Background: Reverse colocation care models reduce lifestyle risk factors, emergency department visits, and readmissions. Persons with serious mental illness have higher than average rates of cardiovascular disease-related morbidity and mortality, with second-generation antipsychotics (SGAs) conferring added related risks. Little is written about reverse colocated medical care (RCL) in inpatient psychiatric settings. The objective of this study was to identify associations between screening, diagnosis, and treatment of chronic medical comorbidities and mode of medical care for patients discharged from 2 inpatient psychiatric units on SGAs.
Methods: This was a cross-sectional retrospective study of medical comorbidities identified and treated for adults consecutively admitted from January 1, 2015, to October 31, 2015, to 2 inpatient psychiatry units of an academic center and discharged on SGAs. One unit has a primary care team consisting of a physician assistant backed up by a medical doctor who provide medical care (RCL). The other unit has medical care provided by psychiatrists with hospitalists as needed (treatment as usual, TAU). We conducted a chart review of demographics, vital signs, laboratory values, diagnoses, and medications with comparative analysis of the evaluation, diagnosis, and treatment for hypertension, diabetes mellitus, hyperlipidemia, obesity, and tobacco use disorder.
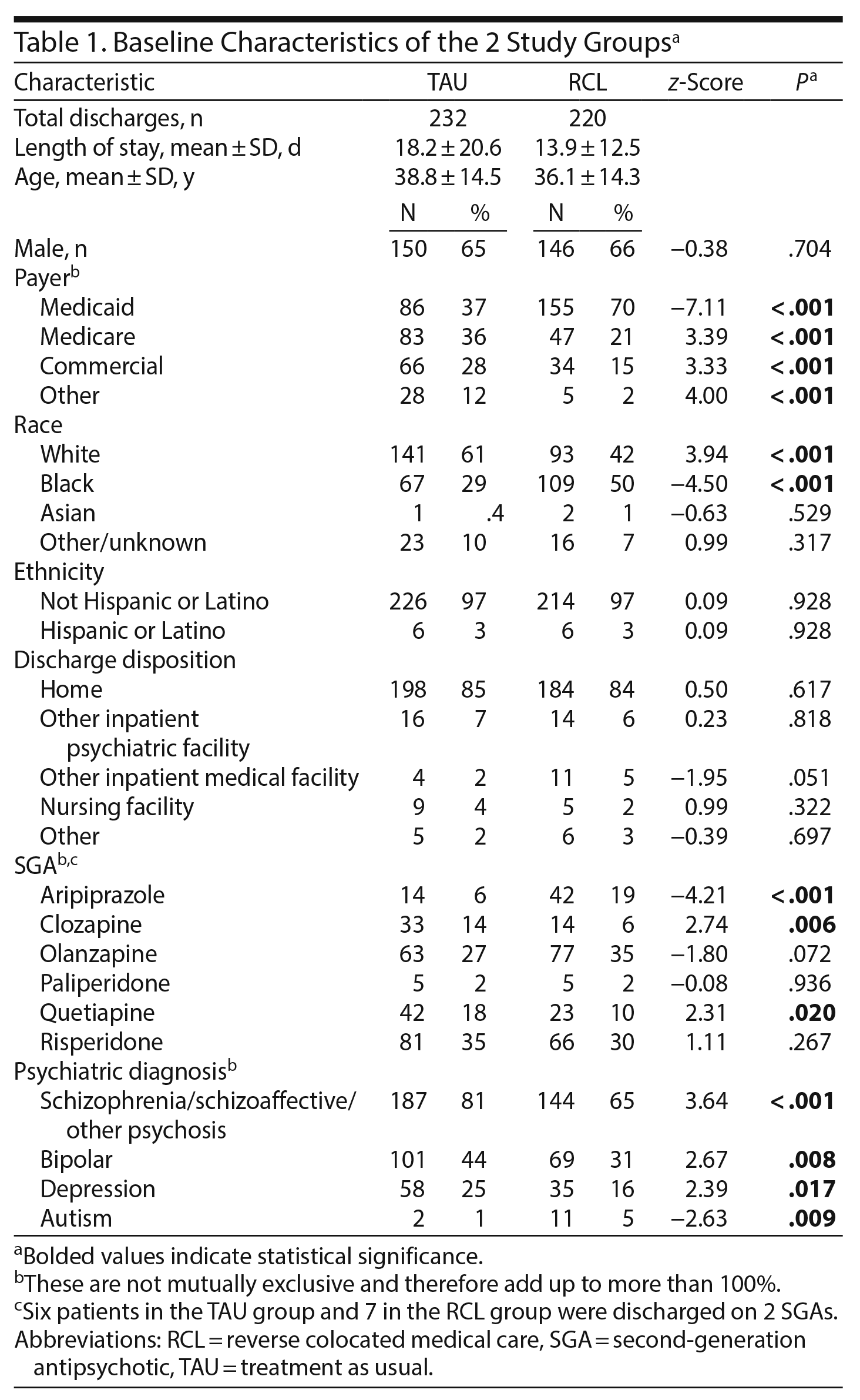
Results: In total, 232 patients were discharged from the TAU group and 220 from the RCL group. Significantly more screening laboratory values (glucose, hemoglobin A1c, lipids) were obtained in the TAU group, while documented responses to abnormal tests were higher in the RCL group. Patients were more likely in the RCL group to be diagnosed with obesity, tobacco use disorder, and hyperlipidemia and to be treated for hypertension and hyperlipidemia.
Conclusions: Reverse colocated medical care is effective in improving screening, diagnosis, and treatment of chronic medical diseases among psychiatric inpatients.’ ‹

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
CME Objective
After studying this article, you should be able to:
‘ ¢ Work to improve the screening, diagnosis, and treatment of cardiometabolic risk factors among patients with serious mental illness
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Date of Original Release/Review
This educational activity is eligible for AMA PRA Category 1 Creditâ„¢ through December 31, 2018. The latest review of this material was November 2016.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for Forest, Lundbeck, Merck, Shire, Takeda, and Elsevier Press; has been a stock shareholder of M3 My Mood Monitor; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Cardiometabolic Assessment, Diagnosis, and Treatment of Chronic Medical Illnesses During an Inpatient Psychiatric Hospitalization:
Colocated Medical Care Versus Treatment as Usual
ABSTRACT
Background: Reverse colocation care models reduce lifestyle risk factors, emergency department visits, and readmissions. Persons with serious mental illness have higher than average rates of cardiovascular disease-related morbidity and mortality, with second-generation antipsychotics (SGAs) conferring added related risks. Little is written about reverse colocated medical care (RCL) in inpatient psychiatric settings. The objective of this study was to identify associations between screening, diagnosis, and treatment of chronic medical comorbidities and mode of medical care for patients discharged from 2 inpatient psychiatric units on SGAs.
Methods: This was a cross-sectional retrospective study of medical comorbidities identified and treated for adults consecutively admitted from January 1, 2015, to October 31, 2015, to 2 inpatient psychiatry units of an academic center and discharged on SGAs. One unit has a primary care team consisting of a physician assistant backed up by a medical doctor who provide medical care (RCL). The other unit has medical care provided by psychiatrists with hospitalists as needed (treatment as usual, TAU). We conducted a chart review of demographics, vital signs, laboratory values, diagnoses, and medications with comparative analysis of the evaluation, diagnosis, and treatment for hypertension, diabetes mellitus, hyperlipidemia, obesity, and tobacco use disorder.
Results: In total, 232 patients were discharged from the TAU group and 220 from the RCL group. Significantly more screening laboratory values (glucose, hemoglobin A1c, lipids) were obtained in the TAU group, while documented responses to abnormal tests were higher in the RCL group. Patients were more likely in the RCL group to be diagnosed with obesity, tobacco use disorder, and hyperlipidemia and to be treated for hypertension and hyperlipidemia.
Conclusions: Reverse colocated medical care is effective in improving screening, diagnosis, and treatment of chronic medical diseases among psychiatric inpatients.
Prim Care Companion CNS Disord 2016;18(6):doi:10.4088/PCC.16m02017
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of North Carolina Hospitals, Chapel Hill, North Carolina
bUniversity of North Carolina Eshelman School of Pharmacy, University of North Carolina Hospitals and Clinics, Chapel Hill, North Carolina
cUniversity of North Carolina Hospitals at Wakebrook, Raleigh, North Carolina
dDepartment of Family Medicine, University of North Carolina Hospitals, Chapel Hill, North Carolina
*Corresponding author: Jason R. Tatreau, MD, 101 Manning Drive, Chapel Hill, NC 27514 ([email protected]).
Roughly 10 million Americans are diagnosed with serious mental illness (SMI) each year.1 Persons with SMI die, on average, 25 years earlier than the general population, mostly due to treatable medical conditions such as infection and cardiopulmonary disease.2-6 Persons with SMI are also at high risk of underuse of evidence-based medical services,7-17 have higher rates of medication nonadherence and use of emergency medical services,18 and are estimated to have health care costs 2 to 3 times that of persons without SMI with almost $300 billion in added health care expenses, the majority due to physical illness.19,20
Hypertension, diabetes, obesity, hyperlipidemia, and smoking are 5 medical conditions that contribute substantially to this earlier mortality. Prevalence of these conditions among patients with schizophrenia is 2-5 times that of the general population.21-26 Identification and treatment of physical illness is especially important for persons taking second-generation antipsychotics (SGAs), who are at significantly elevated risk of weight gain, hyperglycemia, and dyslipidemia.17,27 Importantly, metabolic abnormalities appear early in the course of schizophrenia and use of antipsychotics, with likely interactions among unhealthy lifestyle, antipsychotic use, and the underlying psychotic illnesses themselves.28
Metabolic monitoring for patients taking SGAs was defined in 2004 with a consensus statement from the American Psychiatric Association (APA) and the American Diabetes Association (ADA).29,30 Despite the development of metabolic monitoring parameters, multiple studies17,31 have found low rates of adherence to these guidelines. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial illustrated that appropriate medical treatment is not received by 30.2% of those with diabetes, by 62.4% of those with hypertension, and by 88% of those with lipid abnormalities.17

- Cardiometabolic risks inherent among those with serious mental illness are compounded by the use of second-generation antipsychotics, and these risks appear early in treatment.
- Hypertension and, especially, hyperlipidemia often go untreated and even undiagnosed despite positive screening results among psychiatric inpatients.
- Colocating medical care can improve diagnosis and treatment of cardiometabolic risk factors among psychiatric inpatients with serious mental illness.
Several studies collaborative outpatient practice models have been shown to improve medical care for persons with SMI. Reverse colocation, placing primary care in behavioral care settings, is a model of integrated care aimed at improving physical health of patients with mental illness that recognizes the differential burden of medical comorbidity in patients with SMI. Reverse colocation models of ambulatory care reduce lifestyle risk factors,32 reduce emergency department visits, and increase preventive care screening.33-36
While colocalizing medical care services within ambulatory behavioral health settings has been shown to improve medical care, there is limited systematic evaluation of the effect of primary care services embedded within psychiatric inpatient units. To our knowledge, there is only 1 randomized study37 evaluating the role of adding an internist to an inpatient psychiatric service. Rubin et al37 showed dramatically improved processes of care (health maintenance for tobacco use, cancer screening, and lipid screening) with no increase in cost or added length of stay. In this study, we sought to identify associations between guideline-concordant screening, diagnosis, and treatment of defined chronic medical comorbidities and the mode of medical care delivery.
METHODS
Study Design
This was a cross-sectional, retrospective study of adult patients consecutively discharged between January 1, 2015, and October 31, 2015, from 1 of 2 psychiatric inpatient units at the University of North Carolina Health Care System who were on an SGA (aripiprazole, clozapine, olanzapine, quetiapine, or risperidone) at the time of discharge. Asenapine, brexpiprazole, iloperidone, lurasidone, and ziprasidone were excluded since they were not formulary medications at both units. Patients on more than 1 SGA were not excluded.
While both psychiatric inpatient units primarily treat individuals with SMI, each unit employs a different mode of delivering medical care. One unit, a 16-bed locked inpatient unit in Raleigh, North Carolina, has an embedded medical team providing care to all patients 7 days a week. This reverse colocation model employs a physician’s assistant supervised by a family physician, who provides an admission consultation for all patients within 24 hours, closely follows patients who have comorbid medical conditions, and obtains targeted laboratory values that affect inpatient care. In part as a cost-saving measure, there are no standard admission laboratory tests in the reverse colocation model, such that all laboratory testing has a specific clinical indication. When available, laboratory values recently obtained from other facilities or previous admissions are reviewed, often preventing repeat testing. Weekend and holiday coverage employs an emergency medicine resident physician supervised by an attending family physician. At discharge, the physician’s assistant assures that patients receive appropriate prescriptions for physical health conditions and that the patients are connected to primary care. The family physician that provides supervision also sees patients in an outpatient primary care practice for patients with SMI located in the same facility. This helps cover the cost of the medical team and provides a source of care for patients discharged from the psychiatric unit without a primary care physician.
The other unit is a more traditional, 18-bed, locked unit in Chapel Hill, North Carolina, and provides treatment as usual (TAU), whereby medical care is provided by resident psychiatrists supervised by attending psychiatrists, with hospitalists available as needed for medical consultation. Standard admission orders for all patients admitted to this unit include a basic chemistry panel, complete blood count, thyroid-stimulating hormone analysis, urinalysis, and urine toxicology screen. These laboratory tests are ordered, completed, and reviewed prior to all admissions to evaluate for evidence of medical contributions to the psychiatric presentation. This study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill School of Medicine.
Data Collection
Data were extracted from a medical records data warehouse using Business Objects software. We extracted demographics (age, sex, race, ethnicity, primary payer type), length of stay, discharge diagnoses, discharge medications, and discharge disposition. We also searched for whether patients had the following measured during the admission: hemoglobin A1c, fasting or random glucose, body mass index (BMI), low-density lipoprotein (LDL), triglycerides (TG), and blood pressure. For abnormal results, we used diagnostic criteria of hemoglobin A1c > 6.5% or glucose > 200 mg/dL for diabetes, BMI > 30 for obesity, and blood pressure > 140/90 mm Hg on 3 separate occasions for hypertension. For hyperlipidemia, we used LDL > 100 mg/dL or TG > 150 mg/dL.38 We searched discharge problem lists for ICD-9 codes associated with hypertension, diabetes mellitus, hyperlipidemia, obesity, and tobacco use disorder.
We identified medications that are evidence-based for associated conditions. For hypertension, we searched the discharge medication list for angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, diuretics, calcium channel blockers, or β-adrenergic blockers. α-Adrenergic agents, like prazosin and clonidine, were excluded given the common use of these agents in treating certain psychiatric symptoms, such as posttraumatic stress disorder-related nightmares. For diabetes, we searched discharge medications for insulin, metformin, and other oral hypoglycemics. For elevated lipids, we searched discharge medications for statins.
Data Analysis
To assess frequency of tests ordered (hemoglobin A1c, glucose, LDL, TG) or measurements taken (blood pressure, BMI), we calculated the proportion of patients with test results or measurements as a fraction of all patients. To assess frequency of abnormal test results, we calculated the proportion of patients in each group with abnormal results (as defined previously) as a fraction of all patients in the group with any testing results. If patients had multiple test results, we chose the highest result. To assess appropriate management and follow-up of abnormal tests, we assessed the following criteria: (1) proportion of patients who met diagnostic criteria (as defined previously) who had the diagnosis on the problem list at discharge and (2) proportion who had medications appropriate to medical diagnosis at discharge. We also calculated the proportion of patients with each diagnosis at the time of discharge, regardless of whether any screening was performed. Calculated proportions for the 2 inpatient units were compared using 2-tailed z-score tests with a .05 significance level.39
RESULTS
Baseline Characteristics
There were 220 discharges from the unit with reverse colocated medical care (RCL) and 232 discharges from the unit with TAU that met inclusion criteria. These 2 groups were well matched by age, sex, ethnicity, and discharge disposition with no significant differences. Patients in the RCL group were more likely to have Medicaid, to be of African American race, and to have a shorter length of stay (Table 1). Several patients were identified as having more than 1 primary payer, which accounts for numbers adding up to more than 100%. The most common psychiatric diagnosis for both groups was schizophrenia or schizoaffective disorder (ICD-9 code 295) and was more prevalent among the TAU group (81% vs 65%, P < .001). While olanzapine, paliperidone, and risperidone were used at similar rates in the 2 groups, aripiprazole was more commonly used in the RCL group (P < .001), and clozapine and quetiapine were more commonly used in the TAU group (P < .05, Table 1).
Disease Screening
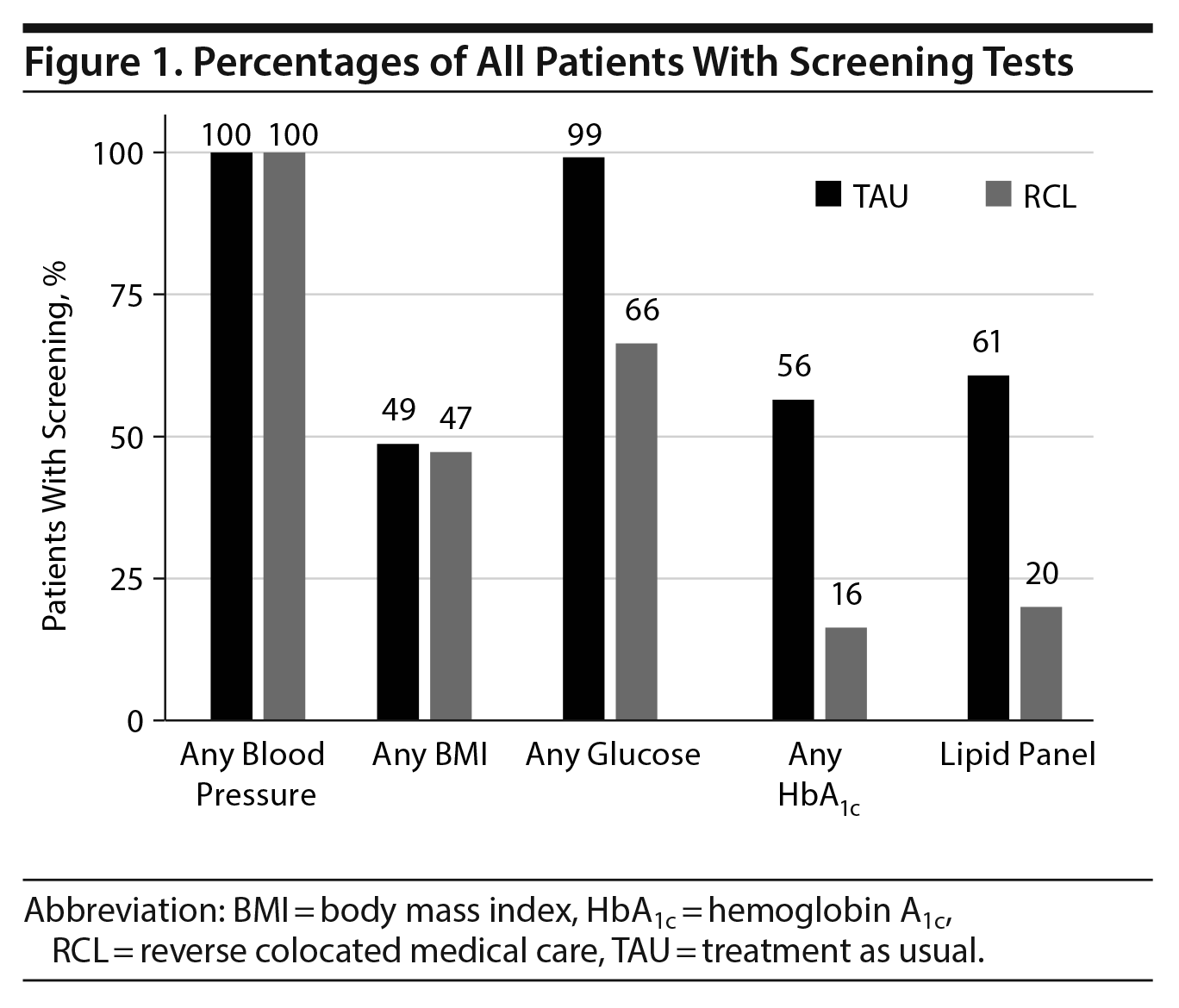
All patients had at least 1 blood pressure measurement, and about half of all patients in each group had a BMI measurement identified (TAU: 49% and RCL: 47%, P = .76, Figure 1). Significantly more screening tests were ordered in the TAU group, including hemoglobin A1c tests (56% vs 16%, P < .001), glucose (99% vs 66%, P < .001), and lipids (61% vs 20%, P < .001, Figure 1).
We also looked at patients with abnormal results among those screened. There were significantly more patients in the TAU group with at least 3 elevated blood pressure readings (63%) than in the RCL group (26%, P < .001, Figure 2). Proportions of patients with a BMI > 30 were similar in the 2 groups (TAU: 23% and RCL: 26%, P = .47, Figure 2). There were no significant differences between the groups in the proportions of patients with elevated glucose (TAU: 4% vs RCL: 6%, P = .27) or elevated hemoglobin A1c (TAU: 2% vs RCL: 6%, P = .30). While more patients had a lipid panel in the TAU group, there was a trend toward significance in the proportion of patients in the RCL group that had abnormal results (52% vs 36%, P = .056).
Screening Follow-Up and Disease Diagnosis
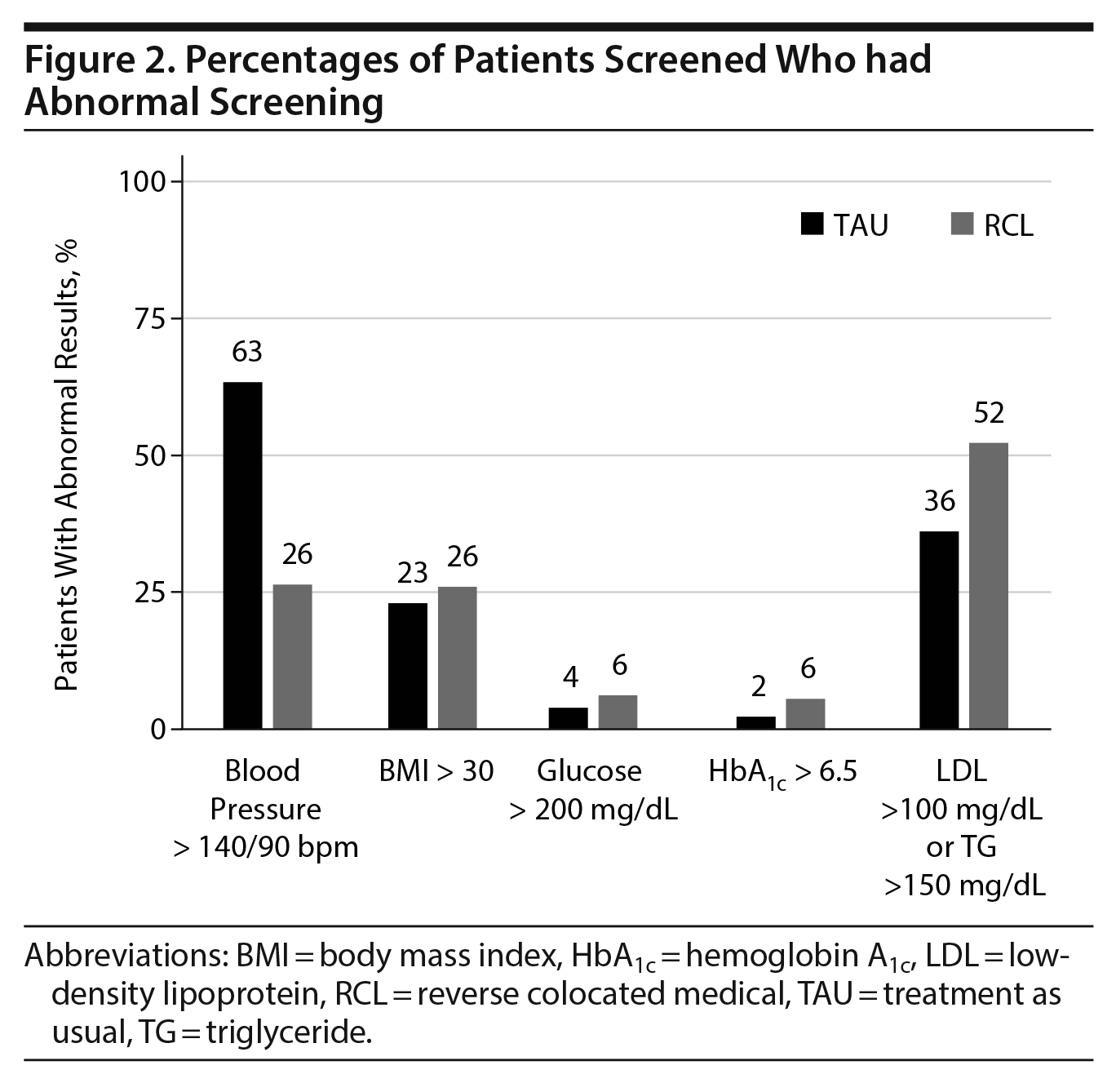
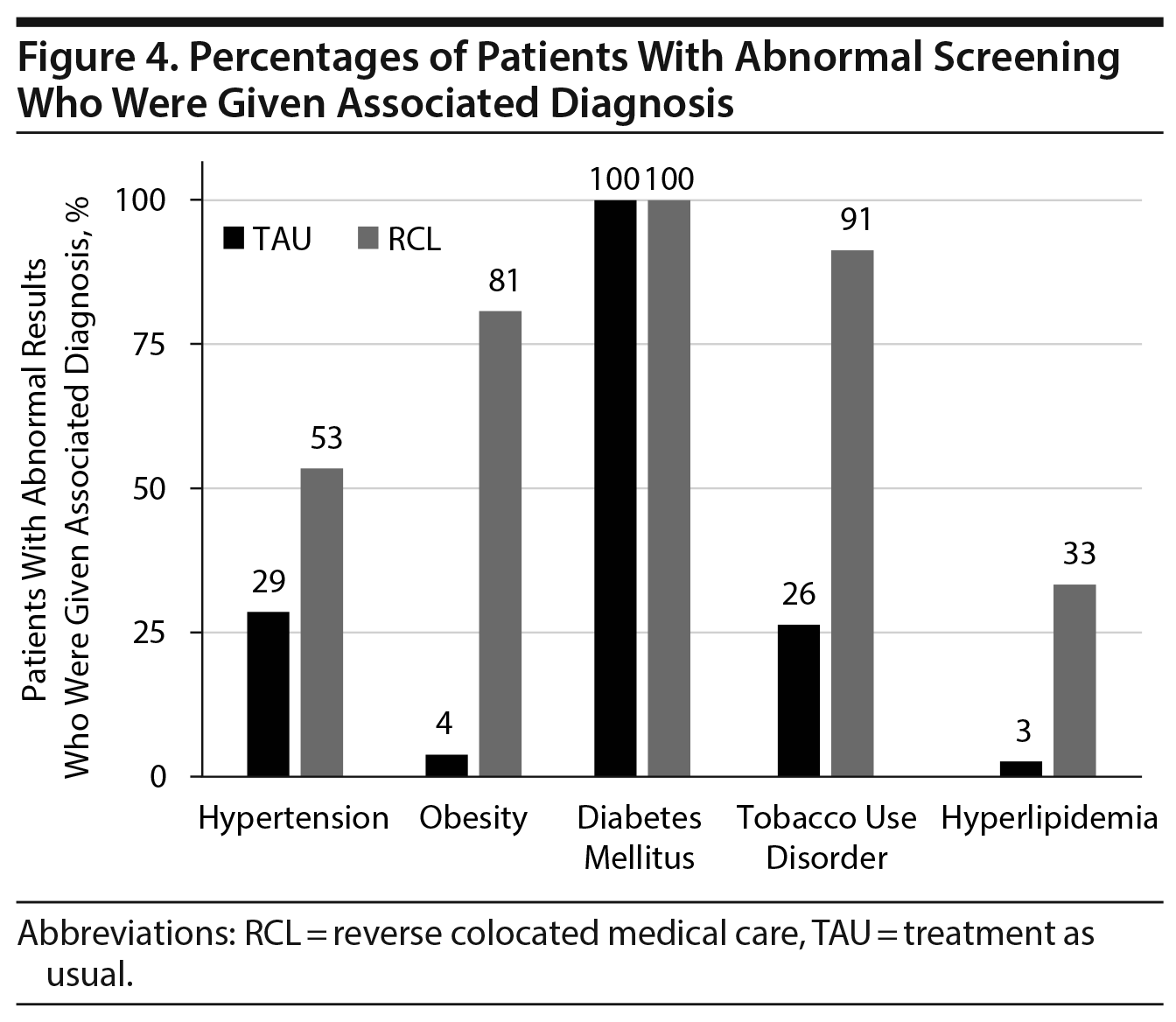
We then looked at those who had abnormal screening results to determine if these patients were given a medical diagnosis associated with the abnormal results. Of the chronic diseases that were screened for, only diabetes mellitus was consistently diagnosed in those with abnormal results. Regardless of whether patients had a glucose > 200 mg/dL or hemoglobin A1c > 6.5%, all of these patients in both groups were discharged with a diagnosis of diabetes mellitus. The proportions of all patients with a diabetes mellitus diagnosis were 6% (TAU) versus 10% (RCL) (P = .11, Figure 3).
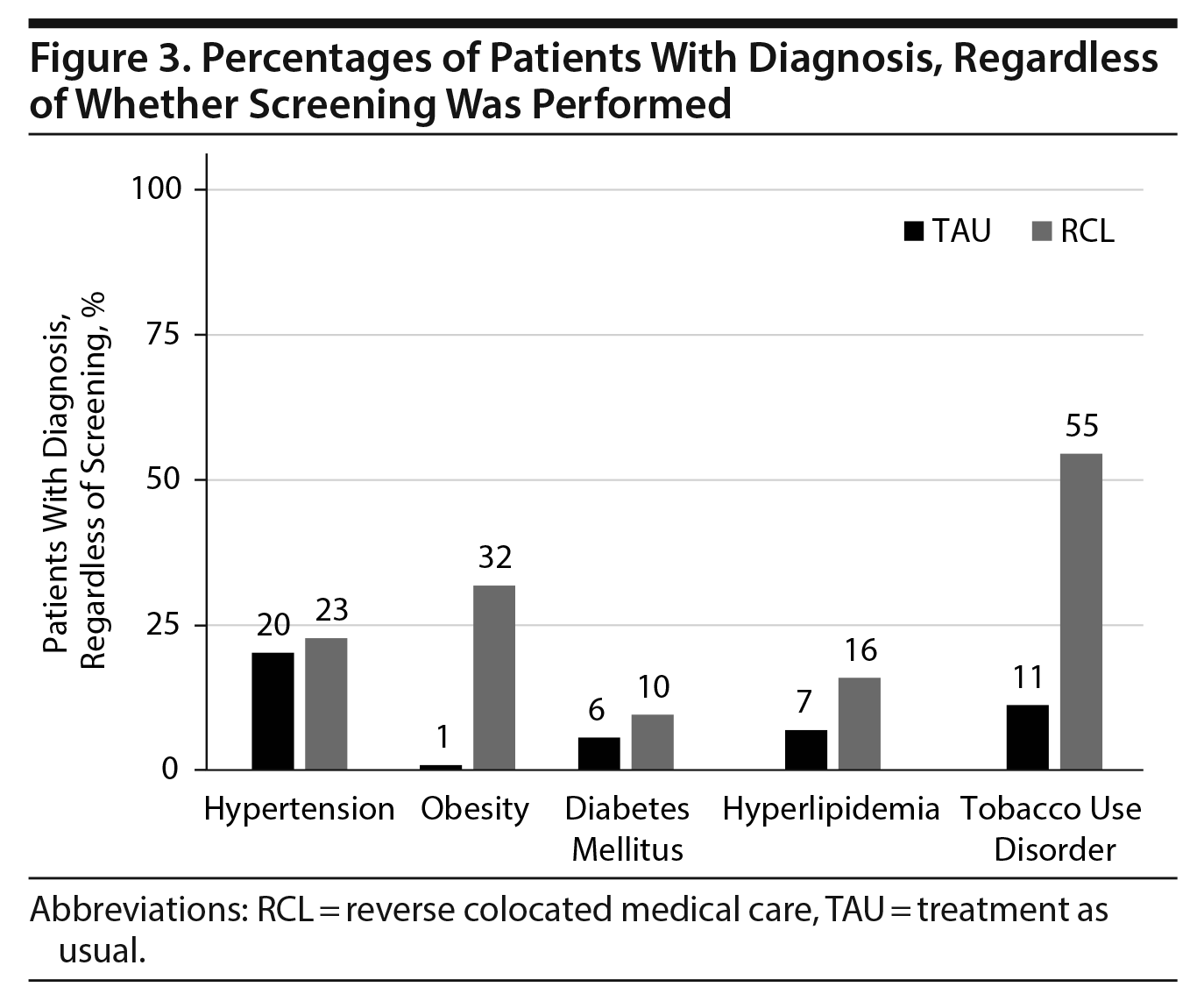
Significant differences were noted between the 2 groups in the proportions of patients diagnosed with the other chronic diseases. The proportion of patients with elevated blood pressure that were diagnosed with hypertension was almost twice as high in the RCL group (53% vs 29%, P < .001, Figure 4), despite there being over twice as many patients in the TAU group meeting criteria for a diagnosis of hypertension (63% vs 26%, P < .001, Figure 2). The proportion of patients with an obesity diagnosis was over 20 times higher in the RCL group (81% vs 4%, P < .001, Figure 4), despite similar proportions of abnormal BMI in the 2 groups (TAU: 23% vs RCL: 26%). A significantly higher proportion of patients with abnormal lipids was given a diagnosis of hyperlipidemia in the RCL group (33%) versus the TAU group (3%, P < .001, Figure 4).
Significant differences were also noted for tobacco use disorder. Patients were more likely in the RCL group to have a tobacco use disorder diagnosis at discharge (55% vs 11%, P < .001, Figure 3), irrespective of whether they received nicotine replacement therapy during their hospitalization. Of patients who received nicotine replacement therapy during hospitalization, only 26% were given a diagnosis of tobacco use disorder in the TAU group, whereas 91% were given this diagnosis in the RCL group (P < .001, Figure 3).
Disease Treatment
For patients with a diagnosis of diabetes mellitus, there were no differences in the proportions of patients in the TAU and RCL groups that were receiving hypoglycemics at discharge (69% vs 71%, respectively, P = .61). However, significantly more patients who were diagnosed with hypertension and hyperlipidemia were on appropriate pharmacotherapy at discharge in the RCL group than in the TAU group (76% vs 58%, P < .001 for hypertension; 37% vs 8%, P < .005 for hyperlipidemia). Regarding tobacco use disorder, there was a significantly higher proportion of patients in the RCL group that received nicotine replacement therapy during admission (52% vs 39%, P < .05), but no significant differences were present between the overall proportions of patients in the TAU and RCL groups that received nicotine replacement therapy at discharge (14% vs 10%, P = .16).
DISCUSSION
Utilizing colocalized medical care in a psychiatric inpatient setting has advantages in screening, diagnosing, and treating chronic medical illness for patients taking SGAs. During screening, laboratory test results (hemoglobin A1c, glucose, and lipids) were collected in significantly higher proportions of patients in the TAU group; these did not correlate with higher rates of diagnosis or treatment of medical illness. Furthermore, the RCL group was associated with higher rates of diagnosis of obesity, hyperlipidemia, and tobacco use disorder and with higher rates of hypertension and hyperlipidemia treatment.
Differences in the number of screening laboratory tests ordered in the 2 groups were significant. The reasons for these differences are likely multifactorial. While obtaining a glucose level is part of the admissions process in the TAU group, it is not for the RCL group. Also, neither group has a protocol that reflexively orders hemoglobin A1c or a lipid panel. We were unable to determine whether patients were due for guideline-concordant screening, whether there were differences in the practices of individual providers, or whether clinical symptoms or signs were considered in the decision of whether to order laboratory tests. Individual chart review would not have reliably identified a specific rationale for ordering or not ordering screening laboratories, but we speculate that the RCL group was, in part, more selective in ordering screening laboratory tests given this group’s focus on identifying and managing chronic medical illness. We are not sure why so many more patients in the TAU group had elevated blood pressure. Potential factors include longer hospitalizations, differences in substance intoxication or withdrawal (which we did not assess), or lower rates of hypertension treatment. It is also unclear why the proportions of patients with a BMI measurement were so low (< 50% in both groups). This finding could be due to our method of data extraction from the chart, since we specifically collected BMI and not weight and height as individual parameters. We specifically chose to capture precalculated BMI, since this is what the treating providers would have also seen in the electronic medical record during the patient’s hospitalization and would not rely on the provider to perform the calculations. While taking the patient’s weight is part of the routine admission process in both groups, height measurements are not.
Effectively capturing medical comorbidities among those with SMI with the added cardiovascular disease risk of being on SGAs is important in risk stratification and ensuring appropriate preventive care needs. The finding that the RCL group was associated with dramatically higher rates of obesity, hyperlipidemia, and tobacco use disorder diagnoses is most likely due to more effective identification and documentation of chronic medical illness in this group, especially since the proportions of patients with abnormal glucose, hemoglobin A1c, BMI, and diagnosis of diabetes mellitus are similar in the 2 groups. It was difficult to account for differences in tobacco use disorder and nicotine replacement therapy use, since it is possible there are differences related to unit-specific practices. The finding that nearly 30% of diabetic patients in both groups were discharged without diabetes medications, 24%-43% of patients with hypertension were not on antihypertensives, and 63%-92% of those with hyperlipidemia were not taking a statin is consistent with rates of diabetes nontreatment (and almost as high as dyslipidemia nontreatment) in the CATIE schizophrenia trial sample23 and illustrates the ongoing need for medical interventions in these populations. We appreciate the possibility that decisions not to treat diabetes mellitus or hyperlipidemia could be based on patient preferences, on a rationale that these issues were nonacute, or in planning for the follow-up medical provider to initiate or resume treatment after discharge. However, since inpatient discharge documentation offers an opportunity to help with following up of chronic medical problems that may otherwise go untreated, decisions not to treat chronic medical illness in the acute psychiatric setting does not diminish the importance of identifying chronic medical illness, especially when screened for.
There are several limitations to this study. This was not a randomized, prospective trial but rather a retrospective look at 2 similar inpatient units that, albeit of the same university system, inherently have differences. We did not exclude repeated admissions for several reasons. First, the facility with RCL care has a single inpatient unit, whereas the facility with TAU has multiple adult inpatient units and a tertiary care medical hospital, making it infeasible to distinguish among incident admissions and readmissions (admissions could have occurred on other units), as well as admissions that could have occurred prior to the time period of study. Second, the numbers of identifiable readmissions to the same units within the study period were low (TAU: 12/232 and RCL: 17/220), making the differences between the groups unlikely to be accounted for by readmissions to a significant extent. Further, we did not attempt to calculate incident diagnosis of chronic medical illness that resulted specifically from abnormal testing, which would have helped identify whether colocated medical care is more effective in addressing abnormal screening, especially if one considers the effect of readmissions.
Additionally, there were significant differences between the 2 groups in the proportions of various payers, lengths of stay, proportions of patients with schizophrenia and schizoaffective disorder, and rates of specific antipsychotics used. We account for the RCL group’s having a much higher proportion of Medicaid patients, in part, because the facility operates alongside a high-volume crisis assessment center, which places referrals for inpatient beds regionally in addition to the unit with the RCL group. A greater proportion of privately insured patients receive admission at other regional psychiatric hospitals, thus increasing the proportion of those with Medicaid that are admitted to the RCL unit. The degree that the RCL model contributed to the shorter lengths of stay seen in this group is unclear, but the differences in length of stay are likely to be multifactorial. While both units are operated through a major academic health care system, there are some staffing differences. The RCL group tends to operate without resident physicians as part of the primary team and in a community setting. The TAU group has more direct involvement of resident physicians in daily patient care, which could account for some differences seen in length of stay, diagnosis, antipsychotic use, and decisions to order screening laboratory tests. While psychotic disorders comprised the majority of diagnoses in both groups, we would not inherently expect a difference between the 2 groups in the proportion with a psychotic illness to affect medical care. However, the comparability of the groups is improved by other similarities, namely the proportions of patients with diagnoses of hypertension and diabetes and of patients with a BMI > 30 and in discharge dispositions (Figures 2 and 4).
While SGAs in general are considered to have cardiometabolic risk, large meta-analyses40,41 have elucidated differences, with asenapine, lurasidone, and ziprasidone considered more metabolically neutral than other psychiatric medications. A recent meta-analysis40 identified significant weight gain among all antipsychotics except aripiprazole, amisulpride, and ziprasidone, with duration of treatment and antipsychotic naivete as associated factors in weight gain. While we found that the RCL group was associated with significantly higher use of aripiprazole and less use of clozapine and quetiapine, we were not able to determine if RCL care was directly related to the choice of antipsychotic used or if abnormal screening affected the choice of antipsychotic. While efficacy often drives antipsychotic choice, ensuring availability of more weight-neutral antipsychotics on inpatient formularies could reduce cardiometabolic risks for those in whom it is clinically feasible.
Taken together, this study highlights differences in medical screening practices at 2 inpatient psychiatric units for patients with, or at high risk for, cardiometabolic disease. It identifies major differences in rates of follow-up of abnormal screening results that directly translates to differences in identifying and treating medical comorbidities. Effectively addressing cardiovascular disease-related risk among persons with SMI relies on effectively intervening, creatively and early, in what is otherwise a population with limited access to medical care and higher medical costs, especially since metabolic abnormalities appear early in the course of schizophrenia and early in the course of antipsychotic use.28
Although beyond the scope of this article, additional consideration needs to be given to evaluating cost differences, both in actual costs in embedding primary care providers in an inpatient psychiatric facility and in potential net savings by more selective screening measures. Although not currently widely available, noninvasive measurement of peripheral arterial compliance, a measure of arterial elasticity negatively associated with atherosclerosis, stroke, and myocardial infarction,42,43 is also reduced in persons with psychiatric illness and among those taking SGAs.44-46 Peripheral arterial compliance measurements could serve as a future biomarker for cardiometabolic risk in general and specifically among those at higher risk due to SMI and SGA use. By employing physician assistants, RCL serves as a generalizable and likely affordable model of care delivery for both inpatient and outpatient settings. Studies looking at posthospitalization follow-up, treatment adherence, and recidivism are needed to evaluate the overall effectiveness of inpatient colocalized medical care. Accountable care organizations may need to systematically and quantitatively examine whether RCL in inpatient psychiatric settings can be a cost-effective mechanism for improving care integration for those with SMI and comorbid chronic medical disease.
Submitted: July 17, 2016; accepted September 9, 2016.
Published online: December 22, 2016.
Drug names: aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), clonidine (Catapres and others), clozapine (Clozaril, FazaClo, and others), iloperidone (Fanapt), lurasidone (Latuda), metformin (Glucophage and others), olanzapine (Zyprexa and others), paliperidone (Invega), prazosin (Minipress and others), quetiapine (Seroquel and others), risperidone (Risperdal and others), ziprasidone (Geodon and others).
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Potential conflicts of interest: Drs Tatreau, Harris, Sheitman, and Steiner have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: None.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Substance Abuse and Mental Health Services Administration (SAMHSA) Center for Behavioral Health Statistics and Quality. Results From the 2010 National Survey on Drug Use and Health: Mental Health Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People With Serious Mental Illness: National Association of State Mental Health Program Directors (NASMHPD). Alexandria, VA: Medical Directors Council; 2006.
3. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131. PubMed doi:10.1001/archpsyc.64.10.1123
4. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173(1):11-53. PubMed doi:10.1192/bjp.173.1.11
5. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156. PubMed doi:10.1176/ps.2009.60.2.147
6. Allebeck P. Schizophrenia: a life-shortening disease. Schizophr Bull. 1989;15(1):81-89. PubMed doi:10.1093/schbul/15.1.81
7. Carney CP, Allen J, Doebbeling BN. Receipt of clinical preventive medical services among psychiatric patients. Psychiatr Serv. 2002;53(8):1028-1030. PubMed doi:10.1176/appi.ps.53.8.1028
8. Carney CP, Yates WR, Goerdt CJ, et al. Psychiatrists’ and internists’ knowledge and attitudes about delivery of clinical preventive medical services. Psychiatr Serv. 1998;49(12):1594-1600. PubMed doi:10.1176/ps.49.12.1594
9. Daumit GL, Crum RM, Guallar E, et al. Receipt of preventive medical services at psychiatric visits by patients with severe mental illness. Psychiatr Serv. 2002;53(7):884-887. PubMed doi:10.1176/appi.ps.53.7.884
10. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136. PubMed doi:10.1097/00005650-200202000-00007
11. Desai MM, Rosenheck RA, Druss BG, et al. Mental disorders and quality of diabetes care in the Veterans Health Administration. Am J Psychiatry. 2002;159(9):1584-1590. PubMed doi:10.1176/appi.ajp.159.9.1584
12. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. PubMed doi:10.1001/archinte.160.14.2101
13. Petersen LA, Normand S-LT, Druss BG, et al. Process of care and outcome after acute myocardial infarction for patients with mental illness in the VA health care system: are there disparities? Health Serv Res. 2003;38(1, part I):41-63. PubMed doi:10.1111/1475-6773.00104
14. Shander D. Cardiovascular procedures in patients with mental disorders. JAMA. 2000;283(24):3198-3199, author reply 3198-3199. PubMed
15. Thorpe JM, Kalinowski CT, Patterson ME, et al. Psychological distress as a barrier to preventive care in community-dwelling elderly in the United States. Med Care. 2006;44(2):187-191. PubMed doi:10.1097/01.mlr.0000196965.54871.d5
16. Wang PS, Avorn J, Brookhart MA, et al. Effects of noncardiovascular comorbidities on antihypertensive use in elderly hypertensives. Hypertension. 2005;46(2):273-279. PubMed doi:10.1161/01.HYP.0000172753.96583.e1
17. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22. PubMed doi:10.1016/j.schres.2006.06.026
18. Hackman AL, Goldberg RW, Brown CH, et al. Use of emergency department services for somatic reasons by people with serious mental illness. Psychiatr Serv. 2006;57(4):563-566. PubMed doi:10.1176/ps.2006.57.4.563
19. American Psychiatric Association. Millman report summary: economic impact of integrated medical-behavioral healthcare. 2014 American Psychiatric Association Web site. https://integrationacademy.ahrq.gov/resources/new-and-notables/economic-impact-integrated-medical-behavioral-healthcare-implications. Accessed September 14, 2016.
20. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60(9):897-903. PubMed doi:10.1001/archpsyc.60.9.897
21. Allison DB, Fontaine KR, Heo M, et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60(4):215-220. PubMed doi:10.4088/JCP.v60n0402
22. Cassidy F, Ahearn E, Carroll BJ. Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. Am J Psychiatry. 1999;156(9):1417-1420. PubMed
23. Davidson S, Judd F, Jolley D, et al. Cardiovascular risk factors for people with mental illness. Aust N Z J Psychiatry. 2001;35(2):196-202. PubMed doi:10.1046/j.1440-1614.2001.00877.x
24. Dixon L, Postrado L, Delahanty J, et al. The association of medical comorbidity in schizophrenia with poor physical and mental health. J Nerv Ment Dis. 1999;187(8):496-502. PubMed doi:10.1097/00005053-199908000-00006
25. Herrסn A, de Santiago A, Sandoya M, et al. Determinants of smoking behaviour in outpatients with schizophrenia. Schizophr Res. 2000;41(2):373-381. PubMed doi:10.1016/S0920-9964(99)00082-1
26. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002;63(3):207-213. PubMed doi:10.4088/JCP.v63n0306
27. De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126. PubMed doi:10.1038/nrendo.2011.156
28. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71(12):1350-1363. PubMed doi:10.1001/jamapsychiatry.2014.1314
29. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601. PubMed doi:10.2337/diacare.27.2.596
30. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349. PubMed doi:10.1176/appi.ajp.161.8.1334
31. Mitchell AJ, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42(1):125-147. PubMed doi:10.1017/S003329171100105X
32. Mauer BJ, Druss BG. Mind and body reunited: improving care at the behavioral and primary healthcare interface. J Behav Health Serv Res. 2010;37(4):529-542. PubMed doi:10.1007/s11414-009-9176-0
33. Boardman JB. Health access and integration for adults with serious and persistent mental illness. Fam Syst Health. 2006;24(1):3-18. doi:10.1037/1091-7527.24.1.3
34. Collins C, Fernandez G, Ruppenkamp J. Reverse co-location report. 2011. Office of Rural Health and Community Care, Community Care of North Carolina Web site. https://www.communitycarenc.org/media/files/reverse-co-location-report.pdf. Accessed September 14, 2016.
35. Druss BG, Rohrbaugh RM, Levinson CM, et al. Integrated medical care for patients with serious psychiatric illness: a randomized trial. Arch Gen Psychiatry. 2001;58(9):861-868. PubMed doi:10.1001/archpsyc.58.9.861
36. Druss BG, von Esenwein SA, Compton MT, et al. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167(2):151-159. PubMed doi:10.1176/appi.ajp.2009.09050691
37. Rubin AS, Littenberg B, Ross R, et al. Effects on processes and costs of care associated with the addition of an internist to an inpatient psychiatry team. Psychiatr Serv. 2005;56(4):463-467. PubMed doi:10.1176/appi.ps.56.4.463
38. Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. PubMed doi:10.1210/jc.2011-3213
39. Stangroom J. Z Score calculator for 2 population proportions. 2016. Social Science Statistics Web site. http://www.socscistatistics.com/tests/ztest/Default2.aspx
40. Bak M, Fransen A, Janssen J, et al. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9(4):e94112. PubMed doi:10.1371/journal.pone.0094112
41. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed doi:10.1016/S0140-6736(13)60733-3
42. Cohn JN. Arterial compliance to stratify cardiovascular risk: more precision in therapeutic decision making. Am J Hypertens. 2001;14(8 pt 2):258S-263S. PubMed doi:10.1016/S0895-7061(01)02154-9
43. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206. PubMed doi:10.1177/153857440303700307
44. Koola M, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. Prim Care Companion CNS Disord. 2016;18(3):158-164. doi:10.4088/pcc.15m01880
45. Koola MM, Raines JK, Hamilton RG, et al. Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia? Curr Psychiatr. 2016;15(5):52-57. PubMed
46. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253. PubMed doi:10.1016/j.schres.2012.02.007
Enjoy this premium PDF as part of your membership benefits!