone, and other typical antipsychotics were replaced by aripiprazole, a significant decrease in triglyceride and total cholesterol levels was recorded (P = .001) as well as a drop in LDL (P = .002) but not in HDL cholesterol levels. Moreover, in an 8-week open-label trial14 among overweight or obese patients (body mass index > 26 kg/m2) treated with haloperidol, olanzapine, perphenazine, quetiapine, risperidone, or ziprasidone, switching from olanzapine to aripiprazole treatment was significantly associated with a reduction in triglyceride and LDL cholesterol levels (P < .05). Furthermore, in a 26-week clinical trial,22 a switch to aripiprazole from other atypical antipsychotics was associated with lower triglyceride and LDL cholesterol levels, a significant decrease in total cholesterol levels, and a significant increase in HDL cholesterol level.
case reports
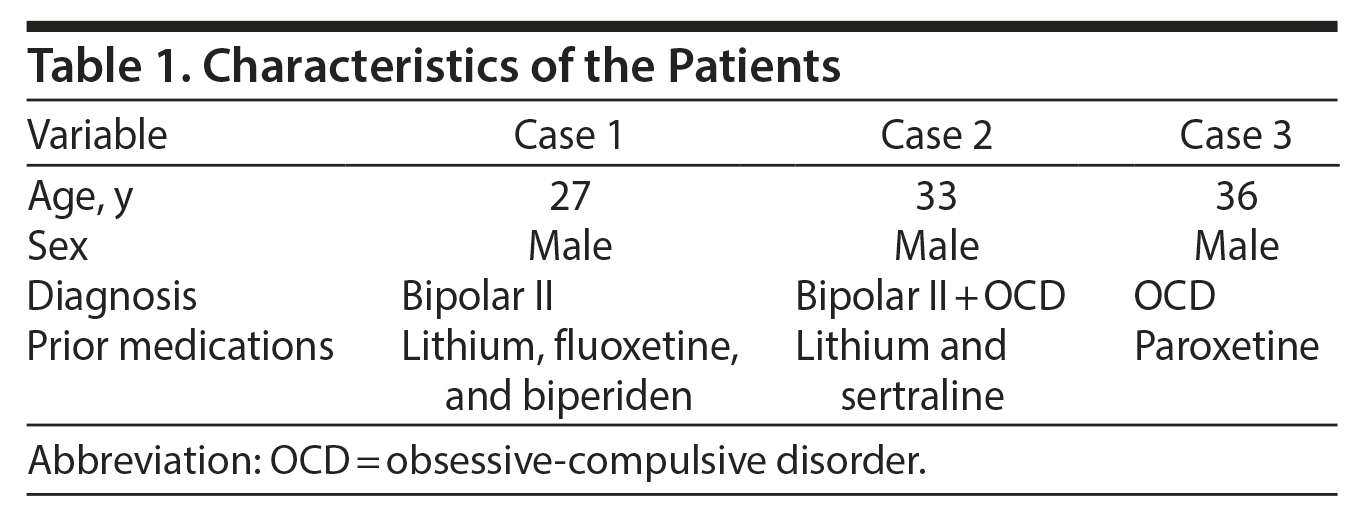
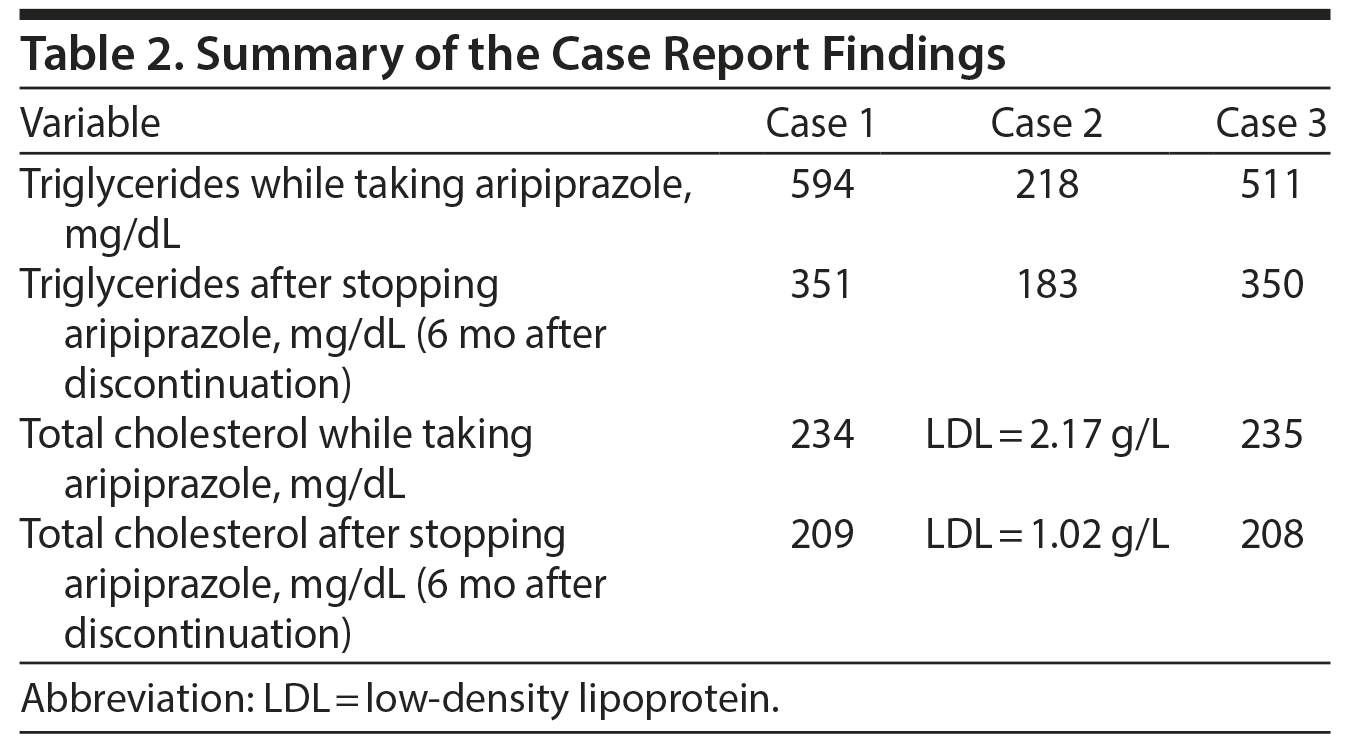
Although by and large the literature supports the supposition that aripiprazole causes less metabolic effects, we hereby report 3 cases of serious aripiprazole-related dyslipidemia in young subjects (Tables 1 and 2).
Case 1
Mr A is a 27-year-old man diagnosed with bipolar II disorder (DSM-IV criteria) in 2008 following a hypomanic episode. Aripiprazole 15 mg once daily was added for the first time in the course of his illness in January 2009. Prior to starting aripiprazole, Mr A had no history of significant physical illnesses, in particular, no abnormal lipid or high blood glucose levels. His baseline triglyceride level was 192 mg/dL, and his baseline total cholesterol level was 137 mg/dL. There was no known family history of hyperlipidemia, ischemic heart disease, or diabetes mellitus.
In September 2012, a regular laboratory workup revealed a triglyceride level of 594 mg/dL (4 times the normal level) and a total cholesterol level of 234 mg/dL that was mildly elevated but 2-fold his baseline level (method used: Hitachi 704 Analyzer, Roche Diagnostics); no changes in his fasting blood sugar or hemoglobin A1c (HbA1c) levels were recorded. His body weight remained intact. In addition to aripiprazole, prescribed medications were lithium carbonate 400 mg 1 tablet twice/d, fluoxetine 20 mg 1 tablet once/d, and biperiden 1 tablet twice/d. Six months following aripiprazole cessation, his triglyceride level decreased to 351 mg/dL, and his total cholesterol level was 209 mg/dL (within the normal limits) with no antihyperlipidemic medication.
Case 2
Mr B is a 33-year-old man with a dual diagnosis of bipolar II disorder and obsessive-compulsive disorder (DSM-IV criteria). He has no medical history or family history of hyperlipidemia or ischemic heart disease. Aripiprazole 15 mg once/d was added to his treatment (lithium and sertraline) in April 2010. In February 2011, a routine laboratory workup showed that his triglyceride and LDL cholesterol levels were 218 mg/dL and 2.17 g/L, respectively, which was approximately 2-fold his baseline levels (method used: Hitachi 704 Analyzer). However, his body weight, fasting blood sugar, and HbA1c levels remained unchanged. In less than a year after discontinuation of aripiprazole, his triglyceride level decreased to 126 mg/dL and his total and LDL cholesterol levels were 183 mg/dL and 1.02 g/L, respectively, with no prescription of antihyperlipidemic medication.
Case 3
Mr C is a 36-year-old man diagnosed with obsessive-compulsive disorder (DSM-IV criteria) and reported to have poor insight. Aripiprazole 15 mg once/d was added to his treatment for the first time in September 2012. Prior to starting aripiprazole, he had no history of significant physical illness, including dyslipidemia. His baseline triglyceride and cholesterol levels were within the normal range. He is not known to have any family history of hyperlipidemia, ischemic heart disease, or diabetes mellitus.
In April 2013, while he was taking paroxetine 20 mg 1½ tablets/d and aripiprazole, a regular laboratory workup showed the following: triglyceride level of 511 mg/dL, a mildly elevated total cholesterol level of 235 mg/dL (method used: Hitachi 704 Analyzer), and no recorded changes in fasting blood sugar or HbA1c levels. His body weight was intact. After discontinuation of aripiprazole, the triglyceride level decreased to 350 mg/dL and the total cholesterol level was 208 mg/dL (within normal limits) with no antihyperlipidemic drug prescribed.
CONCLUSION
The use of second-generation or novel antipsychotics for the treatment of various psychiatric conditions suggests an association between antipsychotic treatment and deterioration of metabolic variables, including lipid abnormalities. However, the literature suggests that aripiprazole, a D2 partial agonist, is presumably a safe drug with the lowest likelihood of inducing metabolic abnormalities among other second-generation antipsychotics, and that switching from an antipsychotic with a high metabolic risk to aripiprazole can reduce this risk.1,5
To our knowledge, only Tolliver et al19 reported a significant increase in triglyceride level rather than cholesterol in patients treated with aripiprazole. Therefore, our cases provide further preliminary evidence to the association between aripiprazole treatment and elevation of triglyceride levels (more than a 2-fold rise), rather than the cholesterol levels, among young patients. Moreover, no known studies or case reports investigate the effect of aripiprazole on the lipid profile among young men with bipolar II disorder. Thus, our cases contribute to the literature by exploring these variables in this specific population.
It is worth mentioning that our patients did not have a previous medical history of dyslipidemia or other metabolic disorders, it was their first exposure to aripiprazole during the course of their illness, and they were not prescribed any other medication with a metabolic adverse effect that might have induced these lipid profile changes. Finally, further studies are warranted to confirm or refute our findings and consequent clinical recommendations.
Submitted: April 8, 2016; accepted July 12, 2016.
Published online: August 25, 2016.
Drug names: aripiprazole (Abilify), biperiden (Akineton), clozapine (Clozaril, FazaClo, and others), fluoxetine (Prozac and others), olanzapine (Zyprexa and others), paroxetine (Paxil, Pexeva, and others), quetiapine (Seroquel and others), risperidone (Risperdal and others), sertraline (Zoloft and others), ziprasidone (Geodon and others).
Potential conflicts of interest: None.
Funding/support: None.
REFERENCES
1. Citrome L, Kalsekar I, Baker RA, et al. A review of real-world data on the effects of aripiprazole on weight and metabolic outcomes in adults. Curr Med Res Opin. 2014;30(8):1629-1641. PubMed doi:10.1185/03007995.2014.908280
2. Newcomer JW. Comparing the safety and efficacy of atypical antipsychotics in psychiatric patients with comorbid medical illnesses. J Clin Psychiatry. 2009;70(suppl 3):30-36. PubMed doi:10.4088/JCP.7075su1c.05
3. Keck PE Jr, Calabrese JR, McQuade RD, et al; Aripiprazole Study Group. A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder. J Clin Psychiatry. 2006;67(4):626-637. PubMed doi:10.4088/JCP.v67n0414
4. Pigott TA, Carson WH, Saha AR, et al; Aripiprazole Study Group. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048-1056. PubMed doi:10.4088/JCP.v64n0910
5. McQuade RD, Stock E, Marcus R, et al. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry. 2004;65(suppl 18):47-56. PubMed
6. Bermudes RA, Keck PE Jr, Welge JA. The prevalence of the metabolic syndrome in psychiatric inpatients with primary psychotic and mood disorders. Psychosomatics. 2006;47(6):491-497. PubMed doi:10.1176/appi.psy.47.6.491
7. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32. PubMed doi:10.1016/j.schres.2005.07.014
8. Reddy SM, Goudie CT, Agius M. The metabolic syndrome in untreated schizophrenia patients: prevalence and putative mechanisms. Psychiatr Danub. 2013;25(suppl 2):S94-S98. PubMed
9. Ryan MCM, Sharifi N, Condren R, et al. Evidence of basal pituitary-adrenal overactivity in first episode, drug naׯve patients with schizophrenia. Psychoneuroendocrinology. 2004;29(8):1065-1070. PubMed doi:10.1016/j.psyneuen.2003.08.011
10. Peet M. Diet, diabetes and schizophrenia: review and hypothesis. Br J Psychiatry suppl. 2004;47:S102-S105. PubMed doi:10.1192/bjp.184.47.s102
11. Ryan MC, Flanagan S, Kinsella U, et al. The effects of atypical antipsychotics on visceral fat distribution in first episode, drug-naive patients with schizophrenia. Life Sci. 2004;74(16):1999-2008. PubMed doi:10.1016/j.lfs.2003.08.044
12. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93. PubMed doi:10.2165/00023210-200519001-00001
13. Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163(10):1821-1825. PubMed doi:10.1176/ajp.2006.163.10.1821
14. Takahashi H, Oshimo T, Ishigooka J. Efficacy and tolerability of aripiprazole in first-episode drug-naive patients with schizophrenia: an open-label trial. Clin Neuropharmacol. 2009;32(3):149-150. PubMed doi:10.1097/WNF.0b013e31817c6b06
15. Stroup TS, McEvoy JP, Swartz MS, et al. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull. 2003;29(1):15-31. PubMed doi:10.1093/oxfordjournals.schbul.a006986
16. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12. PubMed doi:10.1016/j.schres.2008.10.011
17. Kerwin R, Millet B, Herman E, et al. A multicentre, randomized, naturalistic, open-label study between aripiprazole and standard of care in the management of community-treated schizophrenic patients Schizophrenia Trial of Aripiprazole: (STAR) study. Eur Psychiatry. 2007;22(7):433-443. PubMed doi:10.1016/j.eurpsy.2007.03.002
18. Fornal A, Rollin L, Nyllas M. Long term effects of aripiprazole on the lipid profiles of patients with bipolar I disorder. 159th Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Canada.
19. Tolliver BK, McRae AL, Verduin ML, et al. Reversible elevation of triglycerides in dual-diagnosis patients taking aripiprazole: a case series. J Clin Psychopharmacol. 2008;28(4):464-467. PubMed doi:10.1097/JCP.0b013e31817efb99
20. Berman RM, Marcus RN, Swanink R, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):843-853. PubMed doi:10.4088/JCP.v68n0604
21. De Hert M, Hanssens L, van Winkel R, et al. A case series: evaluation of the metabolic safety of aripiprazole. Schizophr Bull. 2007;33(3):823-830. PubMed doi:10.1093/schbul/sbl037
22. Kim S-W, Shin I-S, Kim J-M, et al. Effectiveness of switching to aripiprazole from atypical antipsychotics in patients with schizophrenia. Clin Neuropharmacol. 2009;32(5):243-249. PubMed doi:10.1097/WNF.0b013e31819a68b5
23. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421. PubMed
24. American Heart Association. About Metabolic Syndrome. http://www.heart.org/HEARTORG/Conditions/More/MetabolicSyndrome/About-Metabolic-Syndrome_UCM_301920_Article.jsp#.V6oNU5grLGg. Accessed August 9, 2016.
Enjoy this premium PDF as part of your membership benefits!