Abstract
Objectives: To estimate prevalence and impact of cognitive impairments in schizophrenia in the US.
Methods: Retrospective analyses of the Medical Expenditure Panel Survey (1997–2021) were conducted to identify adults living with schizophrenia and cognitive impairments. Cognitive limitations (CL; 1997–2021) were defined as interference with daily activities, confusion/memory loss, problems making decisions, or requiring supervision for safety. Cognitive difficulties (CD; 2013–2021) were defined as difficulty in concentration/memory/decision-making. Descriptive analyses covered demographic, clinical, and socioeconomic characteristics, health care resource utilization (HCRU), humanistic, and indirect burdens. Multivariable regression analyses were conducted for hospitalizations, emergency department (ED) visits, and total costs. Sampling weights were applied.
Results: Among 661,243 weighted adults living with schizophrenia (mean age: 45.6 years; male: 56.7%), 57.7% reported CL, and 53.8% reported CD. Compared to no CL, CL was associated with lower education (no degree: +2.2%) and annual income (−$4,332) and higher Charlson Comorbidity Index (0.89 vs 0.55) and HCRU. Total health care costs were higher for CL ($18,478 vs $11,689), demonstrating greater economic burden. Individuals reporting CL reported more limitations in activities of daily living (+13.3%) and lower health utilities scores with higher percentage of poor perceived health (+10.1%), indicating higher humanistic burden. For indirect burden, CL was associated with higher unemployment (+15.3%) (all P <.05). Multivariable regression analysis showed that CL was associated with higher odds of hospitalizations (1.47; 95% CI, 1.05–2.06), ED visits (1.64; 95% CI, 1.22–2.20), and total health care costs (1.56; 95% CI, 1.30–1.86). CD showed similar results except that CD was not significantly associated with hospitalizations or ED visits.
Conclusions: Cognitive impairments in schizophrenia are associated with higher multilevel burdens compared to those without, highlighting the need for targeted interventions.
J Clin Psychiatry 2025;86(3):25m15794
Author affiliations are listed at the end of this article.
Schizophrenia, an often debilitating mental health disorder, affects about 3.9 million people in the US population.1 The annual economic burden exceeds $343 billion, highlighting the need for effective management strategies.1 Schizophrenia manifests through 3 symptom domains: positive symptoms (delusions, hallucinations, and disorganized speech, thought, and behavior), negative symptoms (poor motivation, lack of pleasure and enjoyment, lack of speech, lack of social interaction), and cognitive impairments (impaired executive function, attention, and memory).2 While currently available pharmacologic treatments primarily target positive symptoms, effective therapies for negative symptoms and cognitive impairments remain limited.2 Although various pharmacologic agents have been investigated for cognitive impairments associated with schizophrenia, none have been approved by the US Food and Drug Administration.3–5 Current clinical guidelines recommend psychosocial therapies, such as cognitive behavioral therapy, self-management skills training, and cognitive remediation, to manage cognitive impairments in schizophrenia.6 These psychosocial interventions have shown statistically significant improvements in global cognition and global functioning in patients with cognitive impairments associated with schizophrenia.7–11
While available evidence suggests that cognitive impairments are highly prevalent in schizophrenia with an increased risk for premature mortality,12–14 their impact remains understudied. Regarding health care resource utilization (HCRU), previous findings indicate that more severe cognitive impairments in schizophrenia are associated with a greater risk of relapse-related hospitalizations and emergency department (ED) visits.15 Another study evaluating Veterans Affairs (VA) health system reported higher annual average number of hospitalizations (1.0 vs 0.7, P <.01) and ED visits (0.6 vs 0.3, P <.01) among those with cognitive impairments compared to those without.16 However, research addressing HCRU in a broader range of patients experiencing cognitive impairments is lacking.
Similarly, there is limited literature on the economic burden of cognitive impairments with schizophrenia. A UK study estimated the mean public-sector costs for patients living with schizophrenia experiencing cognitive impairments to be $23,825 (2001 USD) over a 6-month period, revealing a significant relationship between costs and the severity of cognitive impairments. However, this study was conducted using a small, outdated UK sample.17
The humanistic burden associated with cognitive impairments in schizophrenia is somewhat better understood. A literature review from 1999 to 2009 evaluated the negative impact of cognitive impairments associated with schizophrenia on various aspects of patients’ lives, including activities of daily living, social and occupational functioning, and relationships.18 Despite offering valuable insights, the findings are outdated.
Given the lack of current US-based research on the impact of cognitive impairments in schizophrenia, this study aimed to explore characteristics, HCRU, and clinical, economic, humanistic, and indirect burdens associated with cognitive impairments among patients living with schizophrenia in the US.
METHODS
Data Source and Study Design
The Medical Expenditure Panel Survey (MEPS) is a comprehensive collection of surveys in the US, covering health care costs, utilization, insurance coverage, and related topics.19 MEPS strategically oversamples disabled and minority groups to ensure US population representation. It consists of 2 main components: household (households and medical provider data) and insurance (employers’ data).7
MEPS is conducted multiple times over 2 years for each cohort, producing full-year consolidated data files, along with medical conditions and event files. For this cross-sectional retrospective study, we utilized the combined datafiles from 1997 to 2021.
Eligibility
The study included adult participants (18+ years of age) who reported a diagnosis of schizophrenia. Participants who reported neurocognitive disorders (dementias, other cerebral degenerations, Parkinson disease, brain-related injuries), health conditions affecting cognition (eg, ischemic stroke), or multiple sclerosis were excluded. Patients with competing diagnostic codes for schizophrenia as well as bipolar disorder at any time point in the collected data were categorized as having a diagnosis of the more severe disorder of schizophrenia.
Cognitive Impairments: Cognitive Limitations and Cognitive Difficulties
Cognitive impairments were assessed using 2 indicators: cognitive limitations and cognitive difficulties. Cognitive limitations (without inclusion of a functional item; data availability: 1997–2021) were comprised of 3 questions: (1) experiencing confusion or memory loss impacting daily activities, (2) facing decision-making problems affecting daily activities, and (3) requiring supervision for personal safety.19 If any of these questions were answered positively, the participant was identified as an individual experiencing cognitive limitations. Since the item related to “requiring supervision for safety” may not directly reflect cognitive impairments, its inclusion may capture broader functional or psychological issues beyond cognition.
Therefore, cognitive difficulties (without inclusion of a functional item; data availability: 2013–2021) were additionally included as a separate outcome, assessed through a single question regarding challenges related to concentration/memory/decision-making without overlap with a functional item.
Outcomes: Clinical Characteristics, HCRU, Health Care Costs, Humanistic and Indirect Burden
Demographic (age, sex, race, ethnicity) and clinical characteristics (Charlson Comorbidity Index [CCI],20 behavioral comorbidities, body mass index) were analyzed. Socioeconomic status was assessed using information on education, annual income, poverty level, and primary payer type, all of which are components of social determinants of health (SDoH). SDoH consists of the environmental conditions in which individuals are born, grow, work, live, and age and include systems that impact everyday life.21
HCRU was analyzed by hospitalizations, ED, outpatient, home-health agency, dental visits, and prescriptions. Outpatient visits were measured by combining outpatient and office-based provider visits. Prescriptions included the number of prescribed medications and medical supplies. Health care costs were estimated across categories of HCRU, including total costs.
Limitations in activities of daily living, limitations in instrumental activities of daily living and function, social/ recreational, and work/house/school were explored. Activities of daily living involve walking independently, feeding, dressing, personal hygiene, continence, and using the toilet independently.22 Domains of instrumental activities of daily living are transportation and shopping, managing finances, meal preparation including shopping, housecleaning and home maintenance, communicating with others, and managing medications.22 Self-perceived physical and mental health statuses were analyzed. For health-related quality of life (HRQoL), Veterans RAND 12-Item (VR-12; data availability: 2017–2021),23,24 physical component scores (PCS) and mental component scores (MCS), as well as Short Form-12 (SF-12) PCS and MCS (data availability: 1997–2017),15,16 were evaluated. SF 6-Dimensions (SF-6D) and EuroQol-5 Dimension (EQ-5D) (data availability: 2000–2003) were used for health utilities.25–27 SF-12 scores were converted to SF-6D based on the proportion of females, average age, and mean PCS and MCS.18
For indirect burden, we included measures of unemployment and missed school/work days due to illness/disability. Per capita wage losses were calculated using annual US median income data from the US Bureau of Labor Statistics.28–31 All health care costs were adjusted to 2021 USD using the Consumer Price Index.32
Statistical Analyses
We conducted descriptive analyses for demographic, clinical, and socioeconomic characteristics, HCRU, health care costs, humanistic, and indirect burden using χ2 tests and t tests. An ordinary least squares regression model was used for total all-cause health care costs, and binomial regression models were used for hospitalizations and ED visits. The main predictor variables included sex, race, ethnicity, and age. Nonspecific survey responses (don’t know, inapplicable, refused, not ascertained) were recoded as missing. All analyses were conducted in SAS Studio v3.8133 using MEPS sampling weights29 to account for the complex survey design and produce national estimates.
RESULTS
Characteristics of Schizophrenia Stratified by Cognitive Impairments
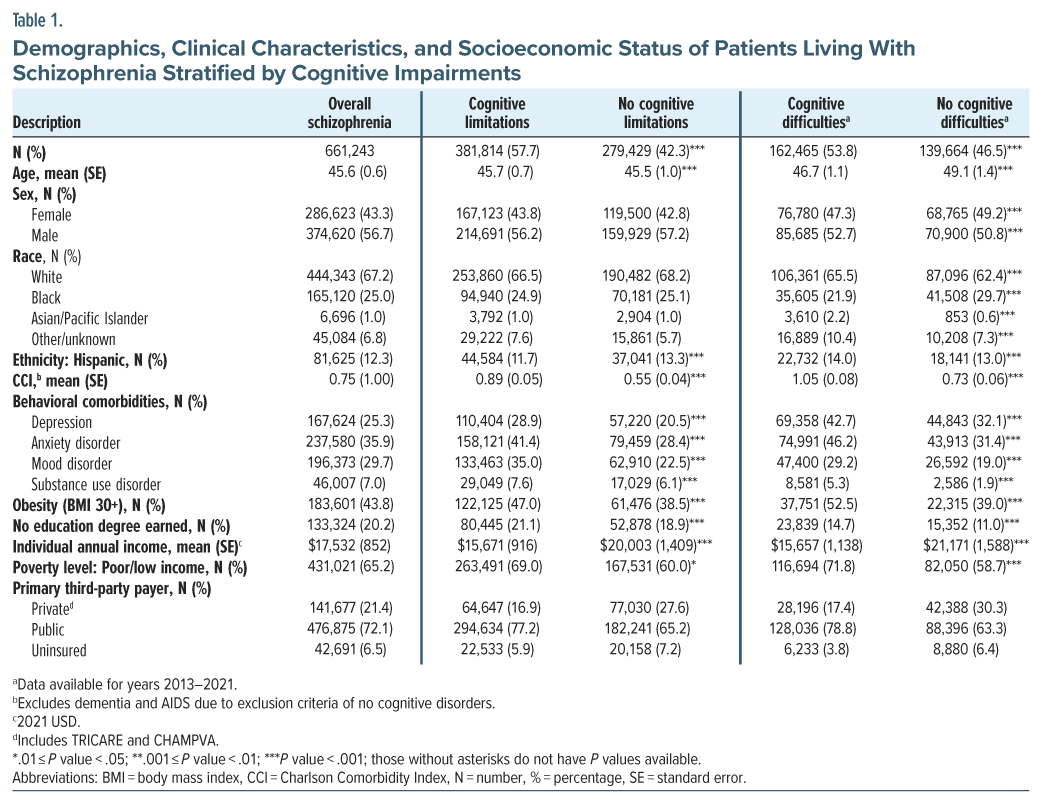
A total of 661,243 weighted participants (mean age: 45.6 years; male: 56.7%) reported a diagnosis of schizophrenia. Among these, cognitive impairments were reported in 57.7% with cognitive limitations and 53.8% with cognitive difficulties (Table 1). Clinically, those with cognitive limitations exhibited higher average CCI scores (+0.34) and a greater prevalence of mental health comorbidities, including depression (+8.4%), anxiety (+13.0%), mood disorder (+12.5%), and substance use disorder (+1.5%), compared to those without cognitive limitations. Obesity was also more prevalent among those with cognitive limitations (+8.5%) compared to those without, with all P values < .05 (Table 1).
Regarding SDoH, cognitive limitations were associated with lower education attainment, with a higher percentage not earning a degree (+2.2%). The annual income was lower by $4,332 among those with cognitive limitations compared to those without cognitive limitations. Similarly, a higher prevalence of poor/low-income level of poverty status was observed in participants with cognitive limitations compared to those without (+9.0%) (Table 1). Participants with cognitive difficulties exhibited similar patterns. All P values were < .05.
HCRU and Economic Burden of Schizophrenia Stratified by Cognitive Impairments
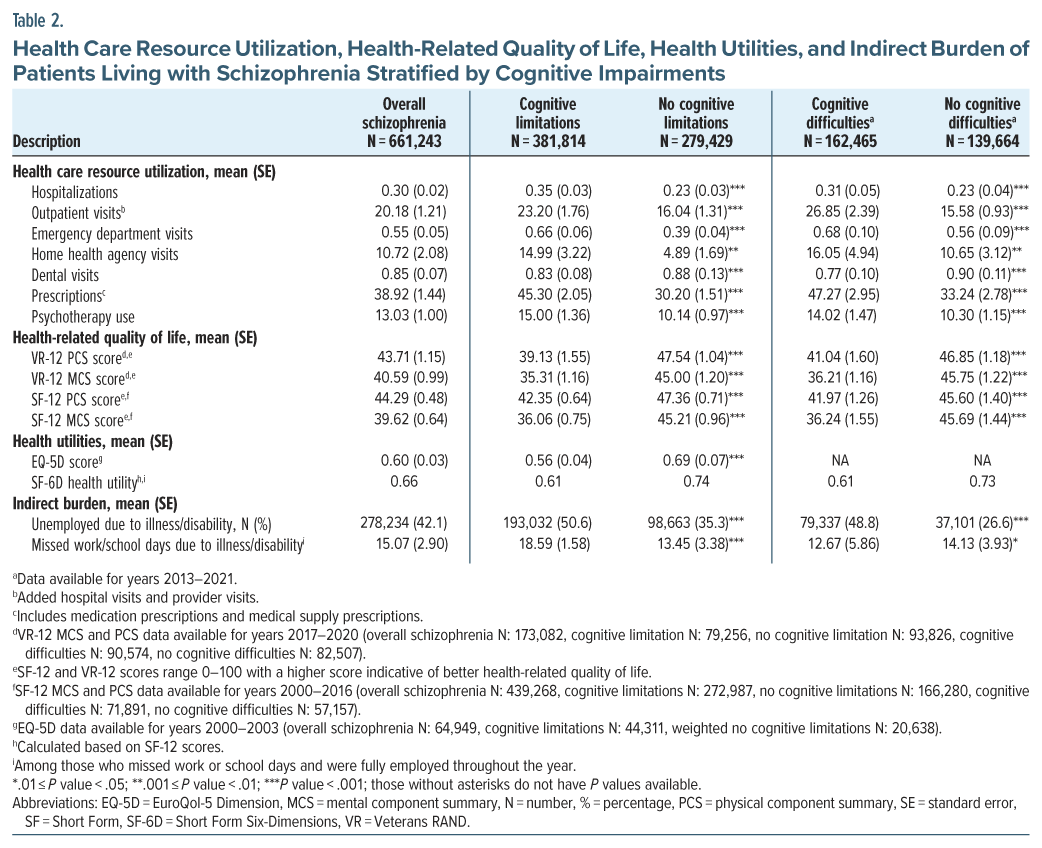
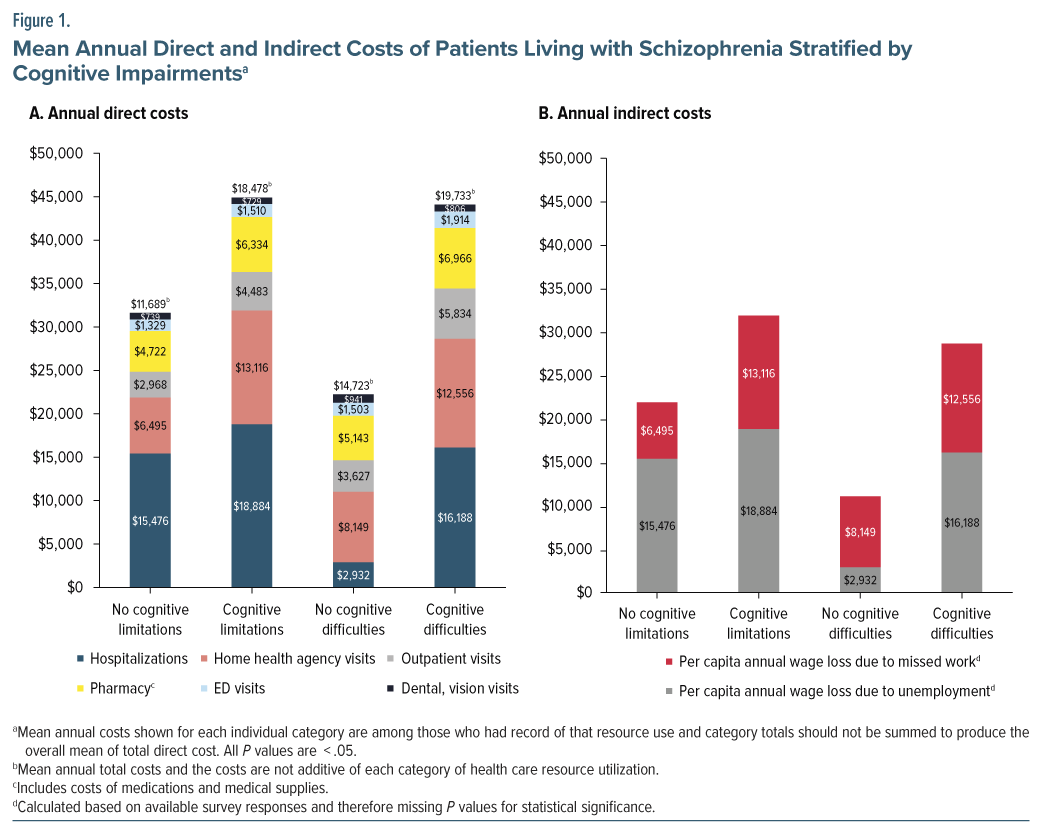
HCRU was significantly higher among participants with cognitive limitations. The greatest difference was observed in the number of prescriptions (+15.1), followed by outpatient visits (+7.2) (Table 2). This trend of elevated HCRU in individuals with cognitive limitations continued with the health care costs. The total health care costs of individuals living with schizophrenia and cognitive limitations were 1.58 times higher than the total health care costs for those without cognitive limitations (+$6,789) (Figure 1). All P values were <.05. Participants with cognitive difficulties exhibited similar findings.
Humanistic Burden of Schizophrenia Stratified by Cognitive Impairments
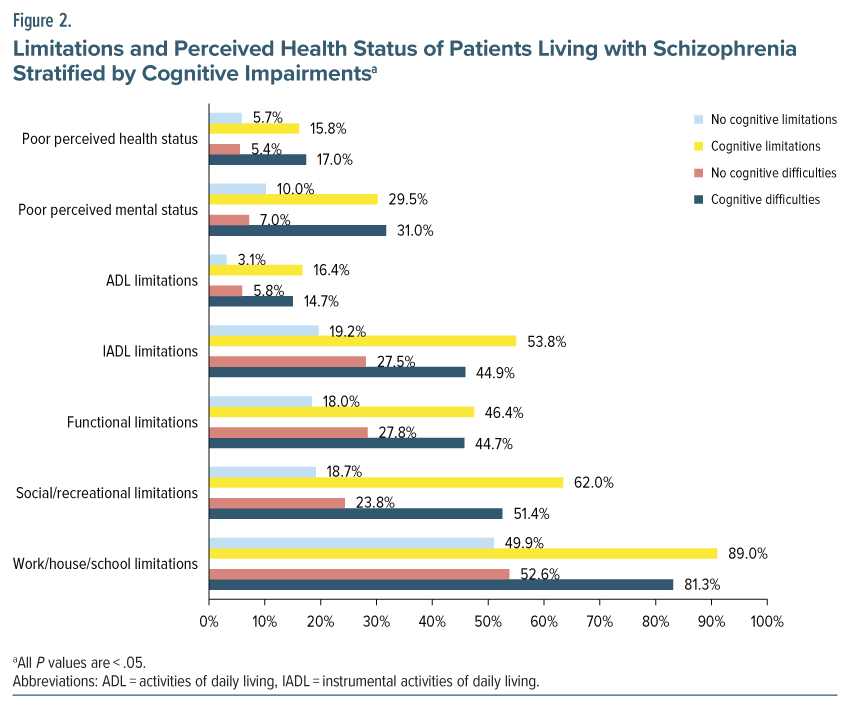
Patients with cognitive limitations were approximately 3 times more likely to report poor perceived health (+10.1%) and mental health (+19.5%). Limitations in activities of daily living (+13.3%) were 5.3 times more likely to be reported among those with cognitive limitations. Additionally, individuals with cognitive limitations (+34.6%) were 2.8 times more likely to report limitations in instrumental activities of daily living compared to those without cognitive limitations. All P values were < .05. Similar trends were seen in functional, social/recreational, and work/house/school limitations, with significantly higher percentages among individuals with cognitive limitations (Figure 2).
For HRQoL, individuals with cognitive limitations reported lower scores compared to those without. The SF-12 and VR-12 scores, which range from 0 to 100 (score of 50 indicates health of general population), were below 50 for both groups, but lower among those with cognitive limitations (Table 2).14 Health utilities showed similar results. Individuals with cognitive limitations reported scores that were 0.13 points lower for both SF-6D and EQ-5D compared to those without. SF-6D and EQ-5D range from 0 to 1, with a score of 1 being the perfect health (Table 2).19,34 Participants with cognitive difficulties showed similar trends.
Indirect Burden of Schizophrenia Stratified by Cognitive Impairments
Individuals with cognitive limitations reported a higher percentage of unemployment from illness/ disability (+15.3%) and missed work or school days due to illness (+5.1 days) than those without cognitive limitations (Table 2). This resulted in $3,408 higher per capita annual wage loss due to unemployment for participants with cognitive limitations compared to those without, with all P values <.05. Similar findings were observed among participants with vs without cognitive difficulties (Figure 1).
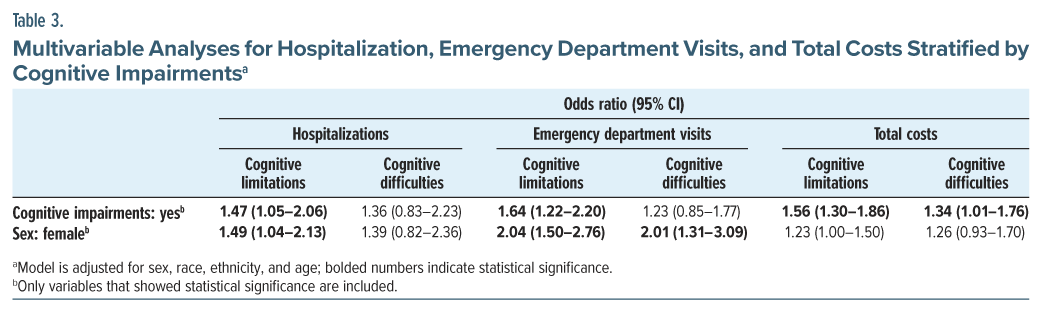
Multivariable Regression Analyses
Significant variations in hospitalizations, ED visits, and total health care costs were observed based on the presence of cognitive limitations. After adjusting for demographic factors (sex, race, ethnicity, age), participants with cognitive limitations had 1.47 times higher odds of hospitalizations compared to those without cognitive limitations (95% CI, 1.05–2.06), with females exhibiting 1.49 times higher odds than males (95% CI, 1.04–2.13). Regarding ED visits, cognitive limitations were associated with 1.64 times higher odds of having an ED visit compared to those without cognitive limitations (95% CI, 1.22–2.20), with female participants having 2.04 times higher odds of ED visits than males (95% CI, 1.50–2.76). Conversely, the presence of cognitive difficulties was not associated with higher odds of ED visits, although females had 2.01 times higher odds than males (95% CI, 1.31–3.09). Estimated health care costs were 1.56 times higher among individuals with cognitive limitations (95% CI, 1.30–1.86) compared to those without and 1.34 times higher among individuals with cognitive difficulties (95% CI, 1.01–1.76) compared to those without cognitive difficulties (Table 3).
DISCUSSION
The 1997–2021 MEPS provides significant insights into the prevalence and challenges faced by individuals with cognitive impairments in schizophrenia. Our analysis identified that 57.7% of participants reported cognitive limitations and 53.8% reported cognitive difficulties. These prevalence results are lower than the approximately 80% prevalence of cognitive impairments in the literature evaluated with cognitive testing tools.12 This discrepancy could be due to the self-reported nature of the MEPS data and the lack of diagnostic codes for clinically relevant cognitive impairments. For example, a VA study reported that only 21.5% of veterans living with schizophrenia were identified with cognitive impairments, reflecting challenges in accurately identifying and under-diagnosing cognitive impairments across different populations and settings.16
Our findings indicate that individuals with cognitive impairments in schizophrenia face a heightened clinical burden. Specifically, those with cognitive limitations and cognitive difficulties exhibit significantly higher CCI scores and are more likely to have comorbid conditions such as depression, anxiety, mood disorders, and obesity. Although not specific to cognitive impairments, the broader literature demonstrates a higher clinical burden in patients living with schizophrenia. For example, a comprehensive review found that individuals living with schizophrenia have a higher prevalence of various physical comorbidities compared to the general population.35 This includes HIV, hepatitis, osteoporosis, altered pain sensitivity, sexual dysfunction, cardiovascular diseases, overweight, diabetes, dental problems, polydipsia, panic disorder, depression, posttraumatic stress disorder, and obsessive-compulsive disorder.35
Results from this study highlight that those with cognitive impairments are associated with an additional layer of clinical burden. Although literature on comorbidities related to cognitive impairments in schizophrenia is limited, our findings contribute to the recognition that patients with cognitive impairments experience a compounded clinical burden.
SDoH-related findings also revealed significant challenges faced by individuals with cognitive impairments in schizophrenia with higher percentages of poverty and unemployment and lower education attainment and annual income. Literature suggests that individuals with moderate to severe cognitive impairments experience notably higher unemployment rates due to disability (50.0% vs 29.7%, P <.05) and higher rates of homelessness (8.5% vs 4.5%, P <.05) compared to those with no/mild cognitive impairments.1 These findings highlight the impact of cognitive impairments on employment and housing stability. Poverty and education attainment are key components of SDoH.36 Research indicates that SDoH accounts for approximately 30%–35% of health outcomes, often surpassing the impact of medical factors.35 Given this context, cognitive impairments contribute to worse SDoH, leading to poorer health outcomes and an increased overall burden.
In terms of HCRU and economic burden, previous literature supports our findings on the association between cognitive impairments and higher costs. A UK survey study found that moderate/severe cognitive impairments were linked to higher odds of hospitalization due to schizophrenia relapse (odds ratio [OR]: 2.23; 95% CI, 1.53–3.24).1 This result aligns with findings from Nili et al16 of reported increased HCRU among patients with cognitive impairments in the VA health system, including higher utilization of antidepressants (47.2% vs 39.4%), psychotherapy services (55.7% vs 39.7%), hospitalizations (1.0 vs 0.7), and ED visits (0.6 vs 0.3; all P values <.01).
Regarding expenditures, Patel et al17 found that better cognitive functioning was associated with lower health care and social costs in a study of 85 patients living with schizophrenia (coefficient: −0.34, P <.05). This contrasts with our findings, which reveal higher expenditures for patients with cognitive impairments. The study was conducted in the United Kingdom with outdated data, potentially contributing to the different findings. Despite this difference, our results are consistent with the literature indicating an association between cognitive impairments and increased economic burden. The discrepancy may reflect variations in study populations or methodologies.
Humanistic burden associated with cognitive impairments in schizophrenia is extensively documented. For example, a comprehensive review identified that patients living with cognitive impairments of schizophrenia experience significant limitations in activities of daily living and instrumental activities of daily living than those without.18 Similarly, our findings indicated that limitations in activities of daily living were reported as 429.0% and 153.4% higher for individuals with cognitive limitations and difficulties, respectively.
Regarding indirect burden, as mentioned for SDoH, previous research has shown a strong association between cognitive impairments and higher rates of unemployment due to disability.15 For instance, a study indicated that patients with moderate-to-severe cognitive impairments had an elevated OR of 2.39 (95% CI, 1.65–3.45) for unemployment due to disability. This result aligns with our findings of elevated unemployment rates among patients with cognitive impairments, where cognitive limitations had a 43.3% higher unemployment rate and cognitive difficulties had an 83.5% higher rate compared to no/mild cognitive impairments.
Overall, our findings underscore the significant burdens associated with cognitive impairments in schizophrenia, consistent with the broader literature demonstrating the impact of cognitive impairments on clinical responsibilities, SDoH, economic outcomes, and daily functioning. With MEPS data, our study provided insights into the burden that cognitive impairments impose on the health care system in the US. Addressing cognitive impairments in schizophrenia effectively could alleviate this burden, potentially enhancing patient outcomes and quality of life. Future research should focus on expanding the understanding of cognitive impairments impacts by exploring additional data sources.
A key strength of this study is its generalizability to the US population, facilitated by the extensive MEPS dataset. Additionally, as the MEPS data provide comprehensive and detailed information on health care utilization, expenditures, insurance coverage, and health status therefore widely used in health services research.37 Therefore, the study findings offer a comprehensive analysis of the impact of cognitive impairments on schizophrenia patients from clinical, economic, and humanistic perspectives.
However, several limitations are acknowledged. The reliance on self-reported and secondary data introduces potential recall and misclassification bias, which could affect the accuracy of the information provided. For example, subjective reporting can be influenced by factors such as lack of insight or subjective distress in individuals with schizophrenia. The absence of a clear temporal relationship limits the ability to establish causality between cognitive impairments and observed outcomes. Furthermore, cognitive impairments were assessed without the use of standardized clinical tools and could have been impacted by other comorbidities, such as anxiety, depression, and substance use disorder, although having other neurocognitive disorders was an exclusion criterion. All cognitive impairments measures available in MEPS (concentration, memory, and decision-making only) were included in this study; domains of cognitive functioning were not collected in MEPS and thus not represented in this study. Additionally, because the definition of cognitive limitations includes an item related to functional dependence (eg, requiring supervision for safety), there is potential for conceptual overlap with our outcome measures of daily living and community functioning, which may limit causal interpretations. Other limitations include health status and HRQoL were subjectively reported by participants, potentially influencing reliability and validity of the results. Additionally, the economic and humanistic burdens reported may have been influenced by other health conditions.
Overall, while this study provides valuable insights into the burden of cognitive impairments in schizophrenia, addressing these limitations and further research will be essential for a deeper understanding and more effective management of cognitive impairments in patients living with schizophrenia.
CONCLUSION
This study reveals the impact of cognitive impairments on individuals living with schizophrenia through increased clinical, economic, humanistic, and indirect burdens compared to those without cognitive impairments. Furthermore, this evidence underscores the need for targeted therapies addressing cognitive impairments to improve patient outcomes and reduce the burden on individuals living with schizophrenia and their caregivers.
Article Information
Published Online: August 11, 2025. https://doi.org/10.4088/JCP.25m15794
© 2025 Physicians Postgraduate Press, Inc.
Submitted: January 14, 2025; accepted May 19, 2025.
To Cite: Choi BM, Singh T, Nili M, et al. Understanding the characteristics and burden of cognitive impairments in schizophrenia in the US: Medical Expenditure Panel Survey. J Clin Psychiatry 2025;86(3):25m15794.
Author Affiliations: Pharmaceutical Sciences, College of Pharmacy, University of Arizona, Tucson, Arizona (Choi); BluePath Solutions, Los Angeles, California (Choi, Singh, Lam, McGarvey); Boehringer Ingelheim Pharmaceutical Inc, Ridgefield, Connecticut (Nili, Xiang); Department of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York (Correll); Department of Psychiatry and Molecular Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/ Northwell, Hempstead, New York (Correll); Center for Psychiatric Neuroscience, Northwell Health, The Feinstein Institute for Medical Research, New Hyde Park, New York (Correll); Department of Child and Adolescent Psychiatry, Charité -Universitätsmedizin Berlin, Berlin, Germany (Correll); German Center for Mental Health (DZPG), Partner Site Berlin, Berlin, Germany (Correll).
Corresponding Author: Briana M. Choi, PharmD, PhD, Pharmaceutical Sciences, College of Pharmacy, University of Arizona, 1703 E Mabel St, Tucson, AZ 85721 ([email protected]).
Relevant Financial Relationships: Drs Choi, Singh, McGarvey, and Lam were employees of BluePath Solutions LLC, which received funding from Boehringer Ingelheim Pharmaceuticals, Inc. to conduct the analyses for the study at the time of this study. Drs Xiang and Nili are employed by the study sponsor. Dr Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Alkermes, Allergan, Angelini, Aristo, Autobahn, Boehringer-Ingelheim, Bristol-Meyers Squibb, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Delpor, Denovo, Draig, Eli Lilly, Eumentis Therapeutics, Gedeon Richter, GH, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedInCell, MedLink, Merck, Mindpax, Mitsubishi Tanabe Pharma, Maplight, Mylan, Neumora Therapeutics, Neuraxpharm, Neurocrine, Neurelis, NeuShen, Newron, Noven, Novo Nordisk, Orion Pharma, Otsuka, PPD Biotech, Recordati, Relmada, Response Pharmaeutical, Reviva, Rovi, Saladax, Sanofi, Seqirus, Servier, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Tabuk, Takeda, Teva, Terran, Tolmar, Vertex, Viatris, and Xenon Pharmaceuticals. He provided expert testimony for Janssen, Lundbeck, and Otsuka. He served on a Data Safety Monitoring Board for Compass Pathways, IntraCellular Therapies, Relmada, Reviva, and Rovi. He has received grant support from Boehringer-Ingelheim, Janssen, and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Kuleon Biosciences, LB Pharma, MedLink Global, Mindpax, Quantic, and Terran.
Funding/Support: This study was funded by Boehringer Ingelheim Pharmaceuticals, Inc (Ridgefield, CT, US).
Role of the Sponsor: Boehringer Ingelheim Pharmaceuticals, Inc. provided input into the initial study concept and design but had no influence over the study execution and the decision to publish. Boehringer Ingelheim Pharmaceuticals, Inc. was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Previous Presentations: Schizophrenia International Research Society (SIRS); April 3–7, 2024; Florence, Italy. Academy of Managed Care Pharmacy (AMCP); April 15–18, 2024; New Orleans, Louisiana.
Additional Information: The Medical Expenditure Panel Survey (MEPS) data used in this study can be accessed at the following URL: https://meps.ahrq.gov/mepsweb/data_stats/download_data_files.jsp. Instructions for accessing and downloading the datasets are provided on the site.
Clinical Points
- While cognitive impairments are common in people living with schizophrenia, its broader impact on functioning, health care use, and socioeconomic burden has been underexplored, including in large, representative US populations.
- Using Medical Expenditure Panel Survey data, we found that over half of adults living with schizophrenia report cognitive impairments, which were associated with significantly higher health care costs and reduced quality of life.
- These findings highlight the need for routine screenings for cognitive impairments and targeted interventions to address cognitive burden in people affected by schizophrenia.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Psychosis section. Please contact Ann K. Shinn, MD, MPH, at Psychiatrist.com/contact/shinn.
References (37)

- Kadakia A, Catillon M, Fan Q, et al. The economic burden of schizophrenia in the United States. J Clin Psychiatry. 2022;83(6):22m14458. PubMed CrossRef
- Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381(18):1753–1761. PubMed CrossRef
- Millan MJ, Agid Y, Brüne M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141–168. PubMed CrossRef
- Correll CU, Rubio JM, Inczedy-Farkas G, et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74(7):675–684. PubMed CrossRef
- Correll CU, Solmi M, Cortese S, et al. The future of psychopharmacology: a critical appraisal of ongoing phase 2/3 trials, and of some current trends aiming to de-risk trial programmes of novel agents. World Psychiatry. 2023;22(1):48–74. PubMed CrossRef
- Keepers GA, Fochtmann LJ, Anzia JM, et al. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. Am J Psychiatry. 2020;177(9):868–872. PubMed CrossRef
- Vita A, Barlati S, Ceraso A, et al. Durability of effects of cognitive remediation on cognition and psychosocial functioning in schizophrenia: a systematic review and meta-analysis of randomized clinical trials. Am J Psychiatry. 2024;181(6):520–531. PubMed CrossRef
- Deste G, Corbo D, Nibbio G, et al. Impact of physical exercise alone or in combination with cognitive remediation on cognitive functions in people with schizophrenia: a qualitative critical review. Brain Sci. 2023;13(2):320. PubMed CrossRef
- Vita A, Barlati S, Ceraso A, et al. Acceptability of cognitive remediation for schizophrenia: a systematic review and meta-analysis of randomized controlled trials. Psychol Med. 2023;53(8):3661–3671. PubMed CrossRef
- Vita A, Gaebel W, Mucci A, et al. European Psychiatric Association guidance on treatment of cognitive impairment in schizophrenia. Eur Psychiatry. 2022;65(1):e57. PubMed CrossRef
- Solmi M, Croatto G, Piva G, et al. Efficacy and acceptability of psychosocial interventions in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. Mol Psychiatry. 2023;28(1):354–368. PubMed CrossRef
- Carbon M, Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014;19(suppl 1):38–53. PubMed CrossRef
- McCleery A, Nuechterlein KH. Cognitive impairment in psychotic illness: prevalence, profile of impairment, developmental course, and treatment considerations. Dialogues Clin Neurosci. 2019;21(3):239–248. PubMed CrossRef
- Dickerson F, Khan S, Origoni A, et al. Risk factors for natural cause mortality in schizophrenia. JAMA Netw Open. 2024;7(9):e2432401. PubMed CrossRef
- Kadakia A, Fan Q, Shepherd J, et al. Healthcare resource utilization and quality of life by cognitive impairment in patients with schizophrenia. Schizophr Res Cogn. 2022;28:100233. PubMed CrossRef
- Nili M, Xiang P, Cummings T, et al. Patient characteristics and health care resource utilization of cognitive impairment among patients with schizophrenia in the Veterans Affairs Administration System. Value Health; 2023;27(6):S168.
- Patel A, Everitt B, Knapp M, et al. Schizophrenia patients with cognitive deficits: factors associated with costs. Schizophr Bull. 2006;32(4):776–785. PubMed CrossRef
- Kitchen H, Rofail D, Heron L, et al. Cognitive impairment associated with schizophrenia: a review of the humanistic burden. Adv Ther. 2012;29(2):148–162. PubMed CrossRef
- MEPS Medical Expenditure Panel Survey. Survey Background. AHRQ Agency for Healthcare Research and Quality; 2019. Accessed January 10, 2024. https://meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. PubMed CrossRef
- Marmot M, Friel S, Bell R, et al. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet Lond Engl. 2008;372(9650):1661–1669. PubMed CrossRef
- Edemekong PF, Bomgaars DL, Sukumaran S, et al. Activities of Daily Living. In: StatPearls. StatPearls Publishing; 2024. Accessed October 2, 2024. http://www.ncbi.nlm.nih.gov/books/NBK470404/
- Selim AJ, Rogers W, Fleishman JA, et al. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12). Qual Life Res. 2009;18(1):43–52. doi:10.1007/s11136-008-9418-2. PubMed CrossRef
- MEPS HC-201: 2017 Full Year Consolidated Data File. 2019. Accessed January 10, 2024. https://meps.ahrq.gov/data_stats/download_data/pufs/h201/h201doc.shtml
- Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. PubMed CrossRef
- MEPS HC-050: 2000 Full Year Consolidated Data File. 2023. Accessed January 12, 2024. https://meps.ahrq.gov/data_stats/download_data/pufs/h50/h50doc.shtml
- MEPS HC-079: 2003 Full Year Consolidated Data File. 2005. Accessed January 12, 2024. https://meps.ahrq.gov/data_stats/download_data/pufs/h79/h79doc.shtml
- MEPS HC-020: 1997 Full Year Consolidated Data File. 2001. Accessed January 12, 2024. https://meps.ahrq.gov/data_stats/download_data/pufs/h20/h20doc.shtml#2582Functional
- MEPS HC-233 2021 Full Year Consolidated Data File. 2023. Accessed January 12, 2024. https://meps.ahrq.gov/data_stats/download_data/pufs/h233/h233doc.shtml
- Usual Weekly Earnings of Wage and Salary Workers Third Quarter. 2023. Accessed January 12, 2024. https://www.bls.gov/news.release/pdf/wkyeng.pdf
- Median usual weekly earnings of full-time wage and salary workers by sex, quarterly averages, seasonally adjusted. 2023. Accessed July 12, 2023. https://www.bls.gov/news.release/pdf/wkyeng.pdf
- CPI – All Urban Consumers (Current Series): All Items Less Food and Energy in U.S. City Average, All Urban Consumers, Not Seasonally Adjusted. U.S. Department of Labor, Bureau of Labor Statistics; 2024. Accessed January 12, 2024. https://data.bls.gov/timeseries/CUUR0000SA0L1E?output_view=pct_12mths.
- SAS Studio. SAS Institute Inc. 2018.
- MEPS HC-192: 2016 Full Year Consolidated Data File. 2018. Accessed January 12, 2024. https://meps.ahrq.gov/data_stats/download_data/pufs/h192/h192doc.shtml
- Leucht S, Burkard T, Henderson J, et al. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand. 2007;116(5):317–333. PubMed CrossRef
- Marmot M, Allen JJ. Social determinants of health equity. Am J Public Health. 2014;104(suppl 4):S517–S519. PubMed CrossRef
- Liu J, Yu F, Song L. A systematic investigation on the research publications that have used the medical expenditure panel survey (MEPS) data through a bibliometrics approach. Emerald Publ Ltd. 2020;38(4):705–721. CrossRef
This PDF is free for all visitors!