We present a case report on the challenges of using the glucagon-like peptide 1 receptor (GLP-1R) agonist semaglutide in a patient with schizophrenia and severe, olanzapine-induced weight gain that was resistant to alternative weight treatments.
Case Report
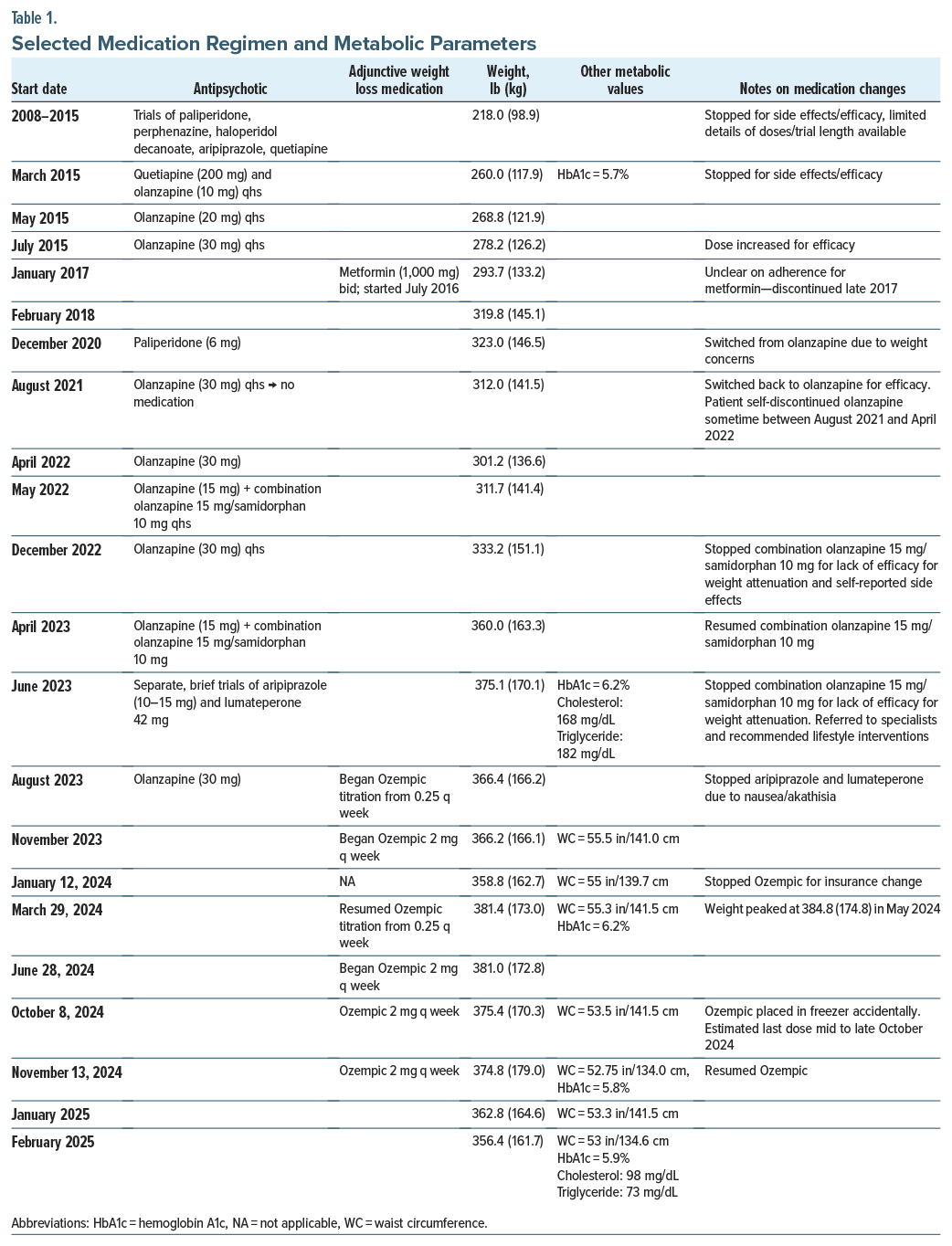
The patient, a 6-ft/1.83-m 39-year-old African-American man, was first diagnosed with schizophrenia in 2008, beginning treatment at our center in 2013 (Table 1). By 2015, he had cycled through numerous antipsychotics, with limited efficacy/ tolerability, including >40 lb/18 kg weight gain. He began olanzapine in 2015, with the dose gradually increased to 30 mg. He responded well, obtaining employment as a peer counselor and rising to a supervisory level. Despite a trial of metformin, he gained an additional >60 lb/27 kg by late 2020 and was cross-titrated to paliperidone. Over the next 14 months, he lost 23 lb/10.4 kg but decompensated concurrent to self-discontinuing his medications and lost his job. He resumed olanzapine but continued to gain weight despite 2 separate trials of olanzapine-samidorphan, reaching a peak of 375.1 lb/170.1 kg in June 2023. While his serum glucose was generally within normal limits, he began to develop medical complications, including elevated cholesterol/triglycerides, prediabetes, sleep apnea, and hypertension, prompting trials of metabolically friendly antipsychotics, referral to a sleep apnea specialist and endocrinologist, and discussions of an exercise program/dietary changes.
His full adherence with these recommendations was unclear. The next few months led to some weight loss, but alternative antipsychotics were ineffective for his symptoms and poorly tolerated, and we agreed to resume olanzapine 30 mg in August 2023 in combination with semaglutide. Ozempic was started due to Wegovy being on back order and was gradually titrated to 2 mg. Due to an unexpected change in the patient’s Medicare plan, Ozempic treatment was interrupted in January 2024 for 3 months. The patient’s weight increased by >20 lb/ 9.1 kg, reaching a new high. Attempts to contest coverage denial, including a probate hearing, were unsuccessful. After exhausting all coverage options, we successfully obtained no-cost coverage directly through a patient assistance program. Ozempic was resumed, and with the exception of a ∼3-week interruption due to his roommate accidentally moving the Ozempic to the freezer, he remains on it as of February 2025, with a weight loss of 25 lb/11.3 kg (6.5%) since resuming it.
Discussion
Our experience speaks to several complications of the long-term management of antipsychotic-induced weight gain and the use of GLP-1R agonists in schizophrenia. First, his limited response/tolerability of metabolically neutral antipsychotics, high functioning, and >100 lb of weight gain on olanzapine exemplifies the tradeoffs of balancing efficacy and metabolic liability in schizophrenia treatment. Olanzapine is an effective1 and tolerable,2 yet metabolically challenging, antipsychotic.3 Olanzapine can lead to mean increases >20 lb over the first year,4 which may be higher in African-Americans5 and may be dose-dependent,6 suggesting that our patient’s experience is typical. He required high-dose olanzapine,7 which may have attenuated the impact of olanzapine-samidorphan, metformin, and semaglutide. The recent approval of xanomeline/trospium chloride provides another metabolically neutral option.8
Second, using a self-administered, off-label, weekly injection with highly specific storage requirements in a condition with potential cognitive impairment created logistical issues that required frequent re-education, management, and surveillance by our team, particularly given his sensitivity to even brief nonadherence. Finally, this case adds to an ongoing debate on the long-term ethics9,10 of antipsychotics and GLP-1R use. There is limited literature on semaglutide in schizophrenia,11,12 but it is highly effective for weight in the general population,13 and studies of older GLP-1R agonists suggest efficacy14–16 in schizophrenia. During treatment, in addition to balancing efficacy and tolerability of olanzapine itself, we faced treatment/ dosage decisions on using semaglutide as a weight-loss–branded formulation (Wegovy, maximum dose 2.4 mg) or not (Ozempic, maximum dose 2 mg) and shifting coverage for off-label use. While successful, use of the patient assistance program is not a viable solution for everyone. Two controlled studies with semaglutide in schizophrenia are ongoing,17,18 which we hope will inform expanded coverage of semaglutide in federal insurance programs.
Article Information
Published Online: June 9, 2025. https://doi.org/10.4088/JCP.25cr15857
© 2025 Physicians Postgraduate Press, Inc.
J Clin Psychiatry 2025;86(3):25cr15857
Submitted: February 25, 2025; accepted April 16, 2025.
To Cite: Ricciardi FL, Melnitsky JL, Peleg SB, et al. Worth the weight? the challenges of administering the glucagon-like peptide 1 receptor agonist semaglutide with long-term olanzapine use in a patient with schizophrenia.
J Clin Psychiatry 2025;86(3):25cr15857.
Author Affiliations: New York State Psychiatric Institute, New York, New York (Ricciardi, Melnitsky, Peleg, Govil, Kantrowitz); Columbia University, College of Physicians and Surgeons, New York, New York (Kantrowitz); Nathan Kline Institute, Orangeburg, New York (Kantrowitz).
Corresponding Author: Joshua T. Kantrowitz, MD, New York State Psychiatric Institute, 1051 Riverside Drive, New York, NY 10032 ([email protected]).
Author Contributions: All authors worked with this patient and wrote and revised the manuscript. All authors reviewed and approved the submitted version of the manuscript.
Relevant Financial Relationships: There are no known conflicts that are directly relevant to this work. Dr Kantrowitz has received consulting payments within the last 36 months from Alphasights, Key quest health, CME Outfitters, Evoke, S.R. One, techspert.io, Health Monitor, Third Bridge, MEDACorp, Marketplus, FCB Health, Trinity, Globaldata, GKA, Clearview, Clarivate, Health Advances, ECRI Institute, ExpertConnect, Slingshot, Antheum, Guidepoint, First Thought, VMLY&R, Bluestar BioAdvisors, Medscape and Otsuka; has served on the Medincell, Merck, Leal, and Karuna advisory boards in the past 36 months; and has conducted clinical research supported by National Institute of Mental Health, Sunovion, Roche, Click, Alkermes, Alto, Neurocrine, Taisho, and Boehringer Ingelheim within the last 36 months. He owns a small number of shares of common stock from GSK. The other authors report no biomedical financial interests or potential conflicts of interest.
Funding/Support: Funded in part by internal funding (New York State Psychiatric Institute) to Dr Kantrowitz. Acknowledgment: We acknowledge and appreciate our patient for providing permission to publish this case report.
Patient Consent: Consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
ORCID: Joshua Kantrowitz:
https://orcid.org/0000-0003-1127-7016
References (18)

- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. CrossRef
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. CrossRef
- Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med J. 2020;132(1):80–90. CrossRef
- Bak M, Drukker M, Cortenraad S, et al. Antipsychotics result in more weight gain in antipsychotic naive patients than in patients after antipsychotic switch and weight gain is irrespective of psychiatric diagnosis: a meta-analysis. PLoS One. 2021;16(2):e0244944. CrossRef
- Stauffer VL, Sniadecki JL, Piezer KW, et al. Impact of race on efficacy and safety during treatment with olanzapine in schizophrenia, schizophreniform or schizoaffective disorder. BMC Psychiatry. 2010;10:89. CrossRef
- Spertus J, Horvitz-Lennon M, Abing H, et al. Risk of weight gain for specific antipsychotic drugs: a meta analysis. NPJ Schizophr. 2018;4(1):12. CrossRef
- Citrome L, Kantrowitz JT. Olanzapine dosing above the licensed range is more efficacious than lower doses: fact or fiction? Expert Rev Neurother. 2009;9(7):1045–1058. CrossRef
- Kantrowitz JT, Correll CU, Jain R, et al. New developments in the treatment of schizophrenia: an expert roundtable. Int J Neuropsychopharmacol. 2023;26(5):322–330. CrossRef
- Ryan N, Savulescu J. The ethics of ozempic and Wegovy. J Med Ethics. 2025:jme-2024-110374.
- Fetterman J. John Fetterman: this drug changed my life. More Americans need access. New York Times; 2025.
- Prasad F, De R, Korann V, et al. Semaglutide for the treatment of antipsychotic-associated weight gain in patients not responding to metformin - a case series. Ther Adv Psychopharmacol. 2023;13:20451253231165169.
- Campforts B, Drukker M, van Amelsvoort T, et al. Management of obesity with semaglutide or metformin in patients with antipsychotic-induced weight gain (MOSA): a non-randomised open-label pilot study. BMC Psychiatry. 2024;24(1):865. CrossRef
- O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double blind, placebo and active controlled, dose ranging, phase 2 trial. Lancet. 2018;392(10148):637–649.
- Menon T, Lee S, Gong XY, et al. A systematic review on the efficacy of GLP-1 receptor agonists in mitigating psychotropic drug-related weight gain - CORRIGENDUM. CNS Spectr. 2024;25:1–7.
- Bak M, Campforts B, Domen P, et al. Glucagon-like peptide agonists for weight management in antipsychotic-induced weight gain: a systematic review and meta-analysis. Acta Psychiatr Scand. 2024;150(6):516–529. CrossRef
- Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719–728.
- Ganeshalingam AA, Uhrenholt NG, Arnfred S, et al. Home-based Intervention with Semaglutide Treatment of Neuroleptic-Related Prediabetes (HISTORI): protocol describing a prospective, randomised, placebo controlled and double-blinded multicentre trial. BMJ Open. 2024;14(3):e077173. CrossRef
- Sass MR, Danielsen AA, Köhler-Forsberg O, et al. Effect of the GLP-1 receptor agonist semaglutide on metabolic disturbances in clozapine-treated or olanzapine-treated patients with a schizophrenia spectrum disorder: study protocol of a placebo controlled, randomised clinical trial (SemaPsychiatry). BMJ Open. 2023;13(1):e068652.
This PDF is free for all visitors!