Strategies for Monitoring Outcomes in Patients With Bipolar Disorder
Practical strategies are available for primary care physicians to monitor psychiatric and medical outcomes as well as treatment adherence in patients with bipolar disorder. Current depressive symptoms can be assessed with tools like the 9-item Patient Health Questionnaire or Beck Depression Inventory. Lifetime presence or absence of manic or hypomanic symptoms can be assessed using the Mood Disorder Questionnaire (MDQ). These measures can be completed quickly by patients prior to appointments. Sensitivity of such ratings, particularly the MDQ, can be increased by having a significant other also rate the patient. Clinicians should also screen mood disorder patients for psychiatric comorbidities that are common in this population such as anxiety and substance use disorders. While patients with bipolar disorder may commonly be nonadherent with prescribed medication regimens, strategies that can help include having frank discussions with the patient, selecting medication collaboratively, adding psychotherapy with a psychoeducation element, monitoring appointment-keeping, using patient self-reports of medication-taking, enlisting the aid of significant others, and measuring plasma drug levels. Medical monitoring is needed to assess the safety and tolerability of psychotropic medications. All of the approved medications for bipolar disorder have at least 1 boxed warning for serious side effects, but are also associated with other common management-limiting side effects such as sedation, tremor, unsteadiness, restlessness, nausea, vomiting, diarrhea, constipation, weight gain, and metabolic problems. Routine monitoring is particularly needed for obesity, metabolic syndrome, and cardiovascular disorders, which lead to high rates of medical morbidity and mortality in patients with bipolar disorder. Monitoring protocols such as the one recommended by the American Diabetes Association for patients taking second-generation antipsychotics can be used for regular assessment.
(Prim Care Companion J Clin Psychiatry 2010;12[suppl 1]:10-16)
From the Department of Psychiatry and Behavioral Sciences, and the Bipolar Disorders Clinic, Stanford University School of Medicine, Stanford, California.
This article is derived from the planning teleconference series "Improving the Recognition and Treatment of Bipolar Disorder in Primary Care," which was held in September 2009 and supported by an educational grant from AstraZeneca.
Dr Ketter is a consultant for Abbott, AstraZeneca, Bristol-Myers Squibb, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Jazz, Novartis, Organon, Schering-Plough, Solvay, Valeant, Vanda, Wyeth, and XenoPort; has received grant/research support from Abbott, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, GlaxoSmithKline, Pfizer, Repligen, and Wyeth; and has received honoraria from Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Noven, Otsuka, and Pfizer; his spouse is an employee of Johnson & Johnson.
Corresponding author: Terence A. Ketter, MD, Stanford University, Department of Psychiatry, 401 Quarry Rd, Rm 2124, Stanford, CA 94305-5723 ([email protected]).
doi:10.4088/PCC.9064su1c.02
© Copyright 2010 Physicians Postgraduate Press, Inc.
To optimize treatment in patients with bipolar disorder, physicians need to monitor psychiatric and medical outcomes as well as medication adherence. Primary care professionals are accustomed to monitoring medical outcomes but may be less familiar with monitoring psychiatric outcomes and treatment adherence. Several practical tools and strategies are available to assist clinicians in monitoring outcomes of patients with bipolar disorder in primary care practices.
PSYCHIATRIC MONITORING
Psychiatric symptoms that require monitoring are not only those of the primary psychiatric disorder, ie, symptoms of depression and mania in bipolar disorder, but also those of common psychiatric comorbidities such as anxiety and substance use disorders. Clinicians should also be alert for psychiatric adverse effects of medications, which may include treatment-induced suicidality and mood elevation.
Dimensions of Bipolar Disorder to Monitor
Many variables have been used to assess psychiatric symptomatic outcomes in patients with bipolar disorder. Generally, these variables can be grouped into 3 basic dimensions: general subjective (patient-rated quality of life and depression), functioning/disability, and manic/psychotic symptoms. Brieger and colleagues1 found that these 3 dimensions accounted for 69% of the total variance in treatment outcomes of bipolar I disorder. The general subjective dimension correlated with comorbid anxiety and personality disorders and a history of past year hospitalizations. The functioning/disability dimension correlated with the number of prior bipolar episodes, poor premorbid adjustment, low income, and disability. The manic/psychotic symptoms dimension correlated with treatment nonadherence and low agreeableness. To help optimize outcomes for patients with bipolar disorder, each of these dimensions should be assessed and monitored.
For Clinical Use
- Monitor patients with bipolar disorder, paying close attention to patients’ functioning and disability, manic or psychotic symptoms, and subjective reporting of depressive symptoms and quality of life.
- Use strategies such as patient education and frank discussions with patients and their families to improve treatment adherence.
- Routinely monitor patients for medical morbidity (ie, cardiovascular, cerebrovascular, and gastrointestinal conditions).
- When prescribing psychotropic treatments, be mindful of serious (boxed warning) and common (eg, central nervous system and gastrointestinal) side effects and monitor patients appropriately.
During the course of bipolar disorder, the focus of monitoring changes.2 Patients are asymptomatic about half of the time,3 during which monitoring commonly focuses on medication-emergent side effects and ensuring that treatments are acceptable to the patient, both of which affect adherence. When patients with bipolar disorder are symptomatic, depressive symptoms are typically more pervasive than mood elevation symptoms by at least 3 to 1.3 Monitoring patients with bipolar disorder, as in monitoring those with unipolar major depressive disorder (MDD), focuses on tracking patients’ depressive symptoms.
Tools for Monitoring Depressive Symptoms
Several tools can be used in primary care settings to monitor patients’ depressive symptoms. The 9-item Patient Health Questionnaire (PHQ-9),4 which is available online at www.cqaimh.org/tool_depscreen.html, is particularly useful because it is brief and validated and can be completed by the patient.5 The Beck Depression Inventory6 can also be used. Self-report instruments are more feasible than clinician-rated scales, given the time constraints that are typical in clinical practice.
The PHQ-9 is a 9-item, self-report inventory related to the depression criteria as outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).7 Patients can complete the PHQ-9 before an appointment, enabling them to consult with significant others, which may enhance the sensitivity of the screen.
The PHQ-9 has been validated for use as a categorical diagnostic screening tool that detects the presence or absence of a major depressive episode. To screen positive for a current major depressive episode with the PHQ-9, the patient must endorse at least 5 symptoms (including at least 1 of sadness or anhedonia) as being present "more than half the days" over the past 2 weeks. If the PHQ-9 depression screen is positive, then, time permitting, more formal diagnostic tools may be used to confirm the presence of a major depressive episode. Keep in mind that major depressive episodes occur not only in (unipolar) MDD, but also in bipolar disorder. The PHQ-9 can also be used as an ordinal monitoring tool for severity of depressive symptoms that can track symptomatic improvement after treatment has begun.5 In this application, scores of 0 to 4 indicate minimal depression; 5 to 9 indicate subthreshold depression; 10 to 14 indicate minor depression, mild major depression, or dysthymia; 15 to 19 indicate major depression of moderate severity; and 20 to 27 indicate severe major depression.4
The PHQ-9 can also be used to monitor improvement in depressive symptoms in patients with mood disorders. Response (clinically meaningful improvement) is present if the total score decreases by at least 50% from baseline. However, the goal of treating depressive episodes is not just response but remission. A score ≤ 5 and no more than mild symptoms indicates remission.
Tools for Assessing the Presence or Absence of Manic or Hypomanic Symptoms
A useful tool to assess the presence or absence of mood elevation symptoms is the Mood Disorder Questionnaire (MDQ).8 The MDQ is a 13-item, self-report assessment that screens for the DSM-IV criteria of manic or hypomanic episodes. To screen positive for mania or hypomania, the patient must have answered "yes" to at least 7 of the 13 items. In addition, the symptoms must have been concurrent and have had at least moderate consequences for the patient, such as being unable to work, being involved in fights or disputes, and/or having family, money, or legal problems. For more in-depth coverage of the MDQ and to get the full version of the assessment, see the article in this supplement by J. Sloan Manning, MD, "Tools to Improve Differential Diagnosis of Bipolar Disorder in Primary Care."9
The MDQ is validated as a diagnostic screening instrument for detecting a lifetime history of mood elevation episodes, and repeated testing with this instrument can detect treatment-emergent mood elevation episodes. However, the MDQ is not an ordinal metric for severity of mood elevation symptoms. MDQ ratings completed by significant others about the patient can provide particularly valuable additional information about manic symptoms because such collateral ratings may be more sensitive for mood elevation, whereas patient self-ratings may be more sensitive for depression.
Psychiatric Comorbidities to Monitor
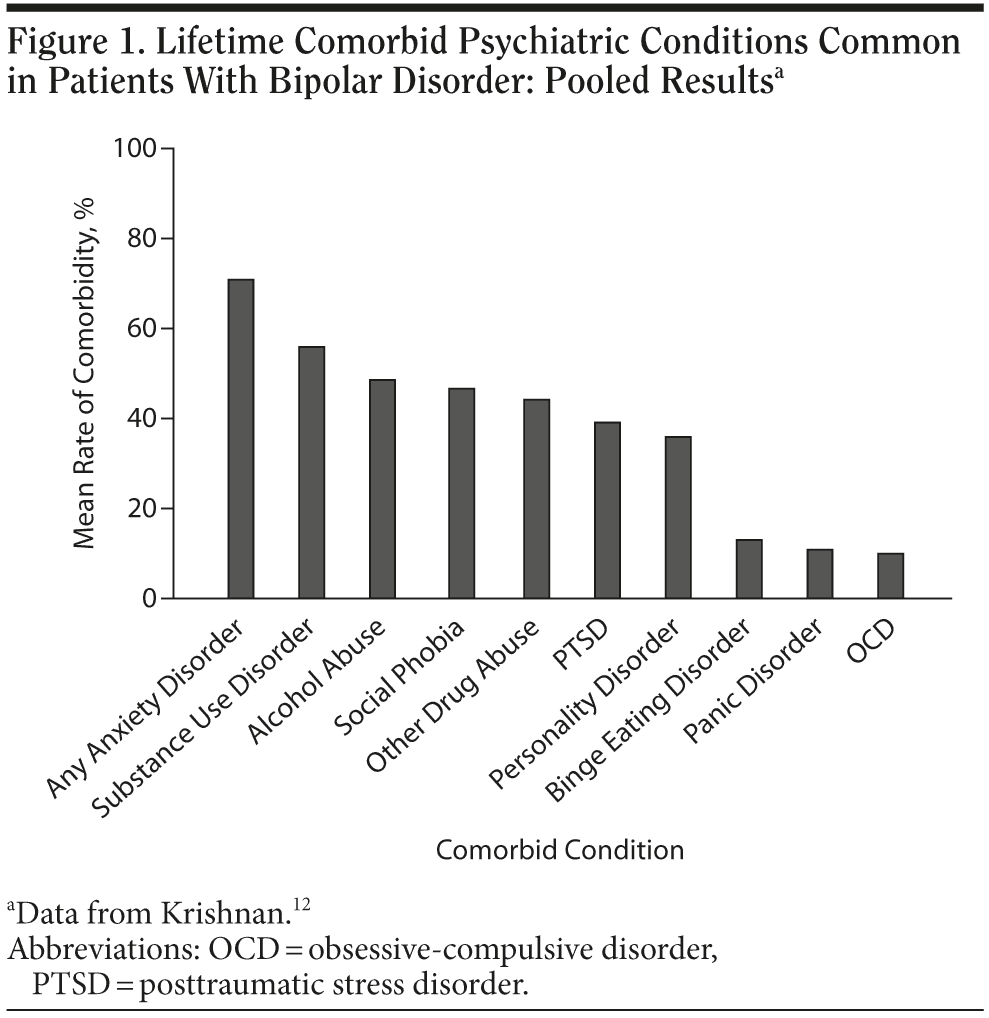
Psychiatric comorbidities are extremely common in patients with bipolar disorder and detection and treatment of these comorbidities are crucial for optimal management.10,11 Psychiatric disorders that often co-occur in patients with bipolar disorder include anxiety, substance use, personality, and eating disorders (Figure 1).12 Patients diagnosed with a mood, substance use, or anxiety disorder should be screened for the other 2 types of disorders in this triad. When clinicians monitor the treatment of a patient with bipolar disorder, they need to know whether concurrent substance use or anxiety disorders are present, whether those disorders are impacting the treatment of the bipolar disorder, and which of these problems constitutes the main or primary condition, and as such, merits particular attention.
MONITORING ADHERENCE TO MEDICATION
Reasons to Monitor Adherence
Bipolar disorder is episodic, and the lifetime risk of recurrence is approximately 90% in individuals who have had a single manic episode13; therefore, long-term treatment is necessary for the vast majority of patients. However, medication cannot be effective if patients do not take it. Treatment nonadherence is common in this population and is associated with an increased risk of relapse.14,15 For example, Keck et al16 found that, among patients hospitalized for acute manic episodes, 64% had been nonadherent with medication in the prior month.
Several factors contribute to suboptimal adherence to treatment for bipolar disorder.17 These factors may relate to the patient, the illness, the particular intervention, or the way the physician administers that intervention.18 Patient factors include demographic characteristics; for example, being of younger age, male, and unmarried are risk factors for nonadherence.19 The stage or characteristics of the illness can also contribute to nonadherence to treatment. For example, patients have been found to be less likely to adhere to medication begun following grandiose and manic symptoms than to medication begun following a depressive episode.20 Having fewer previous episodes or having comorbid personality or substance use disorders increases likelihood of poor adherence to medication.19 Treatment issues, such as adverse effects, and clinicians’ treatment strategies, such as the use of polypharmacy, also may contribute to decreased adherence to medication.16,19 A poor therapeutic alliance between the clinician and the patient can adversely affect adherence as well.14
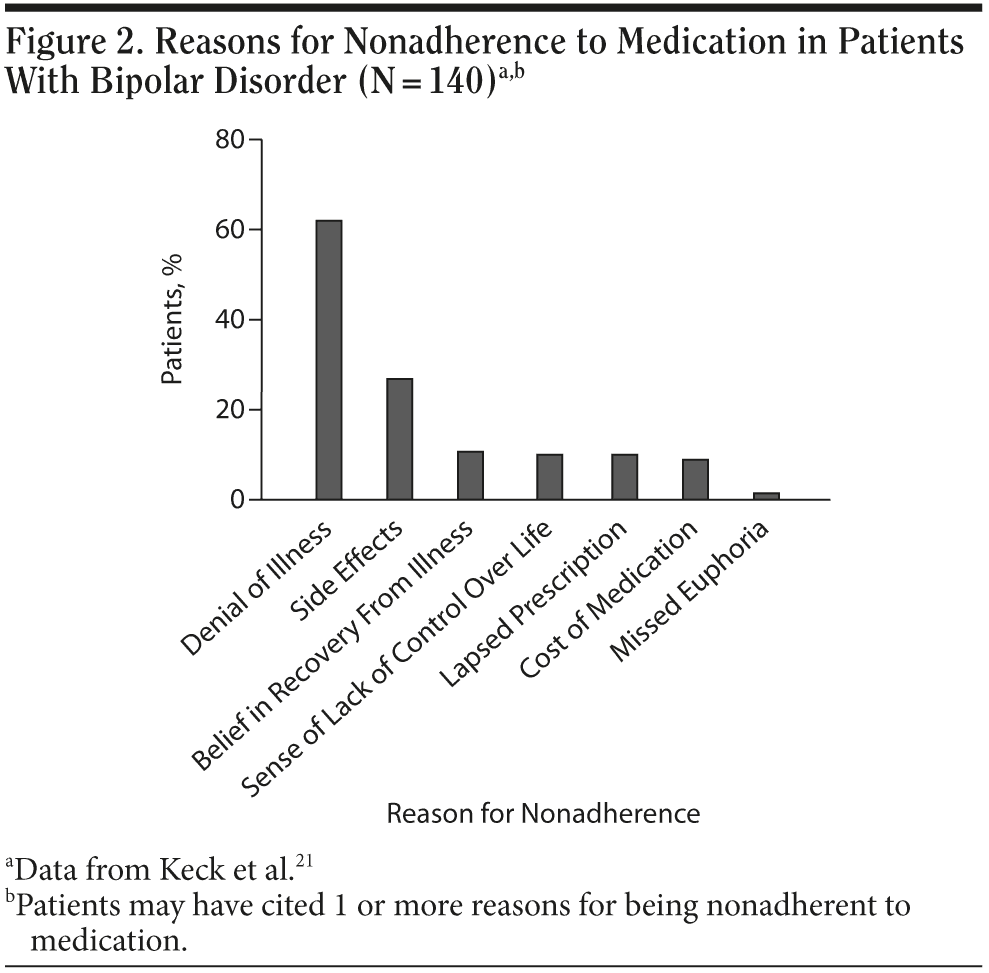
The factors associated with nonadherence influence patients in various ways (Figure 2).21 Residual manic symptoms may affect the patient’s judgment, younger patients who have had few episodes of the disorder may still be in denial about the presence of the disorder, and patients who are free of symptoms may believe they no longer have the illness. The treatment regimen may require multiple medications and several doses per day and may result in side effects that make adherence challenging. The presence of any of the above-mentioned risk factors should alert clinicians to the possibility of nonadherence to medication.
Strategies to Monitor Adherence
Various methods of measuring medication adherence are available. Monitoring patterns of appointment-keeping can help because patients who keep appointments tend to be adherent to treatments; conversely, those who miss appointments are more likely to be nonadherent. Using patients’ self-reports is another strategy. Clinicians should keep in mind that, although these reports have a high likelihood of being true, the sensitivity of the report may be less reliable than other methods. Relying solely on physicians’ clinical judgment is ineffective because physicians tend to overestimate adherence among their own patients.14 Pill counts, electronic monitoring, laboratory measures of plasma drug concentrations, and pharmacy records are sometimes used to assess adherence to medication in clinical trials. However, these methods may still provide inaccurate results, and, owing to the effort involved, some may be impractical to implement in busy primary care practice settings.14
If a patient has a problem with medication adherence, clinicians can try using several practical strategies. For example, the clinician can have a frank discussion not only with the patient but also with significant others regarding attitudes toward medication, illness denial, and adherence history. Distinguishing whether the patient does not want to take medication regularly (eg, due to fear of developing a dependence) or does not have the ability to take it regularly (eg, due to forgetfulness or disorganization) is helpful.14 Clinicians can also recommend psychotherapy with a psychoeducation component to increase understanding of the nature of the illness and the need for preventive treatment.19 Other strategies to facilitate adherence to medication include discussing a preferred treatment formulation (eg, orally disintegrating tablets versus injectable formulations) with the patient, reviewing benefits versus limitations of different medications, and assessing the tolerability of possible treatment options collaboratively with the patient. When monitoring adherence to treatment, clinicians should remember that treatment includes not only medications but also psychotherapy and lifestyle modifications.
MEDICAL MONITORING
Medical monitoring of patients with bipolar disorder is necessary (1) to assess the safety and tolerability of psychotropic medications and (2) to actively monitor for medical conditions common in this population. Some of the more common side effects of psychotropic medications used in the management of bipolar disorder that arouse concern are sedation and weight gain, but serious side effects for which clinicians have to remain vigilant, such as hepatotoxicity or pancreatitis, also occur on rare occasion. Additionally, patients with bipolar disorder are at risk for medical conditions such as metabolic disorders, including excessive weight gain, obesity, and diabetes; endocrine disorders such as thyroid problems; and cardiovascular conditions.
Monitoring Medication Safety and Tolerability
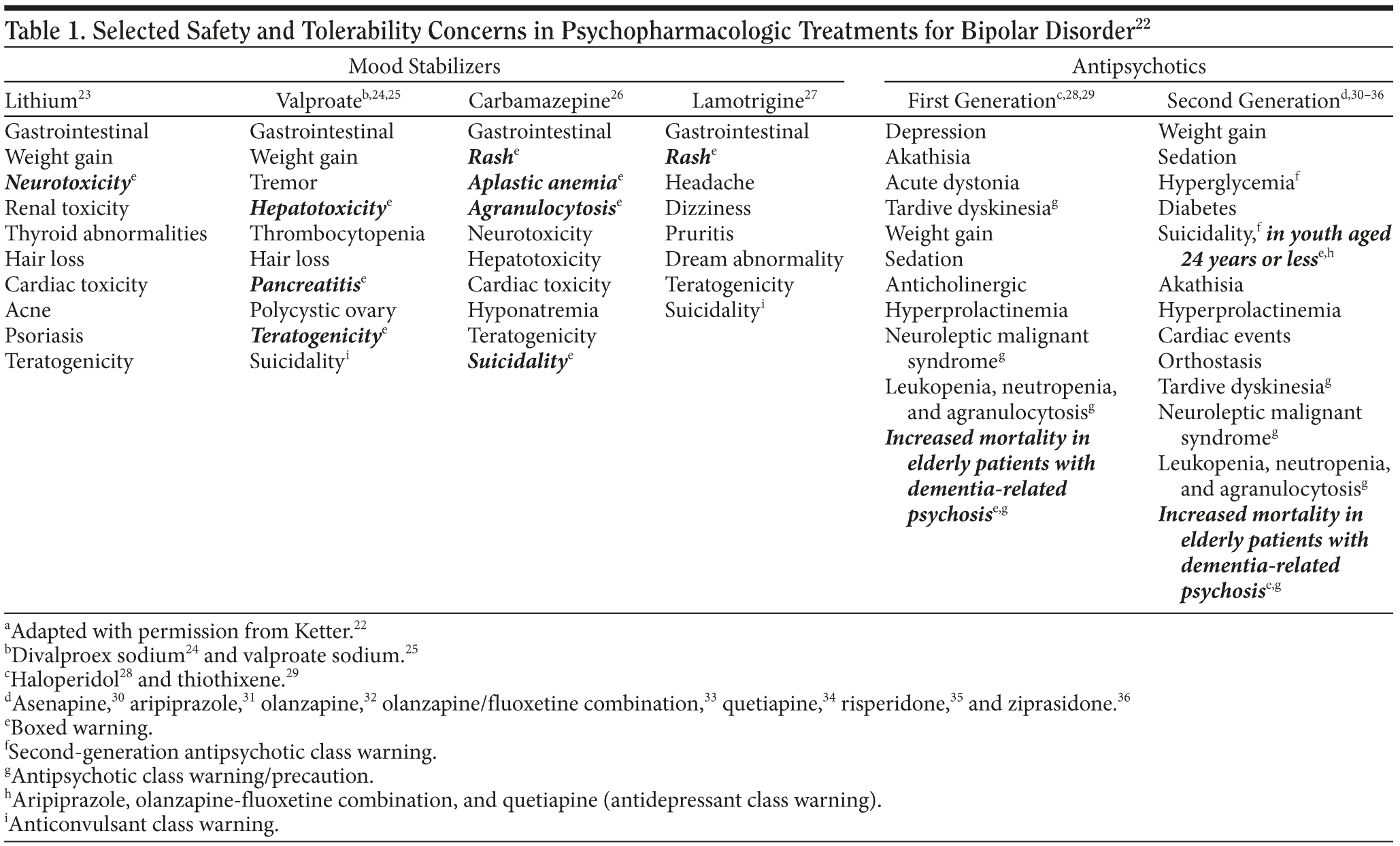
The major classes of medication used for treating bipolar disorder—mood stabilizers and antipsychotics—have side effects that require monitoring for safety and tolerability (Table 1).22-36 The potentially serious adverse effects highlighted in boxed warnings for each medication are far less common than other generally milder side effects affecting the central nervous or gastrointestinal systems that can often cause tolerability issues and thus undermine adherence. Side effects commonly seen in psychiatric patients involve the central nervous system (ie, sedation, hypersomnia, insomnia, tremor, restlessness, and unsteadiness) and the gastrointestinal system (ie, nausea, vomiting, diarrhea, constipation, weight gain, and metabolic problems).
The antipsychotic agents all have a boxed class warning for increased mortality in elderly patients with dementia-related psychosis and unboxed class warnings or precautions for tardive dyskinesia, neuroleptic malignant syndrome, and leukopenia, neutropenia, and agranulocytosis. Second generation antipsychotics also have an unboxed class warning for hyperglycemia. Any medication with an indication for treating either unipolar or bipolar depression, including standard antidepressants as well as some second-generation antipsychotic agents, has a boxed class warning about the increased risk of suicidality in people aged 24 years or younger (in contrast, such risk is not increased for ages 25 to 65 and is decreased for age greater than 65).37 Anticonvulsants have an unboxed class warning for suicidality.
When monitoring patients treated with mood stabilizers, clinicians need to be vigilant for adverse effects such as neurotoxicity, hepatotoxicity, pancreatitis, rash, blood dyscrasias, teratogenicity, and serious rash. Clinicians also need to carefully watch for cardiac problems and pneumonia among elderly patients taking antipsychotic agents. Some of the most common side effects of second-generation antipsychotics include sedation and weight gain. Side effects vary from one medication to another. Agents that cause fewer challenges with sedation and weight gain may cause greater problems with akathisia.
Mortality and Medical Morbidity
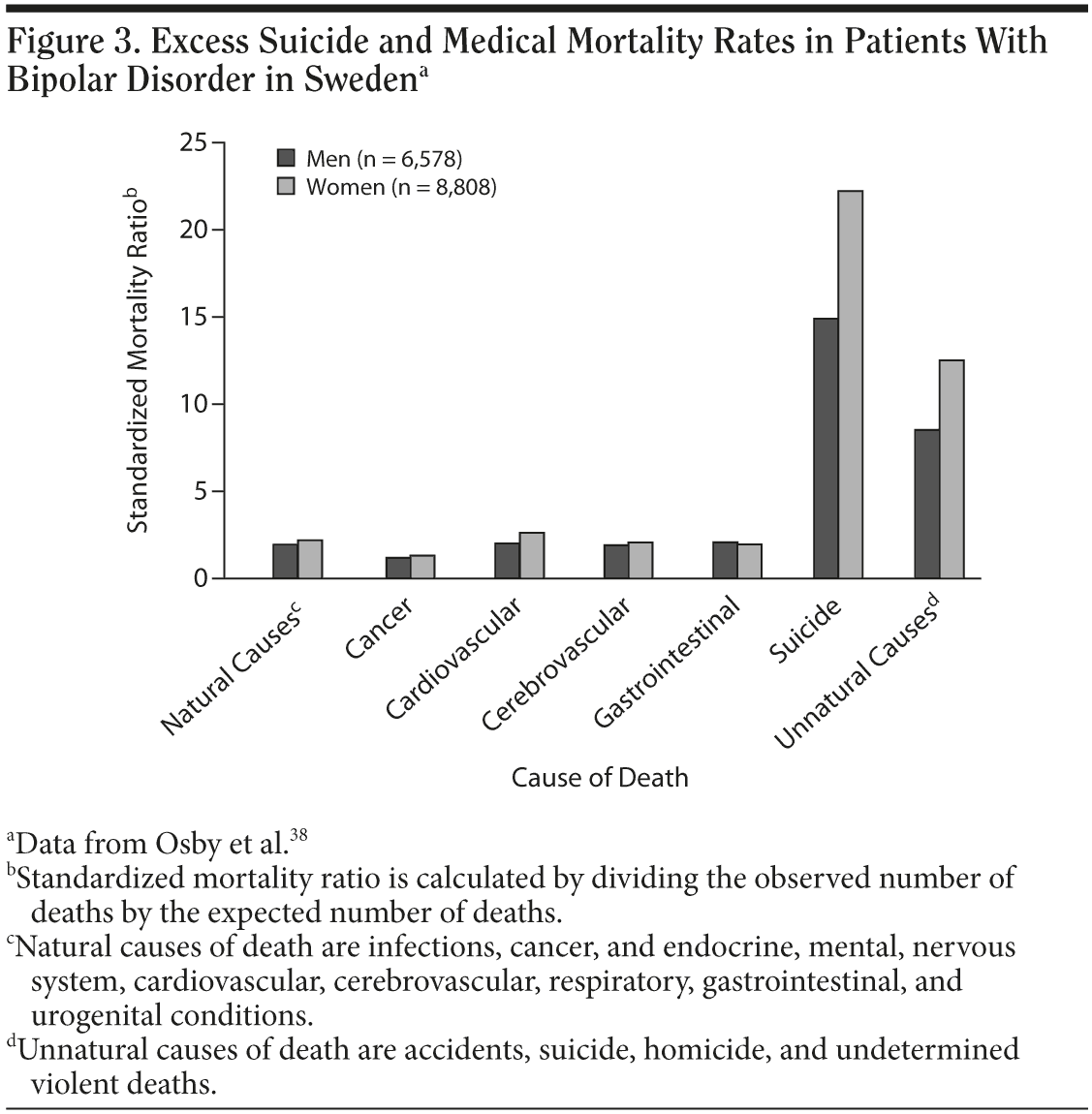
Rates of suicide and medical mortality are higher in patients with bipolar disorder than in the general population. A large Swedish population-based study38 of patients hospitalized for bipolar or unipolar disorder calculated standardized mortality ratios and excess deaths between 1973 and 1995. As shown in Figure 3,38 cardiovascular, cerebrovascular, and gastrointestinal deaths were about twice as common in patients with bipolar disorder as in the general population. In practice, this means that more patients with bipolar disorder are likely to die of cardiovascular disease than suicide because the base rate of suicide in the general population is relatively low and the base rate of cardiovascular mortality in the general population is relatively high.
Common medical disorders comorbid with bipolar disorder include overweight and obesity, diabetes mellitus, cardiovascular disease, hypothyroidism, migraine, and pain disorders.11,12 Medical conditions that especially need to be followed closely are obesity, diabetes mellitus, and cardiovascular disease because they are so prevalent in this population and may be exacerbated not only by having bipolar disorder, but also by medications used to treat bipolar disorder.11,12
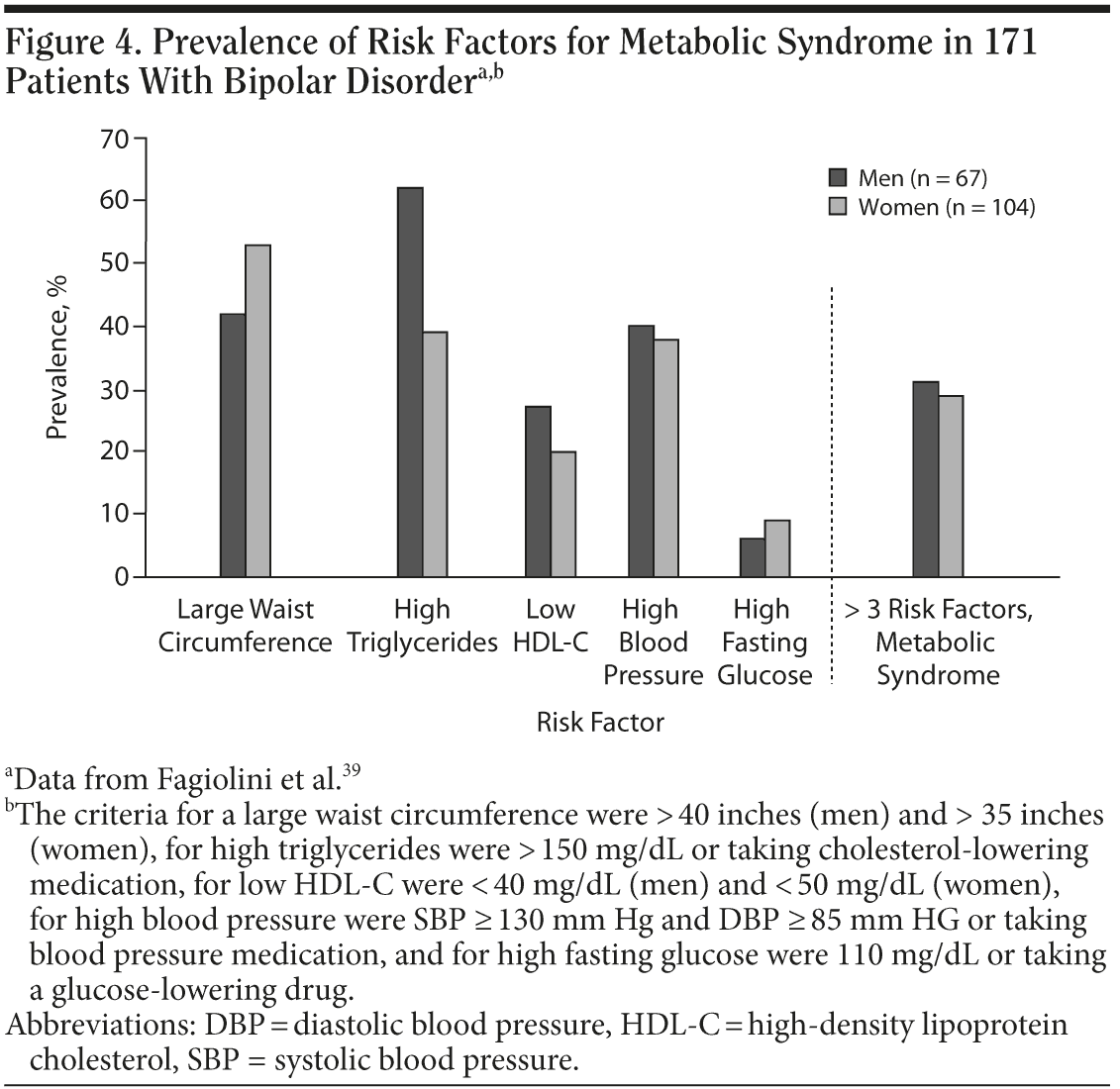
The risk of being overweight or obese and having metabolic syndrome is high in patients with bipolar disorder. Data from a study39 of 171 patients at the Bipolar Disorder Center for Pennsylvanians showed that 45% of the sample were obese and 29% were overweight (but not obese). Individual components of metabolic syndrome, ie, abdominal obesity, hypertriglyceridemia, low high-density lipoprotein cholesterol, high blood pressure, and, to a lesser extent, high fasting glucose levels, were also prevalent in this population (Figure 4). Not only is the epidemic of obesity and metabolic problems in the US general population overrepresented in patients with bipolar disorder, but some of the treatments for bipolar disorder also may contribute to such problems. During the course of treatment, clinicians should monitor weight and the medical symptoms of metabolic syndrome as well as psychiatric symptoms in these patients. In many instances, patients may not have an elevated fasting glucose level early in treatment, as this tends to appear later in the development of the metabolic syndrome. One of the earlier symptoms may be elevated triglycerides. When assessing risk, measuring weight is not as specific as measuring abdominal fat as represented by waist circumference.
While the US Food and Drug Administration (FDA) has placed a warning for hyperglycemia on all second-generation antipsychotics, the American Diabetes Association (ADA) suggested that clozapine and olanzapine create more problems with weight gain, insulin resistance, and dylipidemia than other agents.40 In response to the ADA statement, the FDA reported that about 25% of adverse event reports involving patients with hyperglycemia or diabetes who had been treated with second-generation antipsychotics did not have weight gain.41 Consistent with the ADA statement, weight gain appears to be an important pathway to diabetes in patients with bipolar disorder who are taking second-generation antipsychotics, with increase in appetite, leading to increase in weight and insulin resistance, and ultimately to diabetes, likely being the most common way for patients to develop metabolic complications. However, consistent with the FDA observation, a minority of patients may possibly experience metabolic complications without the intermediary factor of weight gain.
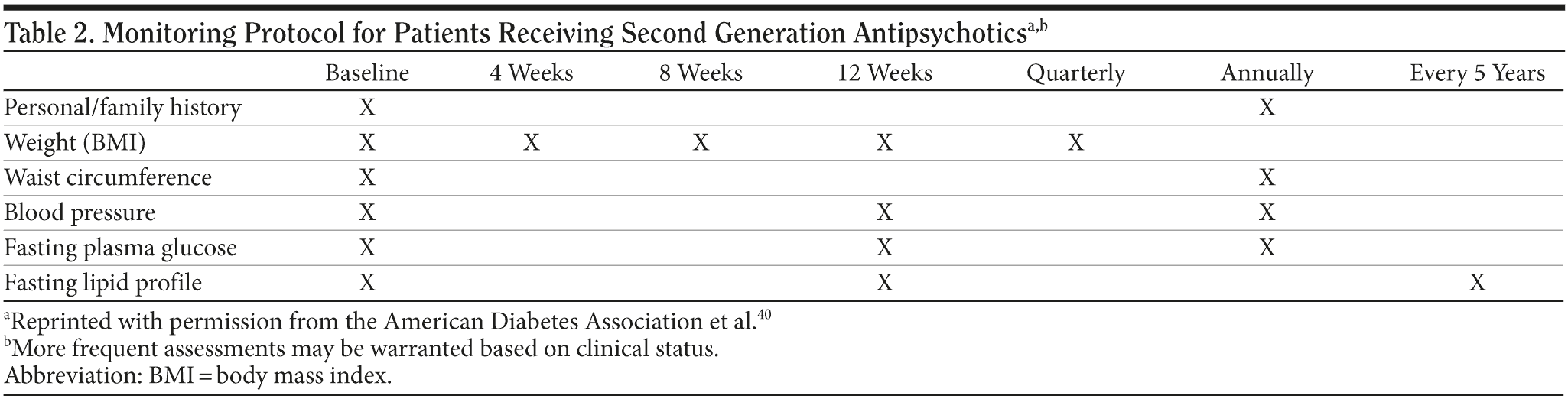
The ADA also produced a monitoring protocol for metabolic symptoms in patients taking second-generation antipsychotics (Table 2).40 The protocol sets out a minimal monitoring regimen, but more frequent assessments may be warranted depending on clinical status. At the outset of treatment, the protocol recommends that a personal and family history of diabetes should be obtained, and weight, waist circumference, blood pressure, fasting glucose, and lipid profile should be measured. Thereafter, weight should be carefully monitored for the first 3 months. Blood pressure, fasting glucose, and fasting lipids should be checked at 3 months and then annually together with other baseline indices. Cholesterol checks are recommended every 5 years, but, in clinical practice, cholesterol is commonly assessed once a year. Considering all of the health issues of patients with bipolar disorder, an argument could be made for annual reassessment of all the measures in the protocol once patients are stable on antipsychotic medication. As Larry Culpepper, MD, MPH, discusses in more detail elsewhere in this supplement, "The Role of Primary Care Clinicians in Diagnosing and Treating Bipolar Disorder,"42 these patients may be at high risk for not obtaining other types of routine preventive health care.
CONCLUSION
Psychiatric and medical monitoring, as well as monitoring of treatment adherence, are necessary in patients with bipolar disorder. Clinicians should measure bipolar symptoms and be alert for psychiatric adverse events of treatment such as suicidality and mood elevation. Self-assessments tools like the PHQ-9 and MDQ and other strategies can be used to assess symptom severity and monitor symptomatic improvement in busy primary care settings. Medical monitoring should assess adverse effects of medication such as sedation and weight gain and metabolic, endocrine, and cardiovascular problems. Many patients with bipolar disorder can be diagnosed in primary care. Less complex cases (with more stable mood, less psychiatric comorbidity, and less complex medication regimens) may also be effectively treated in primary care, although more complex cases (with unstable mood, complex comorbidities, and complicated medication regimens) may need psychiatric referral.
Drug names: aripiprazole (Abilify), asenapine (Saphris), carbamazepine (Carbatrol, Tegretol, and others), clozapine (FazaClo, Clozaril, and others), divalproex (Depakote and others), haloperidol (Haldol and others), lamotrigine (Lamictal and others), lithium (Lithobid and others), olanzapine (Zyprexa), olanzapine-fluoxetine combination (Symbyax), quetiapine (Seroquel), risperidone (Risperdal and others), thiothixene (Navane and others), valproate (Depacon and others), ziprasidone (Geodon).
Disclosure of off-label usage: The author has determined that, to the best of his knowledge, clozapine, haloperidol, and thiothixene are not approved by the US Food and Drug Administration for the treatment of bipolar disorder.
References
1. Brieger P, Röttig S, Röttig D, et al. Dimensions underlying outcome criteria in bipolar I disorder. J Affect Disord. 2007;99(1-3):1-7. PubMed doi:10.1016/j.jad.2006.08.012
2. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269. PubMed doi:10.1001/archpsyc.60.3.261
3. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537. PubMed doi:10.1001/archpsyc.59.6.530
4. Pfizer Incorporated. Patient Health Questionnaire (PHQ-9). http://www.cqaimh.org/pdf/tool_depscreen. Published 2005. Accessed March 3, 2010.
5. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. PubMed doi:10.1046/j.1525-1497.2001.016009606.x
6. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561-571. PubMed
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994.
8. Hirschfeld RMA, Williams JBW, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875. PubMed doi:10.1176/appi.ajp.157.11.1873
9. Manning JS. Tools to improve differential diagnosis of bipolar disorder in primary care. Prim Care Companion J Clin Psychiatry. 2010;12(suppl 1):17-22.
10. McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158(3):420-426. PubMed doi:10.1176/appi.ajp.158.3.420
11. McIntyre RS, Konarski JZ, Yatham LN. Comorbidity in bipolar disorder: a framework for rational treatment selection. Hum Psychopharmacol. 2004;19(6):369-386. PubMed doi:10.1002/hup.612
12. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8. PubMed doi:10.1097/01.psy.0000151489.36347.18
13. Solomon DA, Keitner GI, Miller IW, et al. Course of illness and maintenance treatments for patients with bipolar disorder. J Clin Psychiatry. 1995;56(1):5-13. PubMed
14. Velligan DI, Weiden PJ, Sajatovic M, et al, for the Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46. PubMed
15. Altman S, Haeri S, Cohen LJ, et al. Predictors of relapse in bipolar disorder: a review. J Psychiatr Pract. 2006;12(5):269-282. PubMed doi:10.1097/00131746-200609000-00002
16. Keck PE Jr, McElroy SL, Strakowski SM, et al. Factors associated with pharmacologic noncompliance in patients with mania. J Clin Psychiatry. 1996;57(7):292-297. PubMed
17. Colom F, Vieta E, Mart×nez-Arסn A, et al. Clinical factors associated with treatment noncompliance in euthymic bipolar patients. J Clin Psychiatry. 2000;61(8):549-555. PubMed
18. Jamison KR, Gerner RH, Goodwin FK. Patient and physician attitudes toward lithium: relationship to compliance. Arch Gen Psychiatry. 1979;36(8 spec no):866-869.
19. Vieta E. Improving treatment adherence in bipolar disorder through psychoeducation. J Clin Psychiatry. 2005;66(suppl 1):24-29. PubMed
20. Lenzi A, Lazzerini F, Placidi GF, et al. Predictors of compliance with lithium and carbamazepine regimens in the long-term treatment of recurrent mood and related psychotic disorders. Pharmacopsychiatry. 1989;22(1):34-37. PubMed doi:10.1055/s-2007-1014574
21. Keck PE Jr, McElroy SL, Strakowski SM, et al. Compliance with maintenance treatment in bipolar disorder. Psychopharmacol Bull. 1997;33(1):87-91. PubMed
22. Ketter TA, ed. Handbook of Diagnosis and Treatment of Bipolar Disorder. Washington, DC: American Psychiatric Publishing Inc; 2010.
23. Lithobid (lithium carbonate) [package insert]. Miami, FL: Noven Therapeutics; April 2008. http://www.noven.com/products.htm. Accessed February 9, 2010.
24. Depakote (divalproex sodium) [package insert]. North Chicago, IL: Abbott Laboratories; November 2009. www.rxabbott.com/pdf/depakote.pdf. Accessed February 9, 2010.
25. Depacon (valproate sodium injection) [package insert]. North Chicago, IL: Abbott Laboratories; November, 2009. www.rxabbott.com/pdf/depacon.pdf. Accessed March 5, 2010.
26. Tegretol (carbamazepine) [package insert]. East Hanover, NJ: Novartis; February 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/016608s101,018281s048lbl.pdf. Accessed February 9, 2010.
27. Lamictal (lamotrigine) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; May 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022251,020764s029,
020241s036lbl.pdf. Accessed February 9, 2010.
28. Haldol (haloperidol) [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical, Inc; June 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/018701s059lbl.pdf. Accessed February 12, 2010.
29. Navane (thiothixene) [package insert]. New York, NY: Pfizer Inc; June 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/016584s059lbl.pdf. Accessed February 12, 2010.
30. Saphris (asenapine) [package insert]. Kenilworth, NJ: Schering-Plough; August 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022117s000lbl.pdf. Accessed February 12, 2010.
31. Abilify (aripiprazole) [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Company; November 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021436s027lbl.pdf. Accessed February 9, 2010.
32. Zyprexa (olanzapine) [package insert]. Indianapolis, IN: Eli Lilly and Company; January 2010. http://pi.lilly.com/us/zyprexa-pi.pdf. Accessed February 9, 2010.
33. Symbyax (olanzapine and fluoxetine hydrochloride) [package insert]. Indianapolis, IN: Eli Lilly and Company; January 2010. http://pi.lilly.com/us/symbyax-pi.pdf. Accessed February 9, 2010.
34. Seroquel (quetiapine) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; November 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020639s045s046lbl.pdf. Accessed February 9, 2010.
35. Risperdal (risperidone) [package insert]. Titusville, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; June 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020272s056,020588s044,021346s033,
021444s03lbl.pdf. Accessed February 9, 2010.
36. Geodon (ziprasidone) [package insert]. New York, NY: Pfizer Inc; November 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020825s034lbl.pdf. Accessed February 12, 2010.
37. US Food and Drug Administration. Revisions to Product Labeling. Rockville, MD: US Food and Drug Administration; 2007. http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM173233.pdf. Accessed February 9, 2010.
38. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850. PubMed doi:10.1001/archpsyc.58.9.844
39. Fagiolini A, Frank E, Scott JA, et al. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7(5):424-430. PubMed doi:10.1111/j.1399-5618.2005.00234.x
40. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601. PubMed doi:10.2337/diacare.27.2.596
41. Boehm G, Racoosin JA, Laughren TP, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes: response to consensus statement [letter]. Diabetes Care. 2004;27(8):2088-2089. PubMed doi:10.2337/diacare.27.8.2088-a
42. Culpepper L. The role of primary care clinicians in diagnosing and treating bipolar disorder. Prim Care Companion J Clin Psychiatry. 2010;12(suppl 1):4-9.