Atypical Opioid Use Disorder Treated With Buprenorphine/Naloxone
Loperamide is an over-the-counter (OTC) opioid agonist that primarily treats diarrhea. While a 77% increase in loperamide abuse from 2008 to 2016 has been reported,1 empirically supported management options remain scarce. We report the case of a patient with excessive loperamide use who was successfully managed with buprenorphine/naloxone.
Case Report
Ms A is a 40-year-old white woman with a history of opioid use disorder as well as anxiety, attention-deficit/hyperactivity disorder, and migraine, which were being treated with alprazolam, amphetamine, and hydrocodone, respectively. She presented to the outpatient mental health service and reported excessive use of loperamide 200 mg (100 2-mg tablets/d) for the past 6 months due to reduced effectiveness of hydrocodone (she also may have had limited access to it). After abstaining from loperamide for 4 days and hydrocodone for 10 days, Ms A’s Clinical Opiate Withdrawal Scale (COWS)2 score was 9 (rhinorrhea, mild pupillary dilation, irritability, and restlessness). For detoxification, she was started on buprenorphine 2 mg/naloxone 0.5 mg, which resulted in reduction of her COWS score to 4 with the first dose and to 1 with a second dose. For maintenance, her buprenorphine/naloxone dose was gradually titrated during follow-up visits to buprenorphine 16 mg/naloxone 4 mg daily. In the subsequent 6 months, she remained abstinent from both loperamide and hydrocodone.
Discussion
In the United States, loperamide is approved by the US Food and Drug Administration (FDA) in doses up to 16 mg daily for the treatment of acute and chronic diarrhea.2 Loperamide inhibits intestinal peristalsis through µ-opioid receptor agonism. Loperamide predominantly targets sites peripheral to the central nervous system (CNS). At elevated blood concentrations, loperamide penetrates the blood-brain barrier (BBB) and generates effects that mimic the euphoria commonly associated with often-abused narcotics.3 Loperamide’s permeation of the BBB is attributed to the diffusion-impeding capacity of P-glycoprotein in the barrier being exceeded at higher doses. Furthermore, as loperamide is a substrate for the cytochrome P450 (CYP) 3A4 isoenzyme, concurrent administration with CYP3A4 inhibitors may elevate loperamide concentrations systemically and in the CNS. Exploitation of this physiology can produce euphoria and abate opioid withdrawal symptoms, which leads to addiction and toxicity.4
Investigations of loperamide involving animals initially suggested minimal CNS activity.5 Early studies with humans identified 60 mg of loperamide as having the same addiction potential as placebo.6 However, more recently, illicit use of loperamide has risen sharply, driven by loperamide’s easy accessibility in unchecked quantities and relatively low cost.7 Case series have documented ingestions of loperamide of up to 1,600 mg daily.7 For all these reasons, it’s not surprising that loperamide has been dubbed the "poor man’s methadone."8
Between 2011 and 2014, poison control calls throughout the nation reflected a 71% increase in toxic dosings of loperamide.9 In 2016, the FDA warned health care providers and the general public about possible serious adverse effects of loperamide, including cardiac arrhythmias, respiratory depression, gastrointestinal distention, and death.9,10
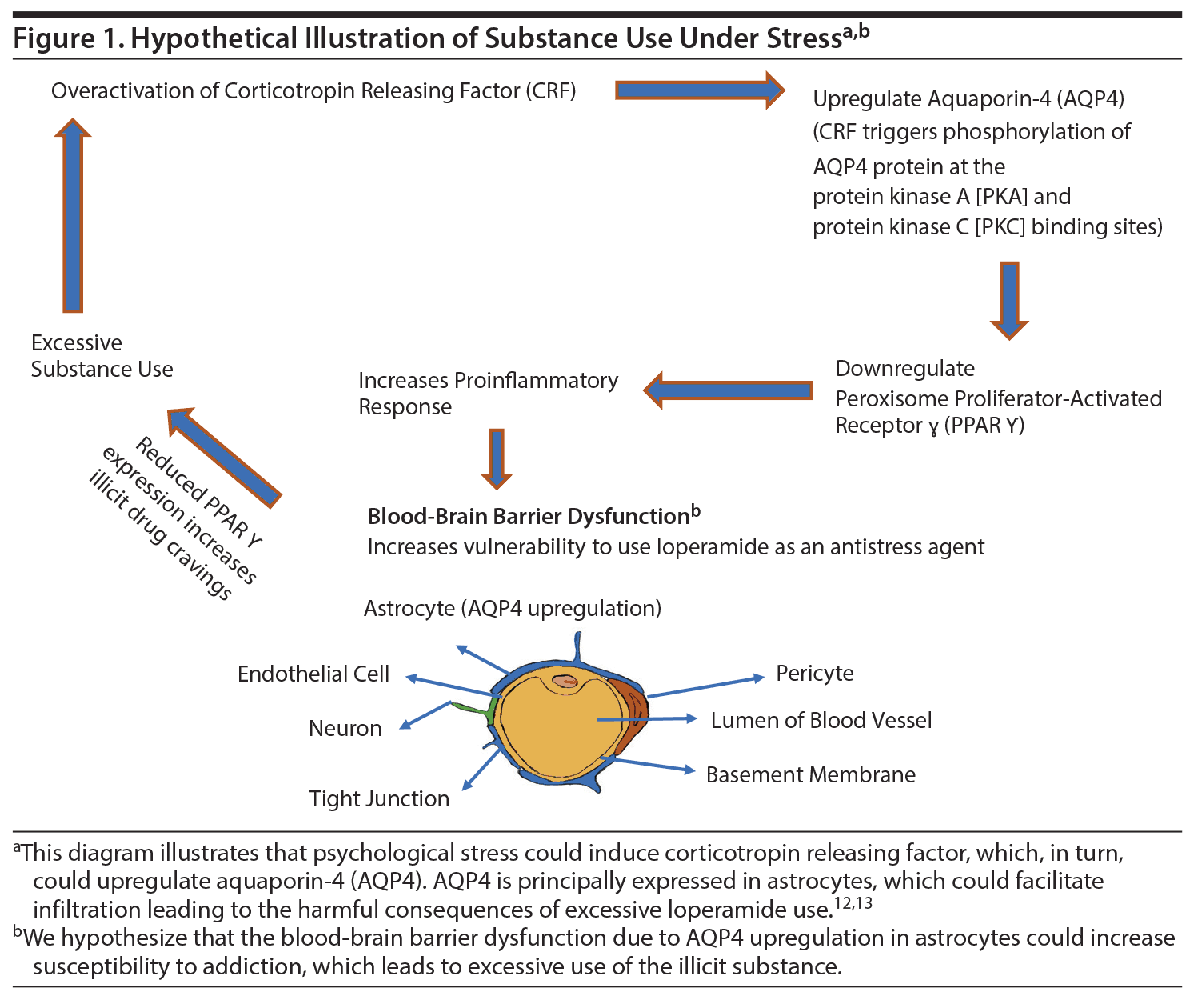
Conceptualization of loperamide as an antistress agent lends support to the supposition that individuals with high allostatic vulnerability may be especially susceptible to its psychopharmacologic features,11 as shown in Figure 1.12,13
Conclusion
This report is the first, to our knowledge, of successful treatment of loperamide addiction with buprenorphine/naloxone. This case and supporting observations9,10 shed light on loperamide addiction as an atypical opioid use disorder of increasing concern and demonstrate the promising role of buprenorphine-naloxone in its management. In the interest of public health, further investigations of treatment as well as the intrinsic properties and external factors contributing to hazardous patterns of loperamide use are needed.
Potential conflicts of interest: None.
Funding/support: None.
Previous presentation: The case report was presented at the 22nd annual scientific meeting of the American Society of Addiction Medicine; April 4, 2014; Orlando, Florida.
Acknowledgments: The authors thank the FDA for assessing reports on loperamide misuse/abuse based on the petition filed by Dr Varghese on December 21, 2016, requesting the amendment of availability of loperamide (Imodium) as a nonprescription OTC drug to a prescription drug. The petition was assigned docket number FDA-2016-P-4388. The comments on this petition from the FDA are available online dated on September 5, 2017 (https://www.chpa.org/PDF/09_05_17_CommentsCitizenPetitionLoperamide.aspx). The response stated that when taken in therapeutic doses, loperamide is both safe and effective because it does not cross the blood-brain barrier. Although overdosing has been reported with loperamide, the benefits of this only OTC drug to treat acute diarrhea and reduce the number of visits to a physician outweigh the consequences.
Patient consent: The patient provided consent to publish this case report, and information has been de-identified to protect anonymity.
Published online: July 3, 2019.
REFERENCES
1.Tompkins DA, Bigelow GE, Harrison JA, et al. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend. 2009;105(1-2):154-159. PubMed CrossRef
2.Hanauer SB. The role of loperamide in gastrointestinal disorders. Rev Gastroenterol Disord. 2008;8(1):15-20. PubMed
3.Baker DE. Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7(suppl 3):S11-S18. PubMed
4.Ooms LA, Degryse AD, Janssen PA. Mechanisms of action of loperamide. Scand J Gastroenterol suppl. 1984;96:145-155. PubMed
5.Choo EF, Kurnik D, Muszkat M, et al. Differential in vivo sensitivity to inhibition of P-glycoprotein located in lymphocytes, testes, and the blood-brain barrier. J Pharmacol Exp Ther. 2006;317(3):1012-1018. PubMed CrossRef
6.Leo RJ, Ghazi MA, Jaziri KS. Methadone management of withdrawal associated with loperamide-related opioid use disorder. J Addict Med. 2017;11(5):402-404. PubMed CrossRef
7.Swank KA, Wu E, Kortepeter C, et al. Adverse event detection using the FDA post-marketing drug safety surveillance system: cardiotoxicity associated with loperamide abuse and misuse. J Am Pharm Assoc (2003). 2017;57(2S):S63-S67. PubMed CrossRef
8.Dierksen J, Gonsoulin M, Walterscheid JP. Poor man’s methadone: a case report of loperamide toxicity. Am J Forensic Med Pathol. 2015;36(4):268-270. PubMed CrossRef
9.Idris A, Mihora DC, Kaye K. Loperamide abuse cardiotoxicity: should loperamide still be an over the counter medication? Am J Emerg Med. 2018;36(9):1716.e1-1716.e3. PubMed CrossRef
10.Vakkalanka JP, Charlton NP, Holstege CP. Epidemiologic trends in loperamide abuse and misuse. Ann Emerg Med. 2017;69(1):73-78. PubMed CrossRef
11.Sudakov SK, Sotnikov SV, Chekmareva NY, et al. Changes in β-endorphin level in the cingulate cortex in rats after peripheral loperamide and methylnaloxone administration at rest and during emotional stress. Bull Exp Biol Med. 2010;149(2):167-169. PubMed CrossRef
12.Chen SJ, Yang JF, Kong FP, et al. Overactivation of corticotropin-releasing factor receptor type 1 and aquaporin-4 by hypoxia induces cerebral edema. Proc Natl Acad Sci U S A. 2014;111(36):13199-13204. PubMed CrossRef
13.Zhao F, Deng J, Xu X, et al. Aquaporin-4 deletion ameliorates hypoglycemia-induced BBB permeability by inhibiting inflammatory responses. J Neuroinflammation. 2018;15(1):157. PubMed CrossRef
aAddiction Recovery Treatment Services, VA Northern California Health Care System, Sacramento, California
bDepartment of Psychiatry and Behavioral Sciences, University of California, Davis, Sacramento, California
cDow University of Health Sciences, Karachi, Pakistan
dAlcohol and Substance Abuse Programs, Inc, Altadena, California
eDepartment of Psychiatry, University of Illinois at Chicago, Chicago, Illinois
fMental Health Service Line, Veterans Affairs Medical Center, Loma Linda, California
gDepartment of Psychiatry and Behavioral Sciences, George Washington University School of Medicine and Health Sciences, Washington, DC
*Corresponding author: Sajoy P. Varghese, MD, 10535 Hospital Way, Mather, CA, 95655 ([email protected]).
Prim Care Companion CNS Disord 2019;21(4):19l02446
To cite: Varghese SP, Kumari P, Wijegunaratne H, et al. Loperamide addiction: atypical opioid use disorder treated with buprenorphine/naloxone. Prim Care Companion CNS Disord. 2019;21(4):19l02446.
To share: https://doi.org/10.4088/PCC.19l02446
© Copyright 2019 Physicians Postgraduate Press, Inc.
Enjoy this premium PDF as part of your membership benefits!