A Case of Psychosis After Use of a Detoxification Kit and a Review of Techniques, Risks, and Regulations Associated With the Subversion of Urine Drug Tests
Context: The practice of drug testing in the workplace has been adopted for US federal government employees, and many state and local governments as well as private businesses have followed suit. However, a parallel industry dedicated to subverting the results of urine drug testing has emerged with little or no regulation.
Evidence Acquisition: First, the case of a 19-year-old man who developed psychosis after the use of a detoxification kit is presented. Second, a review of the existing literature on the techniques, risks, and regulations associated with the use of drug tampering kits is provided. PubMed, Cochrane Database, and Google Scholar were searched using the keywords UDS, urine toxicology, pass the drug test, and clean UA, with no restrictions on publication date. Case reports, letters to the editor, and original research and review articles in multiple languages were reviewed, as were federal regulations and acts on the topic. The search yielded 4,082 results, of which 49 articles were selected for relevance. Some articles were later omitted as they had cited the original article and had nothing new to offer.
Results: Three commonly used tampering techniques are in vivo adulteration, urine substitution, and in vitro adulteration. Review of the literature regarding the risks involved with use of tampering kits yielded no results. In 1986, an executive order was issued requiring all federal employees to refrain from illicit drug use, and the 1988 Drug-Free Workplace Act precipitated the Substance Abuse and Mental Health Services Administration guidelines and their subsequent revisions. Recently, many states have made regulatory efforts to bring drug test defrauding under the ambit of law.
Conclusions: Clinicians need to be aware of the tampering techniques and the possibility of false-negative urine drug tests. Cognizance of inherent risks involved with using these techniques including psychiatric and/or medical complications is also warranted. The manufacture, sale, and use of these products have little or no regulation by state or federal authorities, making them potentially dangerous and imposing new challenges in testing for abused drugs. The extent of use of these products and techniques is not known at this time and is an area that warrants further research.
Prim Care Companion CNS Disord 2011;13(5):doi:10.4088/PCC.11r01178
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: March 9, 2011; accepted May 9, 2011.
Published online: October 20, 2011.
Corresponding author: Ahsan Y. Khan, MD, Department of Psychiatry and Behavioral Sciences, University of Kansas School of Medicine-Wichita, 1010 N Kansas, Wichita, KS 67214 ([email protected]).
Illicit drug use and the inherent risks involved with such use have been and continue to be a significant concern around the world. The National Survey on Drug Use and Health in 2008 found the overall rate of illicit drug use in the United States among the population aged 12 years and older to be 8.0%, and it rose to 8.7% in 2009.1 When the problem is this significant, it affects not only the individuals using the substances and their families, but also workplaces and educational campuses. As the substance use impairs one’s ability to function in any given setting, it is important that some regulation is in place.
Clinical Points
- Contact the laboratory in the case of a negative urine drug specimen when accompanied by a high index of suspicion of drug use.
- Specimen integrity tests and sample collection procedures should never be ignored.
- Detoxification kits must be included in the differential for causation of medical and/or psychiatric symptoms.
- Advocacy for more stringent laws regarding the manufacture, sale, and marketing of drug test tampering products is warranted.
The drug-testing movement began in 1986 when former US President Ronald Reagan signed Executive Order 12564. This order required all federal employees to refrain from using illegal drugs, on or off duty, as a condition of federal employment.2 Two years later, the US Congress passed the Drug-Free Workplace Act of 1988.3 That, in turn, spawned the creation of the Federal Mandatory Guidelines for Federal Workplace Drug Testing Programs.4 Although the Drug-Free Workplace Act applies only to federal employees, many state and local governments followed suit and adopted similar state laws and drug-free workplace programs. These developments led to research and subsequent development in the industry of ways to screen for illicit drugs. However, over the years, a parallel industry dedicated to subverting the results of urine drug testing also emerged and has been in service for more than a decade now. A search of the key phrase beat a drug test in 1 of the popular Internet search engines, Google, yields 12 million results. Some of the products available on the market were Urine Aid, Urine Luck, Klear, and Whizzies. The products have little or no regulation by state or federal authorities, and some do not even mention their ingredients. The absence of such regulation makes these products potentially dangerous.
We hereby present the case of a patient who developed psychosis secondary to the use of a detoxification kit. In addition, we discuss the literature available on this topic.
CASE Report
Mr A, a 19-year-old single, unemployed, Asian man, was brought by the police and emergency medical service to the emergency department after his mother called 911 because of his bizarre behavior. Mr A reported that for the last 2 days he had been having visual and auditory hallucinations of gang members in his house. Mr A believed that he had betrayed them and that they were out to kill him. He believed the gang members had shot and killed his younger brother. Mr A stated that he had been approached by the US Federal Bureau of Investigation (FBI) to be an informant and was now working for them. He believed that there was an America’s Most Wanted episode featuring him.
Collateral information was obtained from the patient’s mother, who reported that Mr A had no association with gangs, that there had been no gang members in the house, and that the patient’s report of gang members having killed his younger brother was untrue. The mother and other family members denied any previous psychotic episode in this patient. There was no family history of any psychiatric illness, and his medical history was unremarkable.
Upon further questioning, he reported that he had smoked cannabis 2 to 3 times a week for the past year. The last time that he had smoked cannabis was a week before this incident. Recently, prior to hospitalization, Mr A had applied for a job that required a urine specimen for a drug screen. He, upon advice from a friend, started using a detoxification kit in hope of passing the drug test.
While in the emergency department, Mr A became agitated and combative. He was observed to be responding to internal stimuli. The physical examination did not reveal any abnormal findings. No anxiety or mood symptoms were reported. He was given lorazepam 2 mg and haloperidol 5 mg intramuscularly to control his agitation. He was later admitted to an inpatient psychiatric unit with a working DSM-IV-TR diagnosis of psychosis not otherwise specified. After admission to the psychiatric unit, his urine drug screen came back positive for cannabinoids, with a tetrahydrocannabinol (THC) level of 268 ng/mL, and for benzodiazepines. A comprehensive metabolic profile revealed an elevated aspartate aminotransferase (AST) level of 321 U/L and an elevated alanine aminotransferase (ALT) level of 78 U/L. His total bilirubin level was high at 2.3 mg/dL, which trended down during the hospitalization, and at discharge had returned to normal.
During this hospitalization, Mr A was provided with safe therapeutic milieu. He was placed on standing orders of lorazepam 1 mg and haloperidol 5 mg as needed. He did not require any doses, as he did not become agitated during his stay at the hospital. As mentioned previously, on day 1, Mr A was convinced that there were gang members in his house and that there was an America’s Most Wanted episode about him suggesting that he was an FBI informant. On day 2, his psychotic symptoms improved, and he gradually gained insight into his illness. By day 4, his psychotic symptoms had resolved without the use of any antipsychotic medication. Mr A was discharged home on day 4 with the final DSM-IV-TR diagnosis of substance-induced psychotic disorder with delusions resolved.
Follow-up of Mr A 6 months after this episode revealed no residual psychotic symptoms. He was in good physical health with no hepatic abnormalities.
Case DISCUSSION
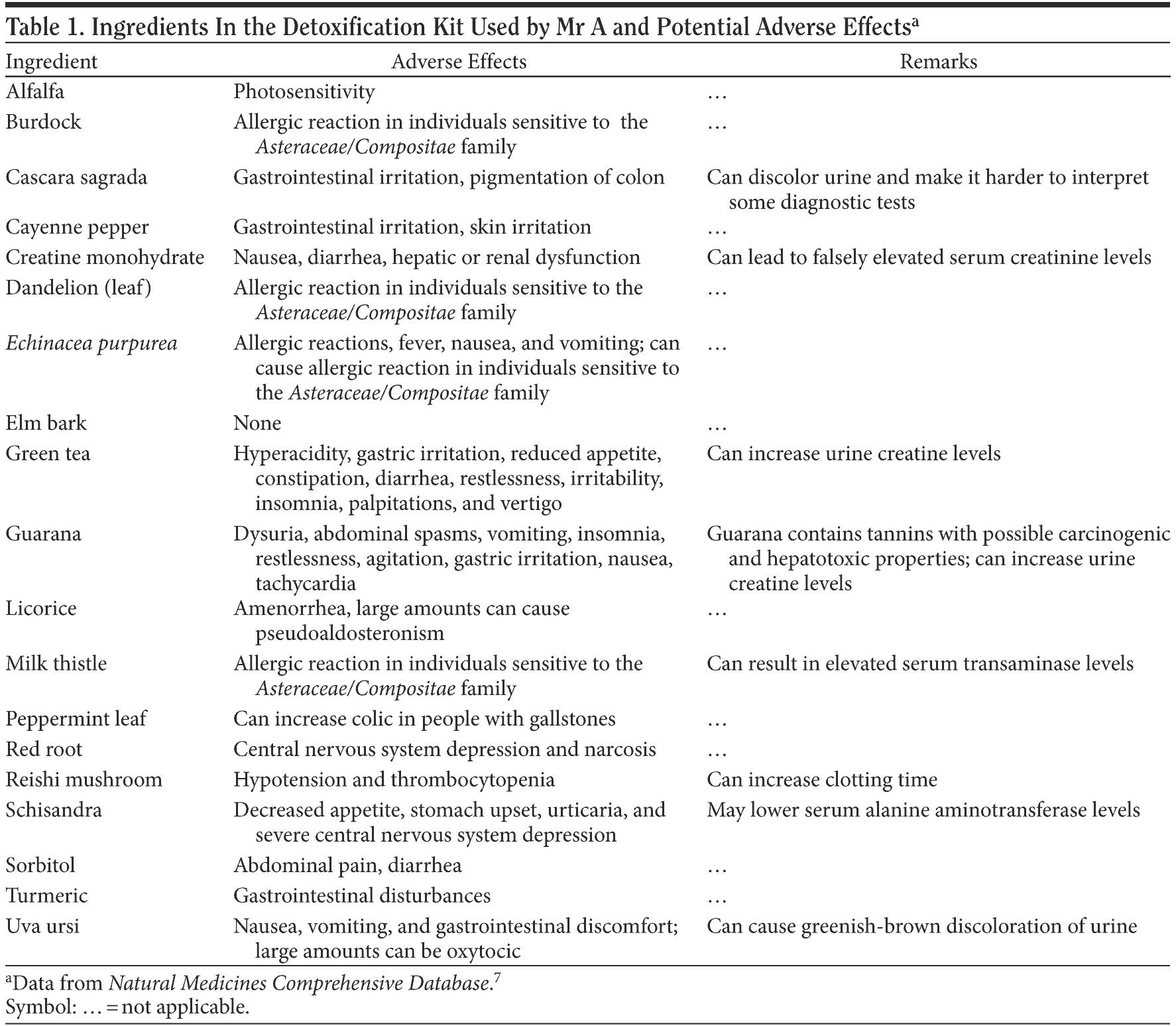
Mr A’s family was asked to bring in the detoxification kit that the patient had used. The product was Premium Detox by Herbal Clean. The ingredients are reported as riboflavin (vitamin B2), cyanocobalamin (vitamin B12), dandelion, cascara sagrada, turmeric, burdock, milk thistle, guarana extract, green tea, Echinacea purpurea, potassium, proprietary blend, creatine monohydrate, alfalfa leaf, slippery elm bark, reishi mushroom, uva ursi leaf, cayenne pepper, licorice, peppermint leaf, red root, schisandra, and sorbitol.5
The manufacturer claims that the product is the “world’s most sophisticated cleansing system.”5 A thorough literature search of each of the product’s ingredients on PubMed and in the Natural Medicines Comprehensive Database6,7 reveals that many of the ingredients can cause liver dysfunction, notably, cascara sagrada, green tea, uva ursi, milk thistle, guarana, and creatine monohydrate.6,7 Some of these ingredients have dose-dependent hepatotoxicity, but no doses of any of the ingredients are specified on the label. Hence, it is quite possible that Mr A, having had no history of hepatic dysfunction and being on no other medications or supplements, developed abnormal liver function tests associated with the detoxification kit. Also, the liver function tests were normal toward the end of his hospitalization, making the temporal association even stronger. On closer look, the pattern of the liver function tests (AST levels higher than ALT levels by almost 4-fold) does not fit into a drug-induced liver dysfunction pattern; rather, it suggests the presence of alcoholic hepatitis. However, 1 of the ingredients, schisandra, has been documented to lower ALT levels, which might explain the discrepancy.6,7
Mr A did not have a prior history of psychiatric problems. One could argue that his psychotic symptoms could be from cannabis consumption, as he was found to be positive for cannabis at admission. Current research shows that it is highly likely that cannabis exposure is a “component cause” that interacts with other factors to precipitate schizophrenia or other psychotic disorders, but it is neither necessary nor sufficient to do so alone.8 Moreover, he had been using cannabis for more than a year and had not had any psychotic symptoms. The patient and his family denied any other illicit drug or alcohol use at the time of presentation or in the past. Also, he had no genetic predisposition to psychiatric conditions, including psychosis of any form, with his family history being negative. A literature search for the ingredients of the detoxification kit reveals that creatine has been reported in some case studies to have caused acute psychosis.9 Medical workup in the emergency room did not yield any results that could explain his psychotic symptoms. In addition, the fact that the psychotic symptoms resolved on their own, without using any antipsychotic medications, refutes a true psychotic episode. Hence, the relationship between the onset of psychosis and the initiation of the detoxification kit strongly suggests that a correlation exists. Table 1 provides a comprehensive list of the potential side effects of all ingredients in the detoxification kit taken from the textbook Natural Medicines Comprehensive Database.7
The possible mechanism of action of the detoxification kit, as per review of the literature, is dilution.10 The product used by Mr A comes with directions to drink 16 oz of water, 6 times a day, for 7 days and to urinate frequently. Drinking 96 oz of water daily will surely dilute the urine, but the dilution can be detected by the presence of lower quantities of creatinine in the urine. To counteract detection, the product contains creatine, which raises the level of creatinine in the urine sample. Creatinine is frequently measured during the integrity checks of the urine sample.11 Also, the kit contains riboflavin, cascara sagrada, and uva ursi, which adds color to the urine, making it look more concentrated and natural. Moreover, riboflavin interferes with the fluorescence polarization immunoassay; however, it has not been shown to produce a clinically false-negative result.12
Review of the Literature
We have discussed the possible association of our patient’s symptoms with the detoxification kit and the risks involved with its use. The subsequent review will discuss the available tampering techniques, the industry involved in the development of the drug test defrauding kits, and the evidence of little to no regulation to control their usage. We will also discuss the existing literature on risks involved with the use of various tampering methods and efforts by state and federal authorities to regulate these products.
Method
PubMed, Cochrane Database, and Google Scholar were searched using the keywords UDS, urine toxicology, pass the drug test, and clean UA, with no restrictions on publication date. Case reports, letters to the editor, and original research and review articles in multiple languages were reviewed, as were federal regulations and acts on the topic. The search yielded 4,082 results, of which 49 articles were selected for relevance. Some articles were later omitted as they had cited the original article and had nothing new to offer.
TAMPERING TECHNIQUES
Three methods used to tamper or falsify urine toxicology testing are in vivo adulteration, urine substitution, and in vitro adulteration. Each method is discussed in detail below.
In Vivo Adulteration
An in vivo adulterant is a product that is ingested to change one’s urine specimen in order to avoid detection of recent substance use. Dilution and excretion are the 2 main methods in this technique. Drinking excess water and using diuretic agents are included in this category. There are products available on the market such as Premium Detox, The Stuff, Fizzy Flush, Quick Flush Drug Detox Capsules, and Green Clean Drug Detox Drink. These products contain a mixture of B vitamins and creatine that help avoid detection of recent substance use by specimen integrity tests such as visual inspection and creatinine level check. The products claim to “flush” the body of toxins and to be effective in passing a urine drug screen when used as directed. This mechanism has already been discussed, as it was relevant to our case.
Urine Substitution
The urine substitution technique as the name indicates works only if one can substitute his/her urine sample with “clean” urine obtained either commercially or from a friend who is not using drugs. Urine specimens obtained by these means will surely pass integrity checks, as well as checks for any of the adulterants described below. The 2 ways in which the substitution can be detected are by the temperature of the urine specimen and specimen collection under direct supervision.
In the literature, various case reports exist regarding many creative ways that substance abusers have attempted to use urine substitution as a tampering method. In one notable method, urine is stored in a condom, taped or strapped to the genitalia, and warmed by any of the readily available hand warmers used for wintertime outdoor activities. At the time of sampling, the condom may be punctured by a pin or fingernail and a realistic-looking stream may be produced for a closely observed collection.13 Multiple products are available on the Internet including freeze dried or synthetic urine, which can be rapidly reconstituted.14 The industry dedicated to subverting urine drug testing by this technique has come up with various commercially available products such as the Whizzinator, the Urinator, and the Butt Wedge. The Whizzinator15 comes as a kit complete with dried urine, a syringe, heater packs (to keep the urine at body temperature), and a lifelike prosthetic penis (available in several skin tones including white, tan, latino, brown, and black). A female version is also available and is known as Number One.16
The Urinator kit includes 1 digitally controlled self-regulated heating element, 2 temperature test strips, 1 calibrated bottle-filling device, a complete operator’s manual, and a free synthetic concentrate sample. The flexible plastic container is strapped to the body, the electronic heating unit heats the synthetic urine, and a small plastic tube placed near the urethra delivers the specimen.17
The Butt Wedge contains a wedge-shaped container to store and warm urine between the user’s buttocks. At the time of sampling, a small tube placed near the opening of the urethra is used to deliver the specimen.18 This device was apparently a favorite among eastern European athletes during the 2004 Athens Olympics.19
In extreme cases, the substance abuser first empties his own bladder and then uses a catheter to fill his/her bladder with clean urine of a nonusing individual.
In Vitro Adulteration
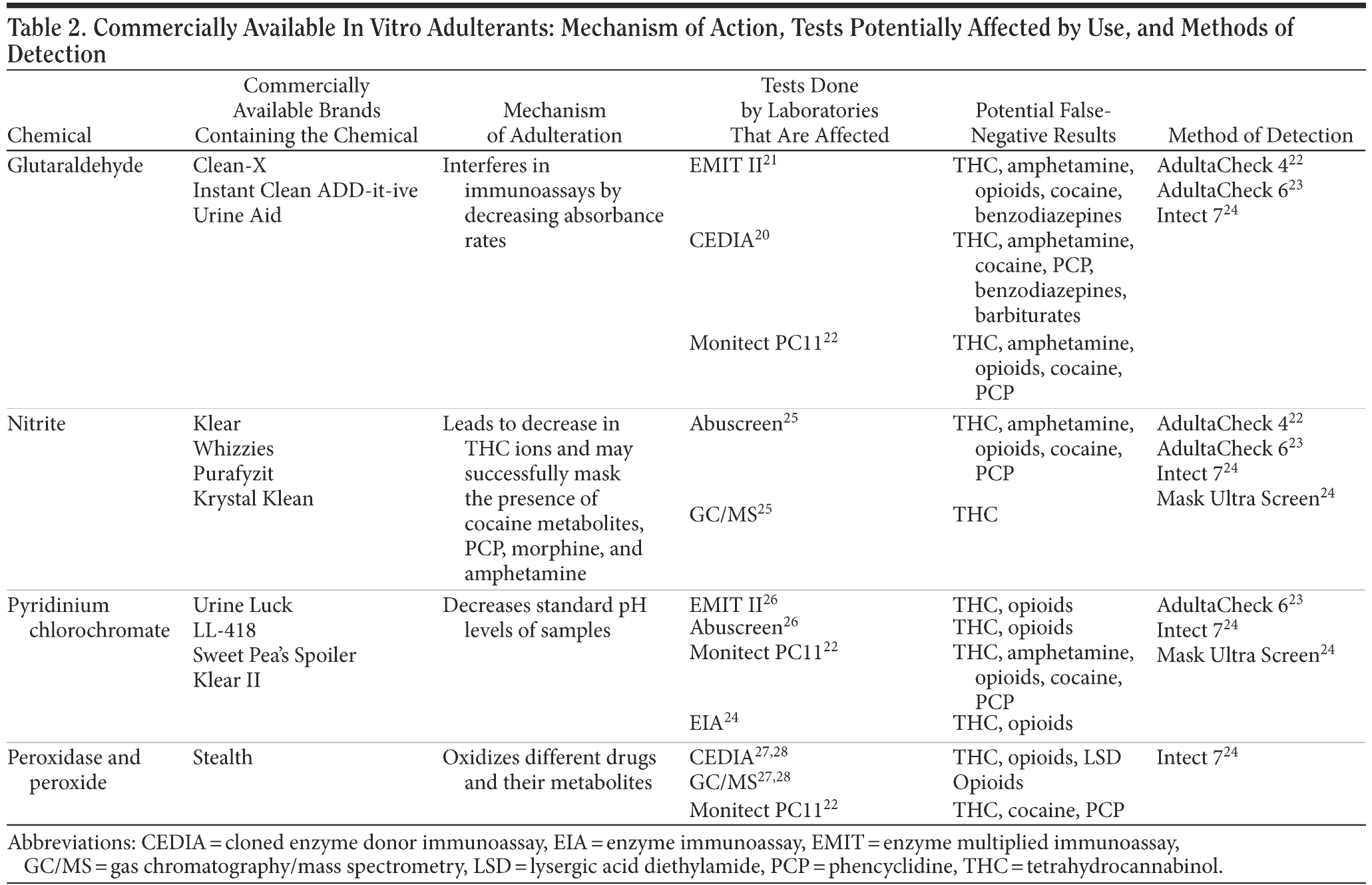
Adding foreign substances to a urine sample after micturition is known as in vitro adulteration. Wu et al20 reported that these products work by either interfering with the detection of toxins by masking their presence or converting them to compounds that are undetectable during screening, thereby producing negative urine drug screens. Usual active ingredients of the commercially available in vitro adulterants are glutaraldehyde, sodium or potassium nitrate, pyridinium chlorochromate, and peroxide/peroxidase. In Table 2, we discuss these adulterants and their plausible mechanism of action, which tests can be potentially affected by which substances, and how they can be detected.
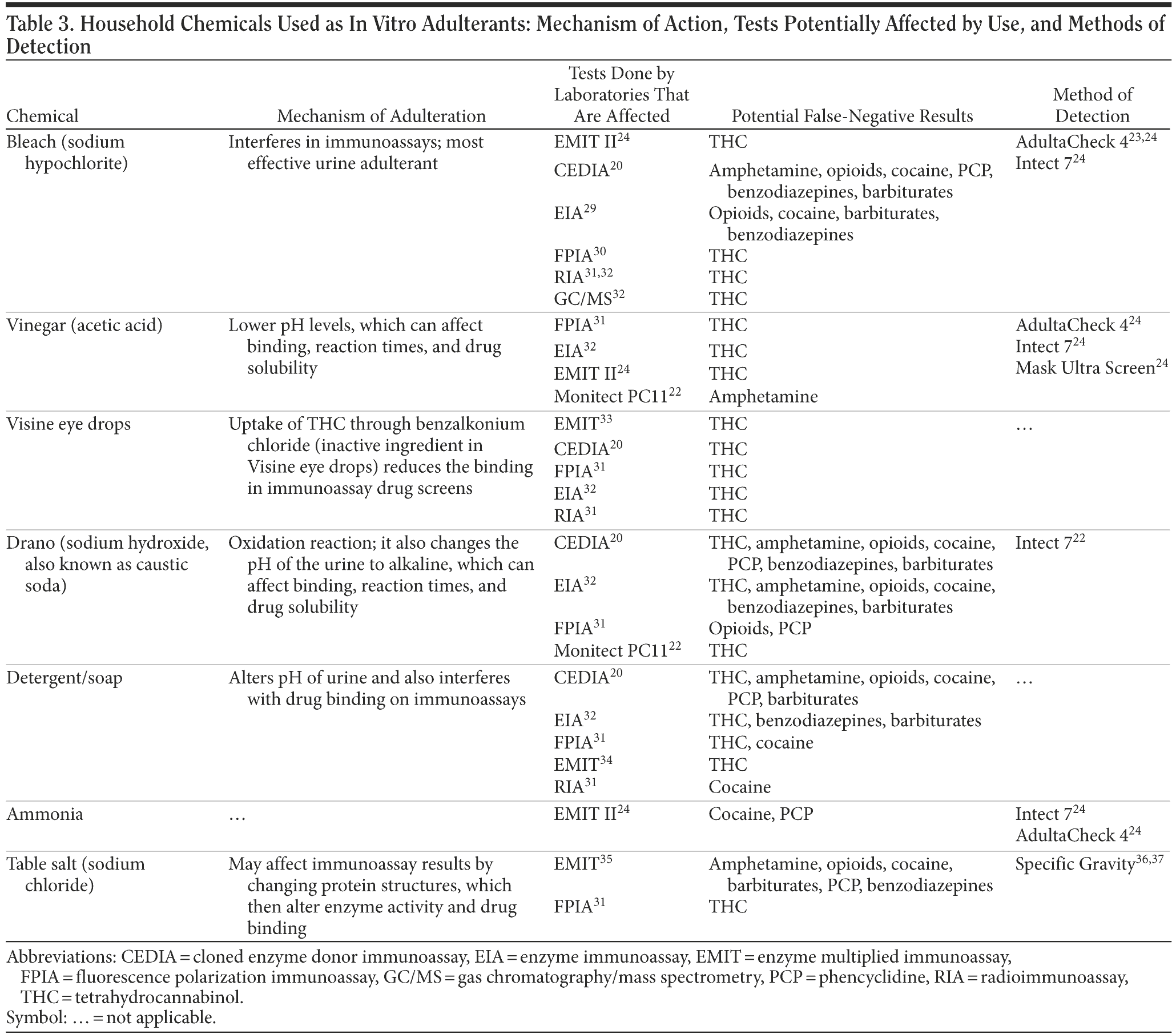
Apart from the commercially available in vitro adulterants, there are many household chemicals used as in vitro adulterants. Reports of use of household chemicals such as bleach, table salt, laundry detergent, toilet bowl cleaner, vinegar, lemon juice, and Visine eye drops were published in the medical literature as early as 1988.29 In Table 3, we discuss these adulterants, their plausible mechanism of action, which tests can be potentially affected by which substances, and how they can be detected.
RISKS INVOLVED WITH USING THE DETOXIFICATION KITS
A search for publications in PubMed and the Cochrane Database did not yield any results for side effects and risks associated with the use of detoxification kits. Although there is no literature published on this topic, we will discuss some of the possible risks involved with the use of the above-mentioned tampering methods.
In Vivo Adulteration
The in vivo adulteration technique carries the most risk among the techniques available, as it requires addition of unregulated chemicals in one’s body. Most of the ingredients in the commercially available products are herbal. The detoxification kit used in the case presented here is unfortunately only one of many herbal products available. Extensive literature has been published on the risks involved in the use of unregulated herbal compounds. Another risk involved with this technique is drinking excess water, which if coupled with insufficient diet can lead to electrolyte abnormalities resulting in adverse effects.38 The use of diuretic medication without the supervision of a medical professional can pose significant risks as well.
Urine Substitution
The risks involved with the use of the urine substitution technique are limited to the introduction of someone else’s urine in one’s bladder by catheterization. Bladder catheterization by a nonmedical professional has risks of urethral rupture and other injuries to the urinary tract. The introduction of foreign bodily fluids into one’s own body carries inherent risks of transmission of infectious diseases.
In Vitro Adulteration
As no products are introduced into one’s body, the risk of using in vitro compounds is minimal. Of course, there are risks involved with the handling of harmful chemicals, which can cause burns if not handled with caution.
REGULATORY EFFORTS
The mandatory guidelines for federal workplace drug-testing programs were first published in the US Federal Register on April 11, 1988.39 Guidelines have since been revised on June 9, 199440; September 30, 199741; and April 13, 2004.42 These guidelines cover the testing process, which includes proper specimen collection, initiation of the chain of custody, and analysis of the specimen by a Substance Abuse and Mental Health Services Administration (SAMHSA) certified laboratory. The screening by immunoassay should be performed using a US Food and Drug Administration-approved method. The confirmation should be performed by a second technique. Federal guidelines have been established for cutoff levels of commonly abused drugs in screening and confirmation by urine drug tests. The urine specimens that during initial screening or during confirmation have abnormal physical characteristics or indicate that the specimen has been adulterated require additional tests as per SAMHSA guidelines. The cutoff levels and SAMHSA guidelines are beyond the scope of this article and can be accessed at the SAMHSA Web site.43
The mechanisms employed by collection sites and laboratories to detect invalid specimens are known as specimen integrity tests. These tests include creatinine concentration, temperature, specific gravity, pH, color, and transparency of the urine specimen.44 SAMHSA guidelines also have a section on specimen collection procedures that include turning off all sources of water, adding a bluing agent in the toilet water, hand washing by the subject prior to specimen evacuation, and the presence of a collector at all times when the specimen is being evacuated (although a stall may be used).42
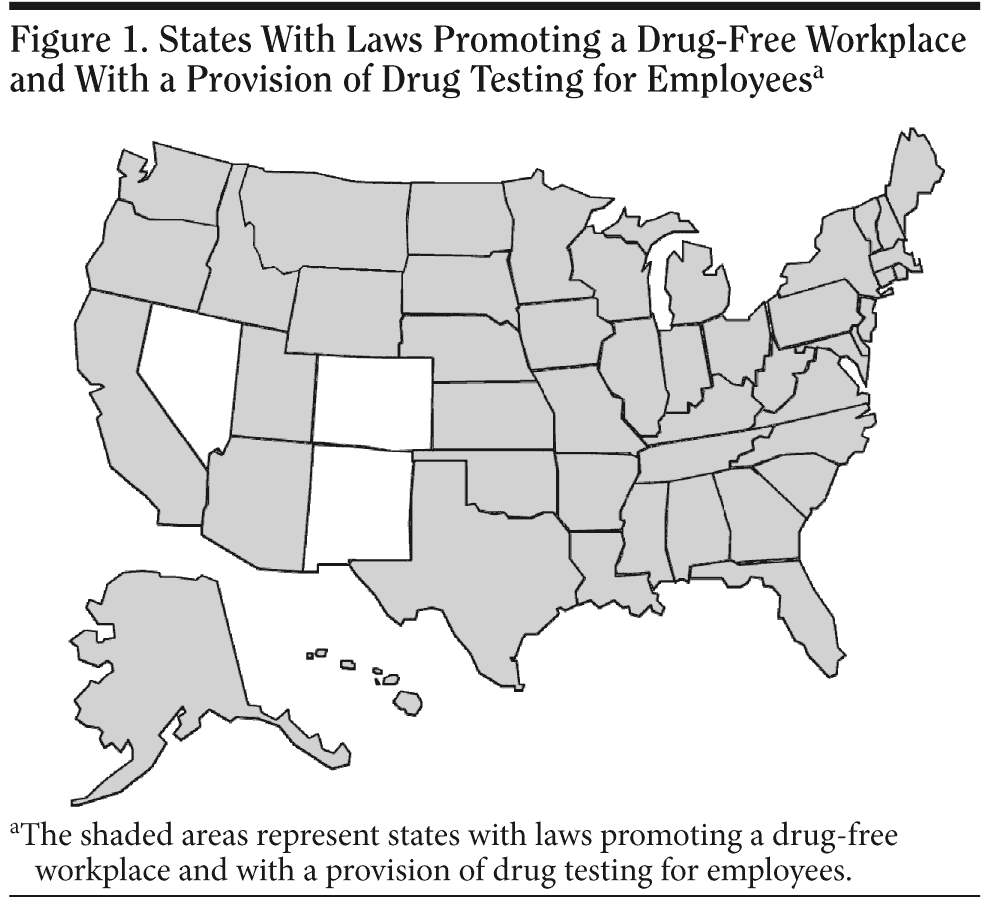
As mentioned earlier, after the US Federal Drug-Free Workplace Act of 1988 was passed, many states followed suit and adopted similar laws. Figure 1 shows the states that have a law in some form that promotes a drug-free workplace with a provision of drug testing for employees. The states and territories of the United States that do not have such laws are American Samoa, Colorado, District of Columbia, Federated States of Micronesia, Guam, Marshall Islands, Nevada, New Mexico, Northern Mariana Islands, and Palau.45
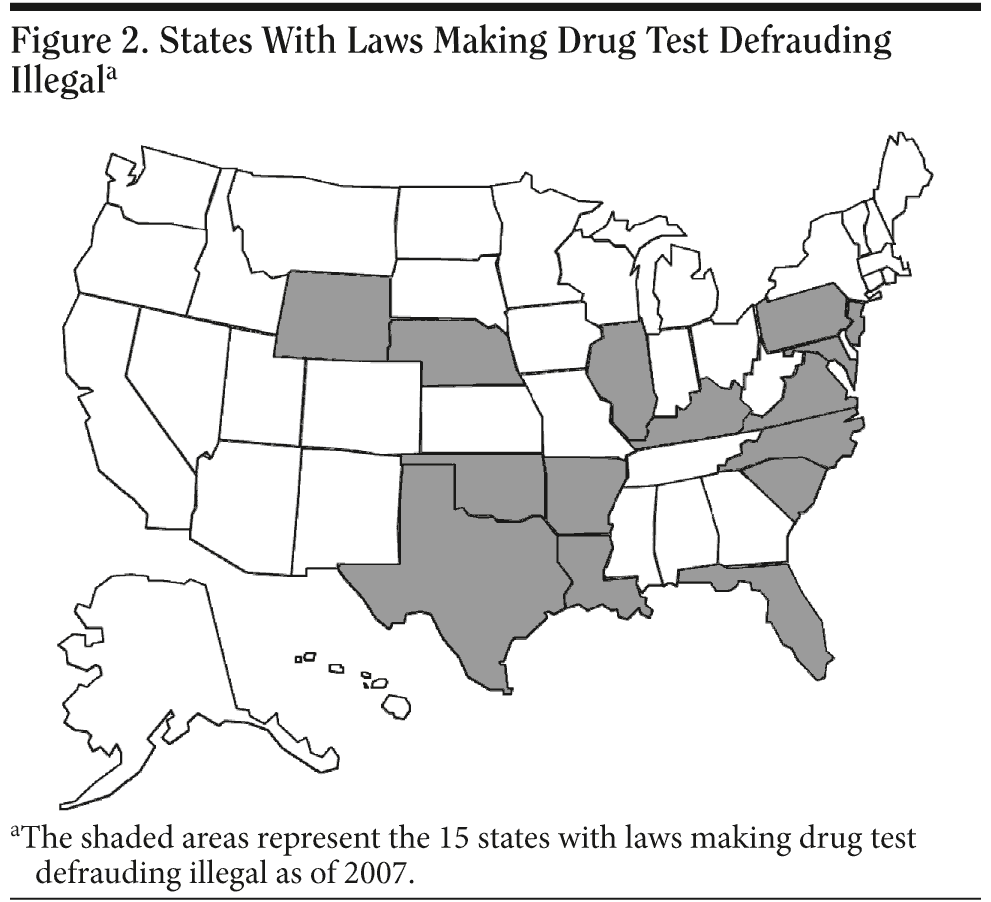
The industry of falsification of drug-testing procedures continues to be a growing concern. Lawmakers are becoming aware and taking the much-needed steps to make subversion of drug testing illegal and a punishable offense. As of 2007, 15 states have passed laws that make drug test defrauding illegal (Figure 2). These states are Arkansas, Florida, Illinois, Kentucky, Louisiana, Maryland, Nebraska, New Jersey, North Carolina, Oklahoma, Pennsylvania, South Carolina, Texas, Virginia, and Wyoming.45-47
CONCLUSION
Several notable take-home messages arise out of this discussion. First, providers need to be aware that a negative urine drug screen may not be a “true negative.” Health providers should always look for signs and symptoms of intoxication and/or withdrawal and also take history from collateral sources such as family for patients for whom clinicians have a high index of suspicion. Second, health care providers should never ignore the specimen integrity testing and the collection procedures employed by the laboratory. In case the provider still suspects substance use in the presence of a negative urine drug screen, he/she should contact the laboratory and ask about specific screening tests currently employed. As a follow-up, one can utilize the information provided in Tables 2 and 3 to ask for a specific test from the laboratory to detect the toxin in the presence of an adulterant, if any. Additionally, hair or saliva drug tests can be requested, although they too can be subject to adulteration.44 Third, providers should apprise themselves of the laws pertaining to their region of practice that regulate the sale and advertisement of such detoxification products. Vigilance and report of such products to regulatory authorities can be a positive step in the direction of improved regulation.
Finally, as significant physical and mental hazards are possible with such products, it would be advisable to have more stringent regulations on their manufacture, sale, advertisement, and use. As mentioned earlier, these products come under the bigger umbrella of the ever-growing herbal product market in the United States, for which more rigorous regulation continues to be imperative. Until legislation is enacted to prevent the easy availability of commercial adulterants, it is expected that this “cat and mouse” game between the drug-testing industry and adulterant product manufacturers will continue.
Drug names: haloperidol (Haldol and others), lorazepam (Ativan and others).
Author affiliations: Department of Psychiatry and Behavioral Sciences, University of Kansas School of Medicine, Wichita.
Potential conflicts of interest: Dr Khan serves on the speakers’ panel of Merck Schering Plough. Drs Mittal and Kalia report no conflicts of interest related to the subject of this article.
Funding/support: None reported.
REFERENCES
1. Substance Abuse and Mental Health Services Administration. Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4856 Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
2. Federal Register. Executive Order 12564: Drug-Free Federal Workplace. http://www.archives.gov/federal-register/codification/executive-order/12564.htmlAccessed July 25, 2011.
3. 41 USC Chapter 10: Drug-Free Workplace. http://uscode.house.gov/download/pls/41C10.txt. Accessed July 25, 2011.
4. Office of the Law Revision Counsel, US House of Representatives. Government organization and employees. 5 USC 7301. http://uscode.house.gov/uscode-cgi/fastweb.exe?getdoc+uscview+t05t08+829+0++%28Drugs%20and%20drug%20abuse.%20Government%20Organization%20and%20employees%29%20%20AND%20%28%285%29%20ADJ%20USC%29%3ACITE%20AND%20%28USC%20w%2F10%20%287301%29%29%3ACITE%20%20%20%20%20%20%20%20%20#hit0000. Accessed July 25, 2011.
5. Herbal Clean Premium Detox 7 Day Complete Cleansing System. http://www.herbalclean.com/products/viewproduct.php?id_product=47. Accessed July 25, 2011.
6. Jellin JM, Batz F, Hitchens K. Pharmacist’s Letter/Prescriber’s Letter. Natural Medicines Comprehensive Database. Stockton, CA: Therapeutic Research Faculty; 1999.
7. Natural Medicines Comprehensive Database. http://www.naturaldatabase.com. Accessed July 25, 2011.
8. Arseneault L, Cannon M, Witton J, et al. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184(2):110-117. PubMed doi:10.1192/bjp.184.2.110
9. Boos CJ, White SH, Bland SA, et al. Dietary supplements and military operations: caution is advised. J R Army Med Corps. 2010;156(1):41-43. PubMed
10. Cone EJ, Lange R, Darwin WD. In vivo adulteration: excess fluid ingestion causes false-negative marijuana and cocaine urine test results. J Anal Toxicol. 1998;22(6):460-473. PubMed
11. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? a systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42. PubMed doi:10.1016/j.jsat.2006.11.008
12. Kunsman GW, Levine B, Smith ML. Vitamin B2 interference with TDx drugs-of-abuse assays. J Forensic Sci. 1998;43(6):1225-1227. PubMed
13. Hoffman A, Silvers J. Steal This Urine Test: Fighting Drug Hysteria in America. New York, NY: Penguin Books; 1987.
14. Berge KH, Bush DM. The subversion of urine drug testing. Minn Med. 2010;93(8):45-47. PubMed
15. The Whizzinator. http://www.thewhizzinator.com/whizzinator.html. Accessed July 25, 2011
16. Jason Cato. Pittsburg Tribune Review. October 15, 2008. Buchanan takes on drug-test cheaters. http://www.pittsburghlive.com/x/pittsburghtrib/news/s_593356.html. Accessed July 25, 2011.
17. What is the Urinator? http://urinator.com/html/what.html. Accessed July 25, 2011.
18. Archived webpage from the website www.passdrugtest.com with information on The Butt Wedge. http://web.archive.org/web/20060214004001/http://www.passdrugtest.com/thebutt.htm. Accessed July 20, 2011.
19. Matt Hennie. Project Q Atlanta. July 10, 2008. Butt wedges, whizzinators on Olympic hit list. http://www.projectqatlanta.com/news_articles/view/butt_wedges_whizzinators_on_olympic_hit_list?gid=992. Accessed July 25, 2011.
20. Wu AH, Forte E, Casella G, et al. CEDIA for screening drugs of abuse in urine and the effect of adulterants. J Forensic Sci. 1995;40(4):614-618. PubMed
21. George S, Braithwaite RA. The effect of glutaraldehyde adulteration of urine specimens on syva EMIT II drugs-of-abuse assays. J Anal Toxicol. 1996;20(3):195-196. PubMed
22. Wong R. The effect of adulterants on urine screen for drugs of abuse: detection by an on-site dipstick device. Am Clin Lab. 2002;21(1):37-39. PubMed
23. Dasgupta A, Chughtai O, Hannah C, et al Comparison of spot tests with AdultaCheck 6 and Intect 7 urine test strips for detecting the presence of adulterants in urine specimens. Clinica Chimica Acta. 2004;348(1-2):19-25.
24. Peace MR, Tarnai LD. Performance evaluation of three on-siteadulterant detection devices for urine specimens. J Anal Toxicol. 2002;26(7):464-470. PubMed
25. Tsai SC, ElSohly MA, Dubrovsky T, et al. Determination of five abused drugs in nitrite-adulterated urine by immunoassays and gas chromatography-mass spectrometry. J Anal Toxicol. 1998;22(6):474-480. PubMed
26. Wu AH, Bristol B, Sexton K, et al. Adulteration of urine by “Urine Luck.” Clin Chem. 1999;45(7):1051-1057. PubMed
27. Cody JT, Valtier S. Effects of Stealth adulterant on immunoassay testing for drugs of abuse. J Anal Toxicol. 2001;25(6):466-470. PubMed
28. Cody JT, Valtier S, Kuhlman J. Analysis of morphine and codeine in samples adulterated with Stealth. J Anal Toxicol. 2001;25(7):572-575. PubMed
29. Mikkelsen SL, Ash KO. Adulterants causing false negatives in illicit drug testing. Clin Chem. 1988;34(11):2333-2336. PubMed
30. Schwarzhoff R, Cody JT. The effects of adulterating agents on FPIA analysis of urine for drugs of abuse. J Anal Toxicol. 1993;17(1):14-17. PubMed
31. Cody JT, Schwarzhoff RH. Impact of adulterants on RIA analysis of urine for drugs of abuse. J Anal Toxicol. 1989;13(5):277-284. PubMed
32. Baiker C, Serrano L, Lindner B. Hypochlorite adulteration of urine causing decreased concentration of delta 9-THC-COOH by GC/MS. J Anal Toxicol. 1994;18(2):101-103. PubMed
33. Pearson SD, Ash KO, Urry FM. Mechanism of false-negative urine cannabinoid immunoassay screens by Visine eyedrops. Clin Chem. 1989;35(4):636-638. PubMed
34. Uebel RA, Wium CA. Toxicological screening for drugs of abuse in samples adulterated with household chemicals. S Afr Med J. 2002;92(7):547-549. PubMed
35. Warner A. Interference of common household chemicals in immunoassay methods for drugs of abuse. Clin Chem. 1989;35(4):648-651. PubMed
36. Cassells NP, Craston DH. The effects of commonly used adulterants on the detection of spiked LSD by an enzyme immunoassay. Sci Justice. 1998;38(2):109-117. PubMed doi:10.1016/S1355-0306(98)72087-8
37. Dasgupta A. Urinary adulterants and drugs-of-abuse testing. MLO Med Lab Obs. 2003;35(2):26-28, 30-31. PubMed
38. Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19(6):1076-1078. PubMed doi:10.1681/ASN.2007091042
39. Substance Abuse and Mental Health Services Administration, Department of Health and Human Services. Mandatory Guidelines for Federal Workplace Drug Testing Programs. Fed Regist. 1988;53:11970-11980.
40. Substance Abuse and Mental Health Services Administration, Department of Health and Human Services. Mandatory Guidelines for Federal Workplace Drug Testing Programs. Fed Regist. 1994;59:29908-29912.
41. Substance Abuse and Mental Health Services Administration, Department of Health and Human Services. Mandatory Guidelines for Federal Workplace Drug Testing Programs. Fed Regist. 1997;62:51118-51120.
42. Substance Abuse and Mental Health Services Administration, Department of Health and Human Services. Mandatory Guidelines for Federal Workplace Drug Testing Programs. Fed Regist. 2004;69:19643-19673.
43. Substance Abuse and Mental Health Services Administration (SAMHSA). http://www.samhsa.gov/. Accessed July 25, 2011.
44. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128(3):491-503. PubMed doi:10.1309/FQY06F8XKTQPM149
45. United States Department of Labor. State laws. http://www.dol.gov/asp/programs/drugs/said/StateLawList.asp. Accessed July 25, 2011.
46. State of Wyoming House Bill No HB0065: 2007. Defrauding drug and alcohol screening tests. http://legisweb.state.wy.us/2007/Introduced/HB0065.pdf. Accessed July 25, 2011.
47. US Government Accountability Office GAO-05-653T. Drug tests: products to defraud drug use screening tests are widely available. http://www.gao.gov/htext/d05653t.html. Accessed July 25, 2011.