Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
Have you ever wondered why physicians try to avoid prescribing opioids for patients with chronic pain? Have you ever struggled to decide whether prescribing opioids is appropriate or wondered how likely it is that one of your patients will become dependent following prescription of an opioid? If you have, then the following case vignette and discussion of patient, provider, and system factors that interfere with prescribing an opioid should prove useful.
Obstacles to the Prescription and Use of Opioids
Have you ever wondered why physicians try to avoid prescribing opioids for patients with chronic pain? Have you ever struggled to decide whether prescribing opioids is appropriate or wondered how likely it is that one of your patients will become dependent following prescription of an opioid? If you have, then the following case vignette and discussion of patient, provider, and system factors that interfere with prescribing an opioid should prove useful.
CASE VIGNETTE
Mr A, a 44-year-old veteran with a history of hypertension, gout, and chronic lower back pain, presented to his primary care physician’s office with complaints of persistent back pain. He has tried ibuprofen and acetaminophen, fentanyl patches, and, most recently, vicodin, all without much relief. He says that his back pain interferes with his ability to work at his physically demanding job (as a contractor) and to play soccer on weekends. He describes having difficulty with sleep and feeling irritable due to his pain. Mr A’s other medications include hydrochlorothiazide and allopurinol. He has no personal or family history of drug or alcohol abuse. He is married and has 2 children in college. During the visit, he reports that he previously received oxycodone for acute back spasms and is wondering if he could try oxycodone once again to treat his current pain. Should Mr A’s physician prescribe oxycodone? How much and for how long?
WHAT IS THE LINK BETWEEN
OPIOID MECHANISM AND PAIN RELIEF?
Opiate use dates back to the beginning of human civilization, yet opiates and their synthetic derivatives continue to be the most potent and effective analgesic agents available.1–3 Opiates are substances that contain opium or its derivatives. The term opioid refers to natural or synthetic chemicals that have opiate-like effects. Opioid medications can be grouped into naturally occurring opioids (morphine, codeine, thebaine), semisynthetic opioids (oxycodone, hydrocodone, hydromorphone), and synthetic opioids (fentanyl, meperidine, methadone, tapentadol).
Opioids imitate endogenous peptides, including endorphins, endomorphins, enkephalins, and dynorphins, by stimulating opioid G protein–coupled receptors.2,4 They act at 4 subtypes of opioid receptors (μ, δ, κ, and the nociceptin orphanin peptide receptor). Among these receptors, μ is the most closely associated with analgesia and addiction.5 Due to concern about addiction, pain often goes undertreated.
Undertreatment of chronic pain has both human and economic consequences. In 2008, about 100 million adults in the United States suffered from chronic pain, resulting in huge health care costs and lost productivity (totaling roughly $560–$635 billion).6 Chronic back pain alone is estimated to cost $100 million per year, representing about 2% of the US domestic gross national product.7 In addition to its economic consequences, undertreatment of pain represents a failure of a physician’s mandate to alleviate suffering and disease burden. Beyond its effects on suffering, chronic pain disrupts patients’ capacity to work and their social interactions, and it increases the risk of both depression and anxiety.8,9
WHAT ARE THE Physician’S OBSTACLES
TO THE PRESCRIPTION OF OPIOIDS?
Physicians who prescribe opioids must consider the risk of tolerance and substance abuse and associated unhealthy behavior patterns. Drug tolerance is an altered physiologic state caused by repeated exposure to a drug, which necessitates continued administration of the drug to prevent withdrawal symptoms. Substance abuse includes affective, behavioral, cognitive, and physiologic factors, which may develop with repeated drug exposure; these typically include the desire to take the drug, trouble controlling the amount of drug taken, continued use despite negative consequences related to drug use, prioritization of drug use above other obligations, and drug tolerance and withdrawal when the drug is withheld.10 While opioids effectively relieve pain, tolerance often develops, and a subset of those individuals with tolerance progress to addiction. Some people become addicted to the inherent euphoric effects of opioids, while others are compelled to continue using the drug to avert symptoms of drug withdrawal following its discontinuation.11 Opioid addiction is particularly dangerous due to its life-threatening side effects (eg, respiratory depression and hypoperfusion). Worldwide, opioid overdose causes an estimated 69,000 deaths annually.10 In the United States, more than 16,000 opioid prescription–related deaths (as differentiated from deaths due to illicit use of opiates) occurred in 2010.12 Opioids are frequently used because there are few alternative agents that offer the same level of analgesia (and μ receptor potency), making it challenging for physicians to balance pain relief with the risk of creating or fueling addictive behavior.3 Additionally, pain management is not adequately taught in the majority of US medical schools (Table 1). Treatment contracts, risk assessment tools, and inclusion of pain management training in medical school curriculums could mitigate some of these challenges.

HOW DOES A PROVIDER ASSESS
WHO RECEIVES AN OPIOID?
Opioids are appropriate for the treatment of acute or severe pain (eg, pain following surgery, trauma, and burns), as well as for chronic pain associated with terminal illnesses (eg, cancer).19 In the above circumstances, opioids are effective and essential. Opioids allow an individual to engage actively in rehabilitation after orthopedic surgery and to mitigate otherwise intolerable pain in those who are terminally ill and unlikely to return to their baseline level of functioning.26 The controversy surrounding opioid medications generally focuses on the management of chronic noncancer pain (eg, chronic back pain).27 More than 3% of adults in the United States are prescribed an opioid medication for chronic non–cancer-related pain despite the lack of high-quality evidence suggesting that opioids are better than placebo or alternative treatments.28–30
HOW DOES A Physician DETERMINE
THE DOSING OF AN OPIOID?
When prescribing an opioid, most practitioners start patients on a small dose and increase it gradually until symptoms are controlled or adverse effects intervene. In choosing the appropriate treatment for chronic pain, alternative nonopioid interventions should be explored.31 If tolerance to an opioid develops, switching to a different opioid may also restore analgesia due to incomplete cross-tolerance at receptor sites.32 Table 2 provides potencies and special features of specific opioids.33 Psychological stress often alters the response to pain and the phenomenon of tolerance.34 The intensity of pain reported and the perception of pain are both influenced by myriad factors (including mood, cultural background, social supports, and financial resources). Treatment of pain should be informed by a biopsychosocial model that addresses not only the biological basis of pain, but also the associated social and psychological factors.9 Thus, pain thresholds that decrease in relation to changing life circumstances should not automatically result in a dose increase. However, tools such as the Pain Assessment and Documentation Tool (PADT)35 and the Diagnosis, Intractability, Risk, and Efficacy (DIRE)36 score, which are physician rated, can be used to assess outcomes (eg, effective analgesia and patient functioning) of pain management.

WHAT PATIENT FACTORS IMPACT
THE PRESCRIPTION OF OPIOIDS?
Patients’ fear of addiction also influences the use of opioid pain medications (Table 3). This fear is heightened by the fact that the full extent of risk factors for opioid dependence is incompletely known, especially in outpatient settings. In a prospective study of 196 patients with non–cancer-related chronic pain seen at a large academic center, one-third demonstrated opioid misuse after 12 months.13 Although 85% of those patients had an income less than $20,000, which might limit generalizability of the findings, the study13 found that a history of cocaine or alcohol abuse was the strongest predictor of opioid misuse. A similar study37 of 15,100 veterans found that in addition to prior substance abuse, a coexisting mental health disorder, younger age, and male sex also contributed to this risk. Boscarino et al38 showed that age < 65 years, as well as a higher number of drug prescriptions entered into the medical record, was associated with opioid dependence. Consequently, numerous risk prediction tools have been created to help identify patients at risk for opioid dependence. The Opioid Risk Tool (ORT),16 which is completed by patients and incorporates family and personal history of substance abuse, age, history of preadolescent sexual abuse, psychological disease, and depression, has a sensitivity of approximately 80% for the detection of patients who will develop opioid-related aberrant behavior. The Screener and Opioid Assessment for Patients with Pain (SOAPP)17 is another modality for assessing the suitability of long-term opioid therapy. The 5 factors included in the SOAPP model are history of substance abuse, legal problems, craving medication, heavy smoking, and mood swings.17 Similarly, the Current Opioid Misuse Measure (COMM)18 is a self-administered 17-item questionnaire that analyzes behaviors over the past 30 days in those with chronic non–cancer-related pain. Therefore, tools such as the ORT, SOAPP, and COMM facilitate the stratification of patient risk and help minimize overuse in patients at risk for dependence. Table 4 provides more information on these risk assessment scales.


WHAT SYSTEMATIC FACTORS IMPACT
THE PRESCRIPTION OF OPIOIDS?
There are several systemic obstacles to prescribing opioid medications (Table 5). Regulatory barriers make it more time consuming to prescribe opioids. Physicians, who are notoriously time pressured, must be willing to spend valuable time obtaining regulatory checks, such as prior authorization, in order to prescribe opioids. Additionally, many physicians fear regulatory and legal sanctions/scrutiny, posing further challenge to opioid prescription.15,42,43 Unfortunately, when pain is severe and requires powerful pain medication to control it, there is little choice; there are no pain medications that are as effective as opioids with lower addiction risk. Continuity of care with pain patients is essential since opioid abuse risk is elevated in patients who receive opioid prescriptions from multiple providers or who use multiple pharmacies.44,45

HOW CAN PHYSICIANS REDUCE
THE RISK OF OPIOID ABUSE?
Before prescribing an opioid, physicians should carefully consider whether opioids are appropriate for a given patient and whether alternative therapies have been exhausted. Alternative therapies for the treatment of chronic pain are shown in Table 6. Once opioid use is deemed appropriate for a particular patient, safeguards should be implemented to reduce the patient’s risk for opioid abuse. The patient should review and sign a treatment contract that specifies the importance of taking the opioid only as prescribed and agree to obtain prescriptions from a single provider.14 A baseline urine drug test obtained prior to initiation of opioid therapy can help the physician assess the patient’s risk for future opioid abuse by providing information on the patient’s current drug use. Results of routine urine testing can inform treatment adherence and may reduce the social cost of medication diversion for nonmedical use. Physicians and patients should collaborate on what a successful pain management plan involves for the patient and wean the opioid if the goals are not reached.

CASE DISCUSSION
The case of Mr A illustrates a commonly encountered complex scenario. Mr A reported significant discomfort and disability from his chronic back pain. Mr A’s primary care physician wanted to alleviate his suffering, but standard first-line pharmacologic treatments failed. A trial of an opioid was not unreasonable, as opioids are likely to help him in the short term. However, long-term opioid use has caused opioid-induced hyperalgesia, which lowers pain thresholds and could ultimately cause greater pain or opioid dependence.67 Opioid medications also cause other side effects, including constipation, nausea, and somnolence, in approximately 80% of patients.68 Opioid use in the outpatient setting is further complicated by misuse and diversion.
In determining whether opioids are an appropriate next step, the physician should assess whether other factors are contributing to Mr A’s pain. For example, Mr A’s irritability and insomnia may indicate an underlying mood disorder, which could augment his experience of pain. If Mr A meets criteria for an underlying mood disorder, he may improve with antidepressant medication. The physician also could discuss the risks and benefits of opioid medications. Mr A’s abuse potential can be calculated using the ORT, SOAPP, or COMM to further help the decision process. Given his age (44 years) and possible depression, Mr A’s ORT score was 1–2, which puts him in the low-risk group.16 Despite having a low-risk profile, these calculation tools are imperfect and opioids still convey risk. If Mr A is amenable, he and his physician can explore a broad range of nonpharmacologic treatment options (Table 6).
If the decision is made to move forward with a short-term opioid trial, several measures can be implemented to mitigate the risk of abuse and diversion. Mr A and his physician should establish a treatment contract as described previously detailing appropriate safeguards (such as taking the opioid only as prescribed, obtaining prescriptions from a single provider/pharmacy, safeguarding the medication from theft, and agreeing on duration of course). The initial dose should be as low as possible and may be slowly titrated to the lowest effective dose. The physician can monitor Mr A’s opioid use with urine drug tests and prescription monitoring program databases. Mr A and his physician also need to determine the goals for therapy and what successful pain management might mean. Realizing that chronic pain treatment in the outpatient setting is challenging and much more nuanced than illustrated with the simplicity of Mr A’s case, providers and patients can generate these goals of therapy in the form of a treatment plan.
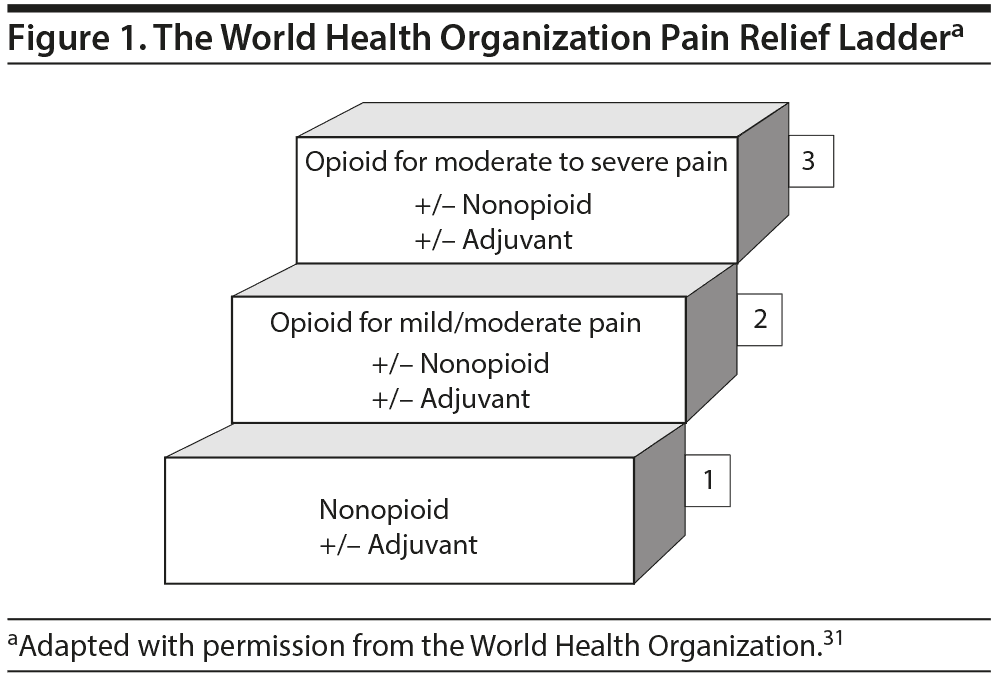
The basis of such a treatment plan should be rooted in the schema outlined by the World Health Organization stepladder for chronic pain treatment (Figure 1).31 Opioids should not be the first line of treatment for chronic pain, but should be prescribed with caution and only used in cases of severe debilitating pain not abated by other nonopioid modalities. Tools such as the PADT and DIRE can assess treatment outcomes. If therapeutic goals are not met, the opioid should be discontinued. If opioid dependence develops, a variety of intervention and treatment strategies are available (but are beyond the scope of this article).69
CONCLUSION
As illustrated in our vignette, the prescription of opioid medication is multifaceted and challenging. Opioid medications are the most effective therapy available, and other treatments may fail to alleviate chronic pain. This puts physicians in a difficult position of attempting to balance the risks and benefits of opioid medications. Physicians should discuss this dilemma frankly and work with patients to create the best treatment plan, which could include exhausting alternative therapies or only using opioids episodically during periods of elevated pain, ultimately limiting the amount of prescribed medication.
REFERENCES
1. Booth M. Opium: A History. London, England: Simon & Schuster, Ltd; 1996.
2. Pasternak GW. The Opiate Receptors. New York, NY: Humana Press/Springer; 2011. doi:10.1007/978-1-60761-993-2
3. Fields HL. The doctor’s dilemma: opiate analgesics and chronic pain. Neuron. 2011;69(4):591–594. doi:10.1016/j.neuron.2011.02.001 PubMed
4. Koob GF, Le Moal M. Neurobiology of Addiction. Amsterdam, the Netherlands: Elsevier; 2006:121–171. doi:10.1016/B978-012419239-3/50041-2
5. De Vries TJ, Shippenberg TS. Neural systems underlying opiate addiction. J Neurosci. 2002;22(9):3321–3325. PubMed
6. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi:10.1016/j.jpain.2012.03.009 PubMed
7. Turk DC, Swanson K. Efficacy and cost-effectiveness treatment for chronic pain: an analysis and evidenced-based synthesis. In: Schatman ME, Campbell A, eds. Chronic Pain Management: Guidelines For Multidisciplinary Program Development. New York, NY: Informa; 2007:15–38.
8. Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi:10.1016/j.ejpain.2005.06.009 PubMed
9. Turk DC, Monarch ES. Biopsychosocial perspective on chronic pain. In: Gatchel RJ, Turk DC, eds. Psychological Approaches to Pain Management: A Practitioner’s Handbook. New York, NY: Guilford Press; 2002:3–23.
10. World Health Organization. Community management of opioid overdose. http://www.who.int/substance_abuse/publications/management_opioid_overdose/en/. Updated November 2014. Accessed October 14, 2015.
11. van Ree JM, Gerrits MA, Vanderschuren LJ. Opioids, reward and addiction: an encounter of biology, psychology, and medicine. Pharmacol Rev. 1999;51(2):341–396. PubMed
12. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. doi:10.1056/NEJMsa1406143 PubMed
13. Ives TJ, Chelminski PR, Hammett-Stabler CA, et al. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6(1):46. doi:10.1186/1472-6963-6-46 PubMed
14. Washington State Agency Medical Directors’ Group. Interagency guideline on opioid dosing for chronic noncancer pain: an educational pilot to improve care and safety with opioid treatment. http://www.agencymeddirectors.wa.gov/Files/OpioidGdline.pdf. Updated 2010. Accessed October 3, 2015
15. Glajchen M. Chronic pain: treatment barriers and strategies for clinical practice. J Am Board Fam Pract. 2001;14(3):211–218. PubMed
16. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432–442. doi:10.1111/j.1526-4637.2005.00072.x PubMed
17. Akbik H, Butler SF, Budman SH, et al. Validation and clinical application of the Screener and Opioid Assessment for Patients with Pain (SOAPP). J Pain Symptom Manage. 2006;32(3):287–293. doi:10.1016/j.jpainsymman.2006.03.010 PubMed
18. Butler SF, Budman SH, Fanciullo GJ, et al. Cross validation of the Current Opioid Misuse Measure to monitor chronic pain patients on opioid therapy. Clin J Pain. 2010;26(9):770–776. doi:10.1097/AJP.0b013e3181f195ba PubMed
19. Spitz A, Moore AA, Papaleontiou M, et al. Primary care providers’ perspective on prescribing opioids to older adults with chronic noncancer pain: a qualitative study. BMC Geriatr. 2011;11(1):35. doi:10.1186/1471-2318-11-35 PubMed
20. Rolita L, Spegman A, Tang X, et al. Greater number of narcotic analgesic prescriptions for osteoarthritis is associated with falls and fractures in elderly adults. J Am Geriatr Soc. 2013;61(3):335–340. doi:10.1111/jgs.12148 PubMed
21. Shorr RI, Griffin MR, Daugherty JR, et al. Opioid analgesics and the risk of hip fracture in the elderly: codeine and propoxyphene. J Gerontol. 1992;47(4):M111–M115. doi:10.1093/geronj/47.4.M111 PubMed
22. Mezei L, Murinson BB; Johns Hopkins Pain Curriculum Development Team. Pain education in North American medical schools. J Pain. 2011;12(12):1199–1208. doi:10.1016/j.jpain.2011.06.006 PubMed
23. Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269(12):1537–1539. doi:10.1001/jama.1993.03500120075029 PubMed
24. Inciardi JA, Surratt HL, Kurtz SP, et al. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Med. 2007;8(2):171–183. doi:10.1111/j.1526-4637.2006.00255.x PubMed
25. Inciardi JA, Surratt HL, Cicero TJ, et al. Prescription opioid abuse and diversion in an urban community: the results of an ultrarapid assessment. Pain Med. 2009;10(3):537–548. doi:10.1111/j.1526-4637.2009.00603.x PubMed
26. Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–127. doi:10.7326/0003-4819-146-2-200701160-00006 PubMed
27. Bouckoms AJ, Masand P, Murray GB, et al. Chronic nonmalignant pain treated with long-term oral narcotic analgesics. Ann Clin Psychiatry. 1992;4(3):185–192. doi:10.3109/10401239209149570
28. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–1985. doi:10.1056/NEJMp1011512 PubMed
29. Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350(jan05 10):g6380. doi:10.1136/bmj.g6380 PubMed
30. Gaskell H, Moore RA, Derry S, et al. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;6(6):CD010692. 10.1002/14651858.CD010692.pub2 PubMed
31. World Health Organization. WHO’s Pain Ladder. 1986. http://www.who.int/cancer/palliative/painladder/en/. Accessed October 14, 2015.
32. Ballantyne JC. Opioid therapy in chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):201–218. doi:10.1016/j.pmr.2014.12.001 PubMed
33. Wasan AD, Alpay M, Nejad SH. Pathophysiology, psychiatric comorbidity, and treatment of pain. In: Stern TA, Fava M, Wilens TE, et al, eds. Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. Philadelphia, Pennsylvania: Elsevier; 2016:852–862.
34. Ballantyne JC, Sullivan MD, Kolodny A. Opioid dependence vs addiction: a distinction without a difference? Arch Intern Med. 2012;172(17):1342–1343. doi:10.1001/archinternmed.2012.3212 PubMed
35. Passik SD, Kirsh KL, Whitcomb L, et al. A new tool to assess and document pain outcomes in chronic pain patients receiving opioid therapy. Clin Ther. 2004;26(4):552–561. doi:10.1016/S0149-2918(04)90057-4 PubMed
36. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7(9):671–681. doi:10.1016/j.jpain.2006.03.001 PubMed
37. Edlund MJ, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic noncancer pain. Pain. 2007;129(3):355–362. doi:10.1016/j.pain.2007.02.014 PubMed
38. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among outpatients on opioid therapy in a large US health care system. Addiction. 2010;105(10):1776–1782. doi:10.1111/j.1360-0443.2010.03052.x PubMed
39. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):131–146, 146.e5. doi:10.1016/j.jpain.2008.10.009 PubMed
40. Butler SF, Budman SH, Fernandez K, et al. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112(1–2):65–75. doi:10.1016/j.pain.2004.07.026 PubMed
41. Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130(1-2):144–156. doi:10.1016/j.pain.2007.01.014 PubMed
42. Cherny NI, Baselga J, de Conno F, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Europe: a report from the ESMO/EAPC Opioid Policy Initiative. Ann Oncol. 2010;21(3):615–626. doi:10.1093/annonc/mdp581 PubMed
43. Jacobsen R, Sjøgren P, Møldrup C, et al. Physician-related barriers to cancer pain management with opioid analgesics: a systematic review. J Opioid Manag. 2007;3(4):207–214. PubMed
44. White AG, Birnbaum HG, Schiller M, et al. Analytic models to identify patients at risk for prescription opioid abuse. Am J Manag Care. 2009;15(12):897–906. PubMed
45. Wilsey BL, Fishman SM, Gilson AM, et al. Profiling multiple provider prescribing of opioids, benzodiazepines, stimulants, and anorectics. Drug Alcohol Depend. 2010;112(1–2):99–106. doi:10.1016/j.drugalcdep.2010.05.007 PubMed
46. Curtis LH, Stoddard J, Radeva JI, et al. Geographic variation in the prescription of schedule II opioid analgesics among outpatients in the United States. Health Serv Res. 2006;41(3 Pt 1):837–855. doi:10.1111/j.1475-6773.2006.00511.x PubMed
47. Vickers AJ, Linde K. Acupuncture for chronic pain. JAMA. 2014;311(9):955–956. doi:10.1001/jama.2013.285478 PubMed
48. Jensen MP. Hypnosis for chronic pain management: a new hope. Pain. 2009;146(3):235–237. doi:10.1016/j.pain.2009.06.027 PubMed
49. Samuel SR, Maiya GA. Application of low frequency and medium frequency currents in the management of acute and chronic pain: a narrative review. Indian J Palliat Care. 2015;21(1):116–120. doi:10.4103/0973-1075.150203 PubMed
50. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;12:CD004504. 10.1002/14651858.CD004504.pub4 PubMed
51. Cameron M, Gagnier JJ, Chrubasik S. Herbal therapy for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2011;2(2):CD002948. 10.1002/14651858.CD002948.pub2 PubMed
52. Williams AC de C, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. 10.1002/14651858.CD007407.pub3 PubMed
53. Newton-John TRO, Spence SH, Schotte D. Cognitive-behavioral therapy versus EMG biofeedback in the treatment of chronic low back pain. Behav Res Ther. 1995;33(6):691–697. doi:10.1016/0005-7967(95)00008-L PubMed
54. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015;9:CD001929. 10.1002/14651858.CD001929.pub3 PubMed
55. Gross A, Kay TM, Paquin J-P, et al; Cervical Overview Group. Exercises for mechanical neck disorders. Cochrane Database Syst Rev. 2015;1:CD004250. 10.1002/14651858.CD004250.pub5 PubMed
56. Al Faraj S, Al Mutairi K. Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine (Phila Pa 1976). 2003;28(2):177–179. doi:10.1097/00007632-200301150-00015 PubMed
57. Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463–1470. doi:10.4065/78.12.1463 PubMed
58. Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Mind to Face Stress, Pain and Illness. New York, NY: Dell Publishing; 1990:4.
59. Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain. 2008;134(3):310–319. doi:10.1016/j.pain.2007.04.038 PubMed
60. Woodman JP, Moore NR. Evidence for the effectiveness of Alexander Technique lessons in medical and health-related conditions: a systematic review. Int J Clin Pract. 2012;66(1):98–112. doi:10.1111/j.1742-1241.2011.02817.x PubMed
61. Ebadi S, Henschke N, Nakhostin Ansari N, et al. Therapeutic ultrasound for chronic low back pain. Cochrane Database Syst Rev. 2014;3:CD009169. 10.1002/14651858.CD009169.pub2 PubMed
62. Chung H, Dai T, Sharma SK, et al. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516–533. doi:10.1007/s10439-011-0454-7 PubMed
63. Brosseau L, Robinson V, Wells G, et al. Withdrawn: Low level laser therapy (Classes III) for treating osteoarthritis. Cochrane Database Syst Rev. 2007;1(1):CD002046. 10.1002/14651858.CD002046.pub3 PubMed
64. Brosseau L, Robinson V, Wells G, et al. Low level laser therapy (Classes I, II, and III) for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2005;4(4):CD002049. 10.1002/14651858.CD002049.pub2 PubMed
65. Mehling WE, Hamel KA, Acree M, et al. Randomized, controlled trial of breath therapy for patients with chronic low back pain. Altern Ther Health Med. 2005;11(4):44–52. PubMed
66. American Society of Anesthesiologists Task Force on Chronic Pain Management, American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810–833. doi:10.1097/ALN.0b013e3181c43103 PubMed
67. Silverman SM. Opioid-induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679–684. PubMed
68. Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic noncancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–380.
69. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies: tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–2066. doi:10.1056/NEJMp1402780 PubMed
LESSONS LEARNED AT THE INTERFACE
OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital (MGH) sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Mss Wallwork and Chipidza are fourth-year medical students at Harvard Medical School, Boston, Massachusetts. Dr Stern is chief of the Avery D. Weisman Psychiatry Consultation Service at Massachusetts General Hospital and the Ned H. Cassem professor of psychiatry in the field of psychosomatic medicine/consultation at Harvard Medical School, Boston, Massachusetts.
Prim Care Companion CNS Disord 2016;18(1):doi:10.4088/PCC.15f01900
© Copyright 2016 Physicians Postgraduate Press, Inc.
Submitted: October 26, 2015; accepted January 4, 2016.
Published online: February 18, 2016.
Potential conflicts of interest: Dr Stern is an employee of the Academy of Psychosomatic Medicine, has served on the speaker’s board of Reed Elsevier, is a stock shareholder in WiFiMD (Tablet PC), and has received royalties from Mosby/Elsevier and the Massachusetts General Hospital Psychiatry Academy and McGraw Hill. Mss Wallwork and Chipidza report no conflicts of interest related to the subject of this article and both contributed equally to the article.
Funding/support: None reported.
Corresponding author: Theodore A. Stern, MD, Harvard Medical School Massachusetts General Hospital, Department of Psychiatry, Massachusetts General Hospital, Fruit St, WRN 605, Boston, MA 02114
([email protected]).
Enjoy this premium PDF as part of your membership benefits!


