Abstract
Bipolar I disorder (BP-I) is a severe and chronic psychiatric condition characterized by recurrent episodes of mania and depression that significantly impact quality of life and functioning. Early recurrence, high relapse rates, and poor adherence to daily oral medications complicate long-term management and increase the risk of hospitalization and suicide. Long-acting injectable antipsychotics (LAIs) offer a potential solution to these challenges by promoting sustained medication delivery and efficacy, reducing pharmacokinetic variability, and improving treatment adherence. Among available LAIs, aripiprazole is the only partial dopamine D₂ receptor agonist, which may contribute to its favorable tolerability and mood-stabilizing properties. Despite the robust evidence for the efficacy and tolerability of aripiprazole monohydrate LAIs in patients with BP-I, this agent remains underutilized in this population. Misperceptions about efficacy and tolerability, coupled with systemic and prescriber-level barriers, have limited broader clinical adoption. To address these issues, a round table panel of experts in psychopharmacology, the clinical treatment of bipolar disorder, and antipsychotic prescribing was convened to evaluate the clinical rationale for earlier use of aripiprazole monohydrate LAIs in BP-I and to identify key challenges limiting its use. This article summarizes their consensus on the pharmacological distinctiveness, practical advantages, and potential of aripiprazole monohydrate LAIs in improving long-term outcomes in individuals with BP-I.
J Clin Psychiatry 2025;86(3):plunlai2424ah3
Published Online: August 13, 2025.
To Cite: Goldberg JF, Achtyes ED, Sajatovic, M, et al. Clinical application of aripiprazole monohydrate long-acting injectables for the treatment of bipolar type I disorder: a consensus panel report. J Clin Psychiatry. 2025;86(3):plunlai2424ah3
To Share: https://doi.org/10.4088/JCP.plunlai2424ah3
© 2025 Physicians Postgraduate Press, Inc.
See more Academic Highlights in this series: Part 1 | Part 2
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the virtual consensus panel meeting “Clinical Application of Aripiprazole Monohydrate Long-Acting Injectables for the Treatment of Bipolar Type I Disorder: A Consensus Panel Report,” which was held September 24, 2024.
The meeting was chaired by Joseph F. Goldberg, MD, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York. The faculty were Eric D. Achtyes, MD, MS, Department of Psychiatry, Western Michigan University Homer Stryker M.D. School of Medicine, Kalamazoo, Michigan; Martha Sajatovic, MD, Department of Psychiatry and Department of Neurology, University Hospitals Cleveland Medical Center, Case Western Reserve University School of Medicine, Cleveland, Ohio; and Stephen R. Saklad, PharmD, BCPP, Division of Pharmacotherapy and Translational Science, College of Pharmacy, The University of Texas at Austin, San Antonio, Texas. Christoph U. Correll, MD, Department of Psychiatry, Zucker Hillside Hospital, Glen Oaks, New York; Department of Psychiatry and Molecular Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/ Northwell, Hempstead, New York; Department of Child and Adolescent Psychiatry, Charité Universitätsmedizin, Berlin, Germany; German Center for Mental Health (DZPG), partner site Berlin, Berlin, Germany
Financial disclosures appear at the end of the article.
This evidence-based peer-reviewed Academic Highlights was prepared by Healthcare Global Village, Inc. The panel meeting for the development of this article was organized and funded by Otsuka Pharmaceutical Europe Ltd and H. Lundbeck A/S. Both companies also funded article processing charges, medical writing, editorial, and other assistance. Editorial support was provided by Miriam Opara, PharmD, Banner Medical LLC, Carl Clay, PhD, and Healthcare Global Village with funding by Otsuka Pharmaceuticals Europe Ltd & H. Lundbeck A/S. The sponsors performed a courtesy review for medical accuracy. The opinions expressed herein are those of the faculty and do not necessarily reflect the views of Healthcare Global Village, Inc., the publisher, or the commercial supporters. This article is distributed by Otsuka Pharmaceutical Europe Ltd and H. Lundbeck A/S for educational purposes only.
Bipolar disorder (BD) is a chronic, relapsing psychiatric illness marked by episodes of mania, hypomania, and depression that significantly impairs psychosocial functioning and quality of life. It is one of the leading causes of disability in young people and is associated with increased mortality, especially death by suicide.1 Bipolar disorder is classified into 2 subtypes: bipolar I (BP-I) and bipolar II (BP-II). Per DSM-5, BP-I requires at least 1 manic episode, which may be preceded or followed by hypomanic or depressive episodes. BP-II requires at least 1 hypomanic and 1 major depressive episode, without any history of mania. Manic episodes are more impairing than hypomanic episodes, and both subtypes are associated with significant depressive symptoms, which are required for diagnosis.2 For most patients, depression is the primary symptom of BD, contributing to the overall burden of illness, in terms of both duration and effect, compared to manic or hypomanic symptoms. However, manic episodes may escalate into psychosis, necessitating urgent medical attention and often leading to hospitalization.3 Additionally, patients diagnosed with BP-I typically have a shorter duration from onset to first recurrence compared with BP-II, implying a greater need for early treatment, stabilization, and treatment optimization.4
Long-term maintenance treatment is critical for relapse prevention in BP-I, yet adherence to daily oral pharmacotherapy remains a pervasive challenge, with estimates suggesting that up to 60% of patients exhibit partial or poor adherence to prescribed regimens.5–7 Nonadherence is a major driver of relapse, rehospitalization, and poor functional outcomes in this population.8,9 Long-acting injectable antipsychotics offer a considered solution to overcome many of the limitations of oral treatment, particularly in patients with a lack of insight into their disease, fluctuating motivation, or comorbid substance use.10 Clinically, LAIs provide sustained plasma concentrations, minimizing the pharmacokinetic variability associated with oral formulations and may reduce the risk of intentional or unintentional medication discontinuation.11–14 Furthermore, LAIs may facilitate regular patient-provider contact, thereby improving patient monitoring and therapeutic adherence.15 Because LAIs may improve medication adherence, they may be associated with reduced risk of relapse and hospitalization and may improve recovery compared to oral antipsychotics.16 Thus, earlier utilization of LAIs may reduce the impact of BP-I and improve psychosocial functioning.
While LAIs have historically been reserved for patients with repeated relapses or demonstrated nonadherence, emerging evidence and expert consensus suggest that earlier use of LAIs in the course of illness may enhance long-term outcomes and prevent the progressive neurobiological and psychosocial sequelae associated with recurrent episodes.17 Among available LAIs, aripiprazole is unique as the only agent with partial dopamine D₂ receptor agonist activity, offering a distinct pharmacological profile compared to dopamine D₂ receptor antagonists. Partial dopamine D2 agonist activity may confer psychiatric benefit in BP-I by stimulating postsynaptic dopamine receptors, thereby increasing dopamine transmission where it is insufficient, while reducing dopamine transmission through postsynaptic dopamine blockade where dopamine transmission is excessive.18,19 Additionally, aripiprazole acts as a partial agonist at serotonin 5-HT2A and 5-HT1A receptors, which may contribute to the favorable tolerability profile and mood-stabilizing effects of the aripiprazole monohydrate LAIs in the acute and maintenance treatment of patients with BP-I.20–23
Aripiprazole monohydrate LAIs are available in two formulations: a once-monthly 400 mg injection (AOM 400) and a 960 mg, ready to use formulation, which is administered every two months (Ari 2MRTU 960). Both formulations are indicated for use in adults with schizophrenia and as monotherapy for the maintenance treatment of adults with BP-I.24,25 To address potential misperceptions among prescribers, a panel of five experts in psychopharmacology, the clinical treatment of BD, and antipsychotic prescribing was organized and convened to discuss the rationale for the early use of aripiprazole monohydrate LAIs in patients with BP-I. The panel focused on aripiprazole monohydrate’s pharmacological distinctiveness, clinical utility, and the potential to shift current paradigms around treatment timing and delivery in mood disorders. The other aripiprazole LAI formulation, aripiprazole lauroxil, was not discussed as it is not indicated for use in patients with BD. This Academic Highlights article summarizes the panels’ discussion and presents their conclusions.
Methods
In September of 2024, a panel of experts in psychopharmacology, the clinical treatment of BP-I, and antipsychotic prescribing convened to develop clinical recommendations on the use of aripiprazole monohydrate LAIs for adults with bipolar I disorder. The panel was held virtually with facilitated discussion, and was chaired by Joseph F. Goldberg, MD, of the Icahn School of Medicine at Mount Sinai in New York City. Panelists included Eric Achtyes, MD, Western Michigan University Homer Stryker M.D. School of Medicine; Martha Sajatovic, MD, Case Western Reserve University School of Medicine; and Stephen R. Saklad, PharmD, BCPP, University of Texas Health Science Center, and Christoph U Correll, MD, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, NY, and Charité – Universitätsmedizin, Berlin, Germany. The panel analyzed publicly available clinical trial data and shared their clinical perspectives to reach a consensus on treatment considerations for BP-I. This article presents the consensus findings from the panel discussion.
Bipolar Type I Disorder: Disease Burden and Progression
Bipolar disorder can be difficult to diagnose, treat, and maintain because of individual neurobiology, varying degrees of functional and social impairment, and treatment response which may worsen with recurrent relapses.26 A 2014 study of 54 patients with BD utilized the Functioning Assessment Short Test (FAST) to illustrate functional variations across 4 clinical stages from stage I—individuals maintain the same functional status as prior to the onset of BD—to stage IV—individuals are unable to care for themselves or live independently. Notably, individuals in stage I exhibited significantly better functioning compared to those in stages III and IV (P < .001).27 Functional recovery, indicated by shifting from higher stages to lower stages, is a critical concern for patients diagnosed with BP-I; it enables patients to resume normal lives and should be regarded as a primary goal of maintenance treatment.
Patients who do not remain on their maintenance medication or who do not respond adequately to maintenance treatment may experience a progressively worse trajectory and an increased frequency of mood episodes, with multiple and successive relapses of bipolar mania typically leading to worsened outcomes, including increased mortality risk.28 Further, patients experiencing their first manic episode achieve recovery and remission more rapidly than patients who experience multiple episodes, indicating the need for rapid stabilization and treatment optimization.29 A 26-year longitudinal population-based study of 14,870 adults concluded that a history of manic episodes during early to mid-adulthood correlated with an approximately 40% increased risk of all-cause mortality.30
The biochemical processes associated with possible neuroprogression may include changes in inflammatory cytokines, neurotrophins, and oxidative stress that are related to the stage of illness. The clinical implications of these findings suggest the possibility of a critical window early in the course of the disorder in which a potentially progressive trajectory may be the most modifiable. This evidence underscores the imperative to minimize the delay to diagnosis and initiation of appropriate therapy that can provide symptom control, maintain or restore patient’s functioning level, and delay subsequent relapses.26
Long-Acting Injectables in Bipolar Type I Disorder
There is mounting evidence to support LAI use early in the treatment of patients diagnosed with BP-I. Unlike oral antipsychotics, which are shorter-acting and require daily dosing, LAIs are designed to release the medication gradually over an extended duration, reducing fluctuations in the plasma concentrations of the active drug, and ultimately providing sustained symptom relief.31 Studies in patients with BP-I suggest that early use of LAIs may provide additional advantages over oral antipsychotics, including consistent pharmacokinetics, bioavailability, and stable, steady-state plasma concentrations, which may result in reduced structural neurologic changes in the initial stages of the disease.32,33 Moreover, LAIs are associated with improved treatment adherence, reduction in relapses, reduced rates of rehospitalization, and enhanced quality of life. Randomized, placebo-controlled studies and real-world data have shown that early treatment with LAIs is generally well tolerated, effective, and leads to improved outcomes compared to oral medication.16,34–36
A meta-analysis of seven randomized controlled trials comparing LAIs to either placebo or oral medications in BP-I, found that LAIs were highly effective in preventing relapse of manic symptoms. However, their effectiveness in preventing depressive relapses was comparable to placebo.35 Similar findings emerged from six mirror-image studies examining LAI use in BD. After initiating LAI treatment, patients experienced fewer hospitalizations and emergency department visits in the following year. A significant reduction in hypomanic and manic relapses was also observed, although efficacy to prevent depressive episodes was less clear.37 Additionally, a prospective Finnish registry study of 18,018 patients with BD found that patients with BD receiving LAIs had a 30% lower risk of hospitalization compared to those taking oral formulations.33
Despite evidence showing that LAIs reduce the risk of relapse and hospitalization in patients with BP-I they remain consistently underutilized in this population. A 2024 consensus panel of expert psychiatric advisors revealed that although LAI use for bipolar mania has risen from 2.2% in 2006 to 11.6% in 2018, LAIs remain underutilized in this patient population.25 Moreover, a 2022 review of electronic medical record data from 41,401 patients who received psychiatric services at the South Carolina Department of Mental Health revealed that only 17.0% of eligible patients received a LAI.32 Factors contributing to underuse of LAIs included lack of clinician awareness and overestimation of patient adherence to oral medications.31,38
Unique Features of Aripiprazole Monohydrate LAIs in the Treatment of Bipolar Type I Disorder
Pharmacologically, aripiprazole has a high affinity for dopamine D2 and D3 receptors and serotonin 5-HT1A and 5-HT2A receptors and modulates dopamine and serotonin release, and exhibits minimal or no activity at alpha-adrenergic, anticholinergic, or antihistaminic receptors. This unique pharmacologic profile of aripiprazole, the combination of partial agonism at D2, D3, 5-HT1A, and 5-HT2A receptors, is thought to contribute to its broad clinical efficacy and tolerability.39,40
As a dopamine receptor partial agonist, aripiprazole monohydrate is associated with a lower risk of excessive dopamine blockade, reducing the likelihood of adverse effects such as drug-induced parkinsonism, elevated prolactin levels, sexual dysfunction, weight gain, metabolic disturbances, and tardive dyskinesia (TD).41–44 Aripiprazole monohydrate is generally well tolerated, with most side effects emerging within the first 1 to 3 weeks of treatment; side effects are typically mild to moderate in nature.45–47 Further, the low risk of metabolic complications, sexual dysfunction, and anhedonia noted with aripiprazole monohydrate may enhance adherence and improve clinical outcomes.48 Given its favorable side effect profile, patients may prefer a partial D2 agonist like aripiprazole monohydrate over first-generation dopamine antagonists.42
Efficacy of Aripiprazole Monohydrate LAIs in Bipolar Type I Disorder
Aripiprazole once-monthly 400 mg and Ari 2MRTU 960 mg are approved as monotherapy for maintenance treatment of BP-I in adults.24,25 The approval of AOM 400 for the maintenance treatment of BP-I was supported by data from an international, double-blind, placebo-controlled, 52-week randomized withdrawal trial of 720 patients with BP-I. In the trial, AOM 400 was significantly more effective than placebo in delaying the time from randomization to recurrence of any mood episode (the primary study endpoint), reducing both the risk of recurrence over 1 year (hazard ratio, 0.45; 95% confidence interval, 0.30–0.68) and the proportion of patients with a recurrent mood episode (26.5% with AOM 400 vs 51.1% with placebo) by almost half.23 Additionally, oral aripiprazole monotherapy in the management of the acute manic phase of BP-I was tested in 7 randomized controlled trials with a total of 2,303 patients. A meta-analysis of these trials found that oral aripiprazole significantly outperformed placebo in both response and remission rates for acute mania.49 Response was defined as a ≥50% improvement in the Young Mania Rating Scale (YMRS) total score, while remission was defined as a YMRS score ≤12. At week 3, the response rate was 48% for oral aripiprazole, 46% for comparator agents, and 31% for placebo, corresponding to a number needed to treat (NNT) of 6 and an odds ratio (OR) of 1.16 for aripiprazole versus placebo. By week 12, response rates increased to 59% for aripiprazole and 51% for the comparators. The meta-analysis also reported an effect size of 0.34 for oral aripiprazole compared to placebo in the treatment of acute mania.49 These findings support the use of oral aripiprazole for acute mania in BP-I and may encourage greater clinical consideration of the LAI formulation’s unique pharmacologic benefits.
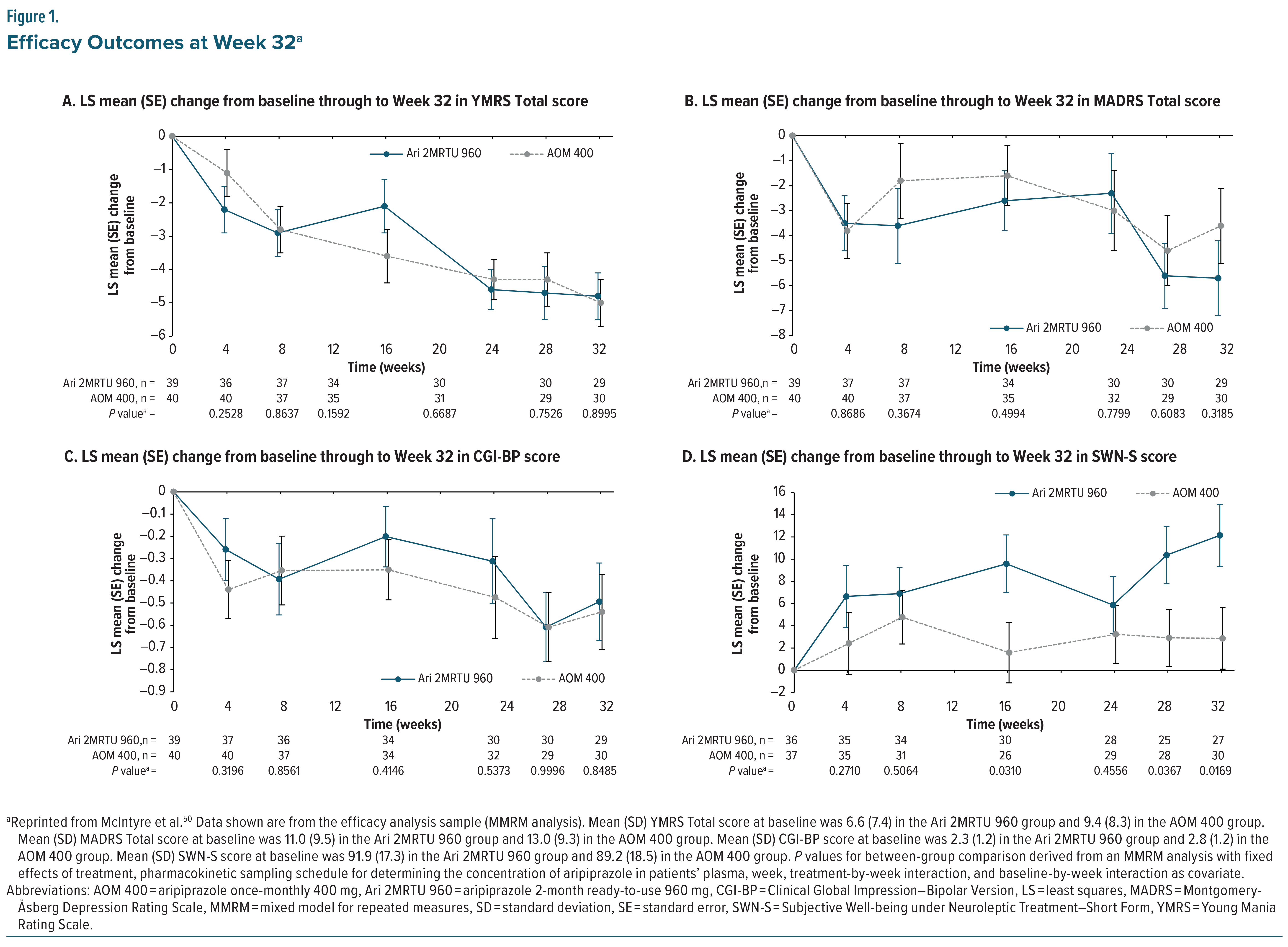
In a secondary analysis of clinically stable patients with BP-I, Ari 2MRTU 960 demonstrated comparable efficacy to AOM 400.50 Minimal changes from baseline were observed in manic, depressive, and global impression scores in both treatment groups, with no statistically significant differences in YMRS, Montgomery-Åsberg Depression Rating Scale (MADRS), or Clinical Global Impression–Bipolar Version (CGI-BP) scores (Figure 1A–1C). Notably, Ari 2MRTU 960 was associated with a significantly greater improvement than AOM 400 in patient-reported well-being as measured by the Subjective Well-being under Neuroleptic Treatment–Short Form (SWN-S) at Week 32 (P = .0169; Figure 1D), suggesting enhanced treatment satisfaction or quality of life. Overall, both AOM 400 and ARI 2MRTU 960 maintained symptomatic stability, supporting the clinical utility of Ari 2MRTU 960 as an effective maintenance treatment for BP-I.50
Early intervention in BP-I is critical, as it may delay the recurrence of mood episodes and reduce functional impairment and other negative outcomes associated with disease progression.51 A recent post hoc analysis evaluated the efficacy and safety of AOM 400 in patients with earlier-stage BP-I, using data from a 52-week, multicenter, double-blind, placebo-controlled, randomized withdrawal trial. Earlier-stage BP-I was defined by the lowest quartiles for either age (18 – ≤32 years; n = 70) or disease duration (0.13 – ≤4.6 years; n = 67) at baseline.52 In both subgroups, AOM 400 significantly delayed the time to recurrence of any mood episode compared with placebo (P = .0251 for patients aged 18–≤32 years; P = .005 for disease duration 0.13–≤4.6 years). This effect was primarily driven by a lower proportion of patients in the AOM 400 group who experienced manic symptom worsening, as indicated by a YMRS total score ≥15 or clinical deterioration. Importantly, depressive symptoms, assessed by changes in MADRS total scores, did not worsen relative to placebo in either subgroup. The safety profile of AOM 400 was consistent with findings from the overall trial.52 These results suggest that AOM 400 is a well-tolerated and effective maintenance treatment for delaying recurrence in earlier-stage BP-I, reinforcing the value of early intervention in this population. Furthermore, a panel report published by a group of UK healthcare professionals, with extensive experience of prescribing aripiprazole monohydrate for acute bipolar mania, found that aripiprazole monohydrate was effective in both the short- and long-term settings, as monotherapy or in combination with a mood stabilizer.48 Unlike traditional dopamine antagonists, aripiprazole monohydrate has antimanic effects that are not associated with sedation, which is beneficial for patients, particularly when considering agents for long-term maintenance treatment. When rapid tranquillization is required when initiating aripiprazole monohydrate in acutely disturbed patients, the panel recommended a short-term co-prescription of a benzodiazepine.48
Combination pharmacotherapy is common in the treatment of BP-I and may offer additional benefits; however, it also introduces challenges such as reduced medication adherence, a higher risk of adverse events, and greater complexity in managing the overall treatment regimen.53,54 A real-world evidence study of patients diagnosed with BP-I compared adherence rates and time to discontinuation of patients receiving AOM 400 to those receiving oral antipsychotics.55 The study demonstrated that the cohort receiving AOM 400 had significantly higher rates of adherence and lower rates of discontinuation or medication switching, compared to those on oral antipsychotics. Further, the study noted that patients exhibited similar maintenance phase outcomes, minimal need for rescue medications, high rates of stability, and high levels of patient-assessed satisfaction relative to their prior treatment. Notably, over 65% of participants reported experiencing no adverse events or significantly fewer adverse events than with previous medications.55
Aripiprazole once monthly 400 mg may be associated with reducing the overall burden of co-prescribed psychotropic medications. A retrospective analysis of claims data from 1815 adults diagnosed with BP-I in the US showed that, for patients who initiated and maintained treatment with AOM 400 for at least 12 months, the use of major classes of antidepressant medications decreased by 52% (P = .007), the use of insomnia medications decreased by 41% (P = .019), and the use of atypical oral antipsychotics decreased by 80% (P < .001) compared to the 12 months prior to initiation of AOM 400.56
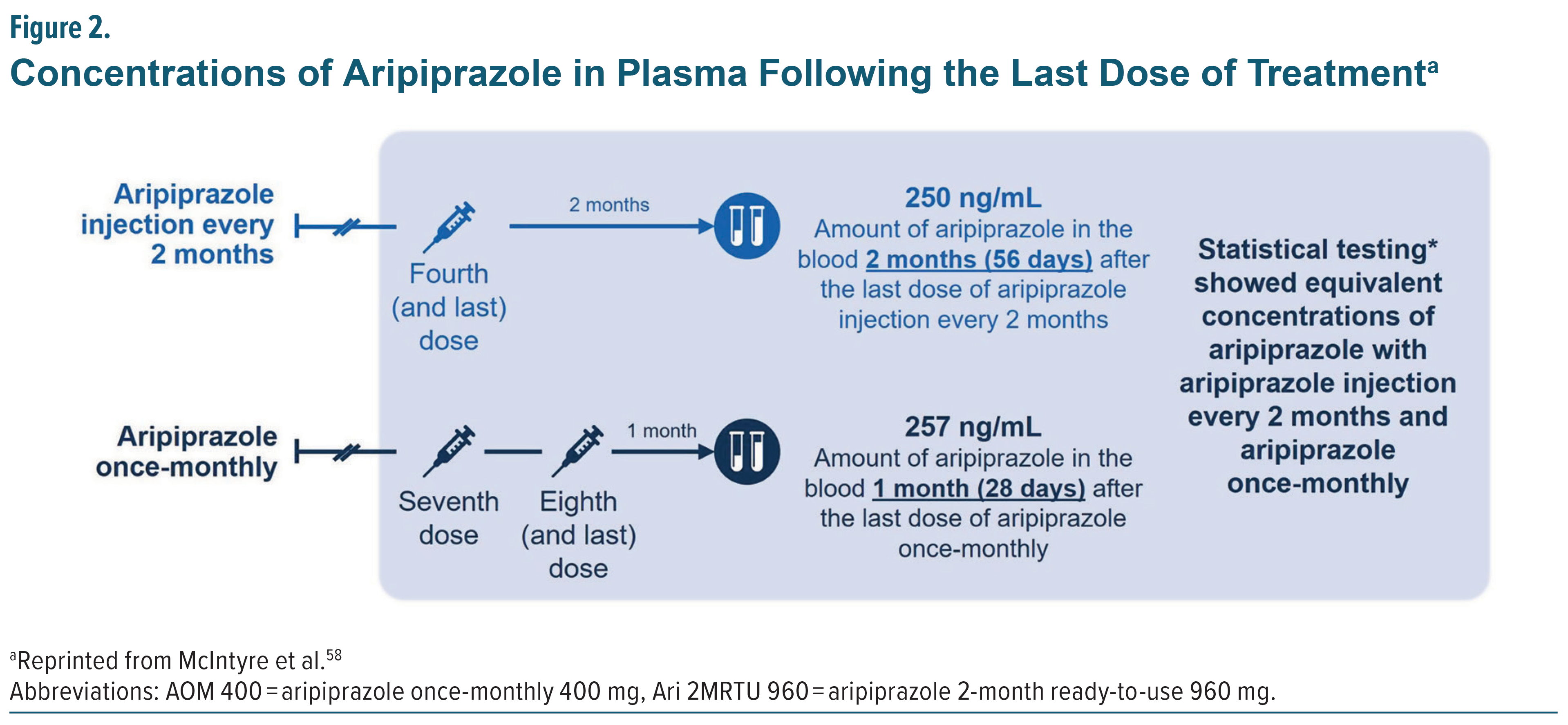
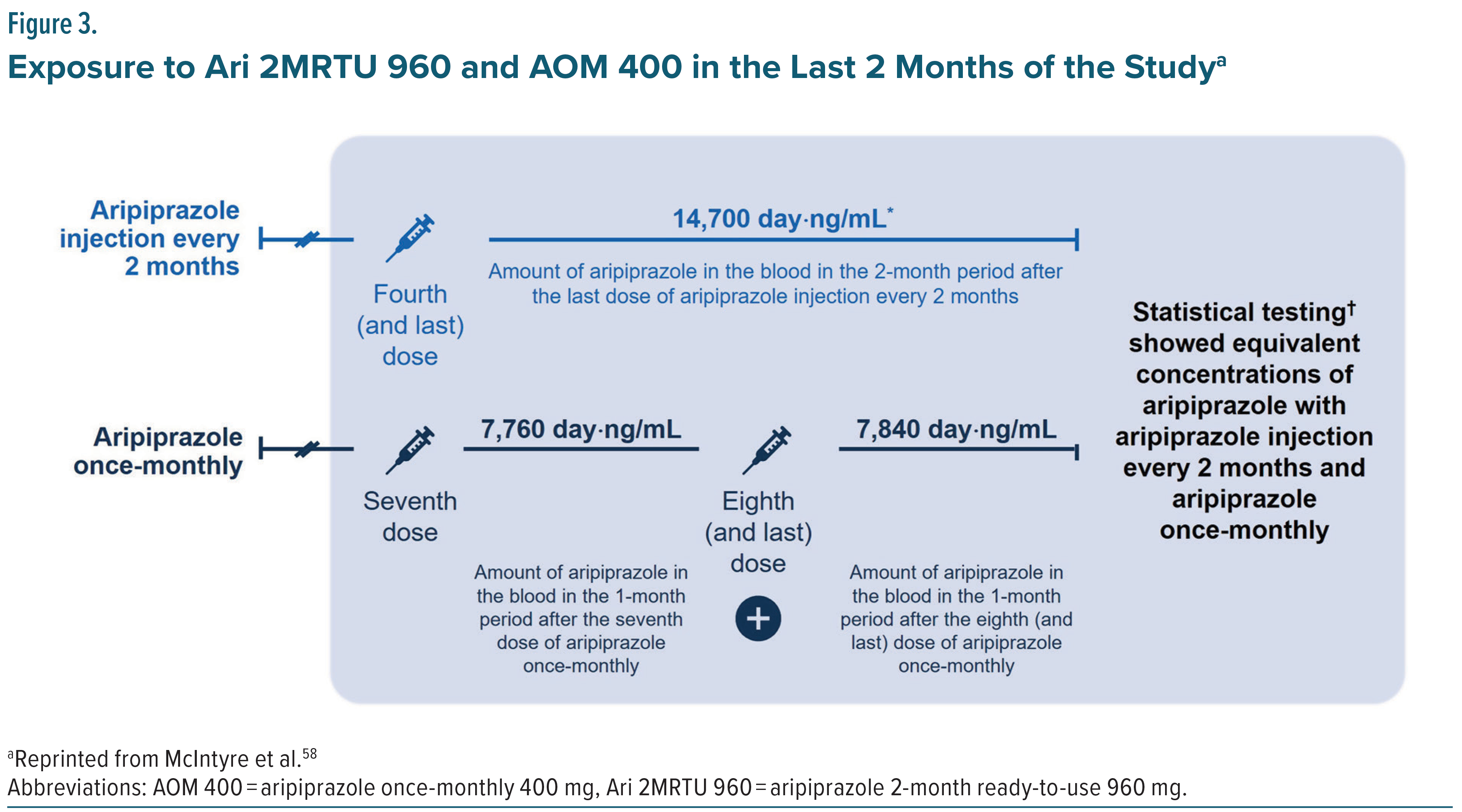
The most recently approved LAI formulation of aripiprazole monohydrate (Ari 2MRTU 960) is administered once every 2 months as maintenance monotherapy for adults with BP-I. A 32-week open-label, active controlled trial involving 266 adults, 185 with schizophrenia and 81 with BP-I, evaluated the safety, tolerability, and pharmacokinetics of Ari 2MRTU 960 compared to AOM 400.57 The results showed that Ari 2MRTU 960 achieved plasma concentrations similar to those of AOM 400, demonstrated comparable efficacy, and was well tolerated, with participants remaining clinically stable throughout the study. Specifically, mean aripiprazole plasma concentrations after the fourth dose of Ari 2MRTU 960 and after the seventh and eighth doses of AOM 400 were comparable (250 ng/mL vs 257 ng/mL, respectively; Figure 2).50,57,58 Additionally, aripiprazole plasma concentration remained above the minimum therapeutic threshold (>95 ng/mL) across the full 2-month dosing interval (Figure 3). Understanding the pharmacokinetic profiles and efficacy outcomes of the aripiprazole monohydrate LAI treatment options should help clinicians assess the suitability of different formulations for patients with BP-I.59 This knowledge could support LAI use and result in sustained plasma concentrations and efficacy, sustained remission, improved quality of life, and consideration of earlier use of LAIs in the maintenance treatment of BP-I.
Panel Consensus Statement #1
Early and Proactive Use of Aripiprazole Monohydrate LAIs Improves Adherence and Outcomes in Patients with BP-I: “Early intervention is essential to avoid the cumulative negative effects of repeated relapses, which can lead to poorer long-term outcomes. Aripiprazole monohydrate LAIs should be considered in the treatment of BP-I to prevent mood episodes and reduce the risk of neuroprogression.”
Addressing Relapse and Adherence Challenges
Bipolar I disorder is marked by a recurring pattern of relapses and remissions, with medication adherence playing a central role in modifying the course and prognosis of the illness. Relapses in BP-I pose substantial treatment challenges and are associated with significant social and economic burdens. As early as 2005, BD was estimated to affect 1.6%–3.7% of the population and to contribute to increased unemployment, workplace difficulties, interpersonal stress, and elevated suicide risk, collectively resulting in an estimated annual societal cost of $10–$45 billion.60 By 2018, the total national economic burden of BD/BP-I had risen to over $195 billion annually, with approximately 25% attributed to direct medical costs. Individuals with BD/BP-I required more frequent healthcare services and incurred higher medical expenses compared to those without BD.61 Key drivers of these elevated costs included frequent psychiatric interventions, co-occurring medical and psychiatric conditions, and both poor medication adherence and inadequate clinical management.61
Nonadherence remains a major challenge in the treatment of BP-I. Patients often discontinue medication without consulting a healthcare provider, frequently due to unwanted side effects.62,63 Nonadherence can result in relapse of mood episodes, delayed remission, and persistent residual symptoms.64 A survey conducted by the Depression and Bipolar Support Alliance (DBSA) involving 896 individuals, approximately half of whom had bipolar disorder, found that even when treatment was perceived as effective (eg, improving sleep and cognitive function), common side effects such as weight gain and trembling were leading reasons for discontinuation.65,66 Nonadherence is linked to clinical deterioration, including worsening symptoms, increased emergency department visits, hospital readmissions, and poorer psychosocial outcomes. Although nonadherence rates among individuals with BP-I range from 20% to 60%, the issue is often underrecognized by clinicians and downplayed by patients.67
Clinical guidelines recommend the use of antipsychotics with or without mood stabilizers for maintenance treatment of BP-I to reduce the likelihood of symptom recurrence and relapse.68,69 However, many factors are typically overlooked that impact adherence to medication, including patient expectations, perceived benefit of treatment, current phase of illness, and the tolerability profile of medications, with particular concern for weight gain, movement disorders, and sedation.70 Risk factors that increase the likelihood of nonadherence include younger age, substance misuse, homelessness, non-Caucasian ethnicity, being unmarried, living alone, and lower levels of awareness or understanding of BD when compared to adherent patients.71,72
In addition to adverse effects of medications, withdrawal concerns can drive nonadherence. An online survey evaluated the experiences of 585 individuals diagnosed with schizophrenia, BP-I, and other psychiatric disorders attempting to discontinue the use of antipsychotic medications.73 Participants were required to be ≥18 and be taking an antipsychotic medication continuously for at least 1 month; exclusionary criteria included being compulsorily detained in a psychiatric hospital. The findings revealed that 72% of participants experienced typical withdrawal symptoms, such as nausea, tremors, anxiety, agitation, and headaches; 26% of participants had made four or more attempts to discontinue their medication, while 23% required at least 1 year to successfully complete withdrawal. Notably, 26% reported experiencing 1 or more positive outcomes with discontinuation, including increased energy and improved clarity of thought, whereas 18% experienced relapse episodes, including psychosis.73 Concerns surrounding the potential for missed doses of daily medication administration and the subsequent risk of withdrawal may be a significant issue for individuals with BP-I.
Safety of Aripiprazole Monohydrate LAIs in Bipolar Type I Disorder
The adverse event profile of aripiprazole is similar to that of other antipsychotics; however, aripiprazole is associated with a lower incidence of cardiometabolic risk, such as weight gain, hypercholesterolemia, glucose dysregulation, cardiovascular abnormalities, and hyperprolactinemia as well as sexual dysfunction, compared with a number of first- and other second-generation antipsychotics, likely because of its partial D2 receptor agonism and the absence of H1 blockade.74 Therefore, implementing aripiprazole monohydrate LAIs in the treatment armamentarium for BP-I may provide an attractive treatment option for the long-term maintenance treatment of BP-I, given its potentially favorable adverse event profile compared with other antipsychotic medications.74,75
Certain adverse events, such as akathisia and drug-induced parkinsonism, can be commonly managed by reducing the dosage of aripiprazole monohydrate. Importantly, once the medication is at a steady state, aripiprazole monohydrate LAIs are associated with fewer withdrawal-related movement disorders compared with oral antipsychotics.76–78 Although the risk of TD is lower with second-generation antipsychotics44,79 and may be particularly low with aripiprazole,44 TD remains a potential concern with all antipsychotic medications and may require treatment with a vesicular monoamine transporter 2 (VMAT2) inhibitor, such as deutetrabenazine or valbenazine.80,81
Panel Consensus Statement #2
LAIs May Enhance Adherence and Relapse Prevention in Patients with Bipolar Disorder: “LAIs, including the two aripiprazole monohydrate formulations, improve adherence by reducing the burden of daily oral medication and ensuring a consistent therapeutic effect, resulting in fewer relapses, especially in patients with unstable living conditions, comorbid substance use disorders, or a history of nonadherence. The aripiprazole monohydrate LAIs are effective, well tolerated, and may reduce non-adherence related to adverse effects.”
Identifying and Addressing Barriers to Early Initiation of LAIs
The primary treatment goals in BP-I include managing episodes of mania and depression, addressing sub-syndromal symptoms between episodes, and preventing mood relapses.28 Despite these goals, LAIs are still rarely prescribed for individuals with BP-I. Historically, LAIs have been used for schizophrenia or as a “last-resort” option for patients with BP-I and were only considered after multiple relapses or demonstrated non-adherence. Limited use may reflect clinicians’ assumptions that patients necessarily prefer oral medications or the perception that relapse in BP-I is in some way a less severe event than in schizophrenia. A 2020 survey of mental health professionals, including psychiatrists and nurses, and their patients noted that physicians and nurses overestimated many of the patients’ fears of LAIs and expressed those fears to a much greater extent than those of patients already on LAIs.82 Acceptance of switching to an LAI formulation of an antipsychotic was associated with shorter time from diagnosis, indicating a need for better communication between patients and providers and the need for shared-decision making at all stages of illness. Additionally, nurses and patients noted very limited knowledge of antipsychotics, underscoring the necessity of the prescriber in leading these conversations.82 These findings have been replicated in multiple other surveys of patient preferences on treatment and communication with their healthcare team.10,83–87 These views may also stem from a lack of awareness about the benefits of LAIs as only recently have LAIs been approved for BP-I treatment, and their adoption into major clinical guidelines has been gradual.88
Stigma and negative perceptions of mental illness and its treatments, including LAIs, present a barrier to effectively managing BP-I. Patient acceptance of LAIs may be affected by a fear of needles or patients’ negative experiences in an emergency setting. An injectable medication could be viewed as intrusive, leading to feelings of punishment rather than assistance and relief.89,90 However, it has been demonstrated that patients’ concerns about the use of LAIs may be exaggerated.82,88 Providing education on LAIs, such as improved effectiveness and fewer adverse events, has resulted in increased patient willingness to initiate LAI therapy.91 Additionally, LAIs can be discussed as a treatment option for patients concerned about reducing the stigma of taking medication daily, and may represent a safety net that allows patients to focus on recovery, remission, and improving quality of life.
While LAIs may have barriers to early initiation and long-term adherence, they may improve patient-clinician interactions and offer convenience in the form of fewer administrations, fewer adverse events, and improved clinical outcomes. Shared decision-making regarding the benefits and costs of treatment options for BP-I treatment may help improve and sustain efficacy and stability of BP-I treatment.65,92
Panel Consensus Statement #3
Destigmatization and Patient-Centered Communication Is Critical for Maintenance Treatment in Patients with BP-I: “The stigma surrounding the use of antipsychotic medications and injectable treatments must be addressed. It is critical to differentiate the safety, efficacy, and pharmacology of second-generation LAIs from first-generation antipsychotics and LAIs. Reframing LAIs as maintenance treatments that enhance recovery, remission, and quality of life, rather than as last-resort options, may foster acceptance and facilitate shared decision-making between patients and clinicians.”
Utilizing Patient-Centered Communication and Shared Decision-Making for Treatment Selection
Data collected over a 4-year period from the Systematic Treatment Optimization Program (STOP) indicate that the implementation of best practices, such as safe and effective maintenance treatment regimens, psycho-education, cognitive behavioral therapy (CBT), interpersonal and social rhythm therapy, and family-focused therapy, as well as peer-support and stress-management programs, has led to increased opportunities for remission and recovery in patients experiencing their first episode of mania. Furthermore, although recurrence is frequently observed, addressing risk factors within the first year, such as anxiety, stress, and co-occurring alcohol or substance abuse, may significantly influence the trajectory of the illness in positive ways.93 Approximately 40%–70% of patients diagnosed with BD have a comorbid substance use disorder (SUD), which is considered a proxy factor that will disrupt adherence, or a direct factor that will make psychotropic medications less efficacious. Research has consistently shown that co-occurring SUDs are correlated with negative effects on BP-I illness outcomes, including more frequent and prolonged affective episodes, decreased compliance with treatments, lower quality of life, and increased suicidal behavior.94 Co-occurring conditions can influence patient and provider treatment priorities and clinical decisions and affect the overall approach to managing BP-I.68
It is essential that clinicians should inform patients of all available treatment options at the earliest stages of their illness and engage in discussion with patients to facilitate effective shared decision-making. This is particularly crucial as some patients may prefer LAIs over the daily regimen of oral medications, but they can only consider this treatment option if they are adequately informed about it. Additionally, failing to disclose all potential options could lead to negative reactions from patients, who may perceive such omissions as paternalistic.31 Shared decision-making is critical in providing effective long-term treatment and management by considering clinical evidence of benefits and risks and balancing the symptom profile, treatment history, and adherence. Thus, clinicians have an opportunity to engage in this process with patients and their caregivers, enabling them to make informed treatment decisions and fostering positive long-term outcomes.68,95 For example, if the option of an LAI is raised and the patient declines, but then relapses with an alternative treatment, a longitudinal perspective and approach about LAIs should be revisited.
Patients with primarily depressive symptoms can benefit from LAI therapy; however, research shows that LAIs protect against manic relapses/recurrences better than against depressive relapses/recurrences, so combination therapy should be considered.96 A depression-polarity-prone BP-I patient may be a relatively poorer candidate for LAI monotherapy than a mania-polarity-prone BP-I patient. Thus, augmentation with mood stabilizers, such as lamotrigine or lithium, would be favored over changing the LAI dose.
For patients who experience a BP-I relapse, clinicians should address patients’ barriers and challenges to medication adherence by engaging in shared decision-making and proposing LAIs as an option to reduce medication burden. Aripiprazole monohydrate LAIs offer additional treatment options for patients diagnosed with BP-I. Once monthly AOM 400 and the extended bi-monthly Ari 2MRTU 960 have the potential to reduce the antipsychotic treatment burden on patients diagnosed with BP-I and may help clinicians enhance patient care. Overall, the favorable safety profile and flexibility in dosing aripiprazole monohydrate LAIs provide clinicians and patients with an effective and well-tolerated treatment option. At the time of this publication, Ari 2MRTU 960 is the only LAI with a 2-month dosing interval approved for the treatment of BP-I and, thus, provides clinicians with a new therapeutic option for BP-I.97
Conclusion
Aripiprazole monohydrate LAIs represent a clinically distinct and generally well-tolerated treatment option for the maintenance management of BP-I. The unique pharmacology of the aripiprazole monohydrate LAIs, combined with sustained efficacy noted in BP-I, and reduced burden of daily adherence make them especially valuable in addressing the high relapse risk and functional impairment associated with the illness. Expert consensus supports the earlier use of aripiprazole monohydrate LAIs to improve adherence, reduce relapse rates, and mitigate the progressive nature of the disorder. Greater adoption of these agents will depend on addressing persistent misperceptions, improving patient-clinician communication, and incorporating shared decision-making into routine care. With monthly and bi-monthly formulations available, aripiprazole monohydrate LAIs offer flexible and effective tools to support long-term recovery in patients with BP-I.
Financial Disclosures:
All authors received an honorarium for attendance of the meeting.
Dr Goldberg has received consulting fees from Alvogen Pharmaceuticals and Genomind; has received honoraria for speaking/teaching from Abbvie, Alkermes, Axsome, Bristol-Myers Squibb, and Intracellular Therapies; has received advisory board fees from Luye Pharmaceuticals, Merck, Neurelis, Neuroma, Otsuka, Sunovion, and Supernus; and has received royalties from American Psychiatric Publishing, and Cambridge University Press. Dr Achtyes has received consulting fees from Clinical Care Options, Boehringer-Ingelheim, VML Health, CMEology, CME Outfitters, Otsuka/Lundbeck, and TotalCME; has received grant/research support from Teva, InnateVR, Boehringer-Ingelheim, Neurocrine Biosciences, Karuna/Bristol Myers Squibb, Janssen, Alkermes, Takeda; and has received advisory board fees from Indivior and Alkermes. Dr Sajatovic has received research grants within past 3 years from Neurelis, Intra-Cellular, Merck, Otsuka, and Alkermes; has received consulting fees in the past year from Otsuka, Lundbeck, Janssen, Teva, and Medscape; has received royalties in the past year from Springer Press, Johns Hopkins University Press, Oxford Press, and UpToDate; and has received compensation for preparation of/participation in CME activities in the past year from American Physician’s Institute (CMEtoGo), American Epilepsy Society, and Clinical Care Options. Dr Saklad has received consulting fees from Alkermes, Genomind, Janssen, Karuna, Lundbeck, and Otsuka and has received honoraria for speaking/teaching from Otsuka PsychU, Neurocrine, Teva, and Texas Society of Health System Pharmacists. Dr Correll has stock options in Cardio Diagnostics, Kuleon Biosciences, LB Pharma, Medlink, Mindpax, Quantic, and Terran; has received consulting fees from AbbVie, Acadia, Adock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Delpor, Denovo, Eli Lilly, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedInCell, MedLink, Merck, Mindpax, Mitsubishi Tanabe Pharma, Maplight, Mylan, Neumora Therapeutics, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Saladax, Sanofi, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Tabuk, Takeda, Teva, Terran, Tolmar, Vertex, Viatris and Xenon; has received grant/research support from Boehringer-Ingelheim, Janssen, and Takeda; has received honoraria for speaking/teaching from AbbVie, Angelini, Aristo, Boehriger-Ingelheim, Cerevel, Damitsa, Gedeon Richter, Hikma, IntraCellular Therapies, Janssen/J&J, Karuna, Lundbeck, Mitsubishi Tanabe Pharma, Mylan, Otsuka, Recordati, Seqirus, Sunovion, Tabuk, Takeda, and Viatris; has received advisory board fees from AbbVie, Allergan, Angelini, Boehringer Ingelheim, Bristol-Myers Squibb, Cerevel, Compass, Gedeon Richter, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedInCell, Merck, Neurelis, Neurocrine, Newron, Novo Nordisk, Otsuka, Recordati, Rovi, Sage, Seqirus, Life Science, Sunovion, Supernus, Teva, Vertex, and Viatris; and has received royalties from UpToDate.
References (97)

- Yunxi Z, Yifan C, Xiaoying S, et al. Global, regional and national burdens of bipolar disorders in adolescents and young adults: a trend analysis from 1990 to 2019. General Psychiatry. 2024;37(1):e101255. doi.org/10.1136/gpsych-2023-101255
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders..5th ed. 2013.
- Tondo L, Miola A, Pinna M, et al. Differences between bipolar disorder types 1 and 2 support the DSM two-syndrome concept. Int J Bipolar Disord. Aug 3 2022;10(1):21. doi.org/10.1186/s40345-022-00268-2
- Brancati GE, Nunes A, Scott K, et al. Differential characteristics of bipolar I and II disorders: a retrospective, cross-sectional evaluation of clinical features, illness course, and response to treatment. International Journal of Bipolar Disorders. 2023/07/14 2023;11(1):25. doi.org/10.1186/s40345-023-00304-9
- Rosa AR, Marco M, Fachel JM, et al. Correlation between drug treatment adherence and lithium treatment attitudes and knowledge by bipolar patients. Prog Neuropsychopharmacol Biol Psychiatry. Jan 30 2007;31(1):217–24. doi.org/10.1016/j.pnpbp.2006.08.007
- Velligan DI, Wang M, Diamond P, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. Sep 2007;58(9):1187–92. doi.org/10.1176/ps.2007.58.9.1187
- Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. Sep 2010;16(5):306–24. doi.org/10.1097/01.pra.0000388626.98662.a0
- Colom F, Vieta E, Tacchi MJ, et al. Identifying and improving non-adherence in bipolar disorders. Bipolar Disord. 2005;7 Suppl 5:24–31. doi.org/10.1111/j.1399-5618.2005.00248.x
- Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar disorder patients. Hum Psychopharmacol. Mar 2008;23(2):95–105. doi.org/10.1002/hup.908
- Tohen M, Goldberg JF, Hassoun Y, et al. Identifying profiles of patients with bipolar I disorder who would benefit from maintenance therapy with a long-acting injectable antipsychotic. J Clin Psychiatry. Jun 16 2020;81(4)doi:10.4088/JCP.OT19046AH1
- Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39–59. doi.org/10.1007/s40263-020-00779-5
- Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. Oct 2013;12(3):216–26. doi.org/10.1002/wps.20060
- Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475–1492. doi.org/10.2147/ndt.S167485
- Sajatovic M, Ross R, Legacy SN, et al. Identifying patients and clinical scenarios for use of long-acting injectable antipsychotics - expert consensus survey part 1. Neuropsychiatr Dis Treat. 2018;14:1463–1474. doi.org/10.2147/ndt.S167394
- Brissos S, Veguilla MR, Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. Oct 2014;4(5):198–219. doi.org/10.1177/2045125314540297
- Vgontzas AN, Paschalidou A, Simos PG, et al. Impact of long-acting injectable antipsychotics vs. oral medication on relapses of patients with psychosis and bipolar disorder. Psychiatry Res. Feb 2024;332:115676. doi.org/10.1016/j.psychres.2023.115676
- Muzina DJ, Momah C, Eudicone JM, et al. Aripiprazole monotherapy in patients with rapid-cycling bipolar I disorder: an analysis from a long-term, double-blind, placebo-controlled study. Int J Clin Pract. May 2008;62(5):679–87. doi.org/10.1111/j.1742-1241.2008.01735.x
- Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488–501. doi.org/10.2174/138161210790361461
- Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. Aug 2003;28(8):1400–11. doi.org/10.1038/sj.npp.1300203
- Thase ME, Jonas A, Khan A, et al. Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of 2 randomized, placebo-controlled studies. J Clin Psychopharmacol. Feb 2008;28(1):13–20. doi.org/10.1097/jcp.0b013e3181618eb4
- Keck PE, Jr., Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry. Sep 2003;160(9):1651–8. doi.org/10.1176/appi.ajp.160.9.1651
- Calabrese JR, Jin N, Johnson B, et al. Aripiprazole once-monthly as maintenance treatment for bipolar I disorder: a 52-week, multicenter, open-label study. Int J Bipolar Disord. Jun 10 2018;6(1):14. doi.org/10.1186/s40345-018-0122-z
- Calabrese JR, Sanchez R, Jin N, et al. Efficacy and safety of aripiprazole once-monthly in the maintenance treatment of bipolar i disorder: a double-blind, placebo-controlled, 52-week randomized withdrawal study. J Clin Psychiatry. Mar 2017;78(3):324–331. doi.org/10.4088/JCP.16m11201
- ABILIFY ASIMTUFII (aripiprazole) extended-release injectable suspension, for intramuscular use [package insert]. Otsuka America Pharmaceutical, Inc.2025.
- ABILIFY MAINTENA (aripiprazole) for extended-release injectable suspension, for intramuscular use [package insert]. Otsuka America Pharmaceutical, Inc.2025.
- Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. Int J Neuropsychopharmacol. May 2009;12(4):441–5. doi.org/10.1017/S1461145708009498
- Rosa AR, Magalhaes PV, Czepielewski L, et al. Clinical staging in bipolar disorder: focus on cognition and functioning. J Clin Psychiatry. May 2014;75(5):e450–6. doi.org/10.4088/JCP.13m08625
- Oliva V, Fico G, De Prisco M, et al. Bipolar disorders: an update on critical aspects. Lancet Reg Health Eur. Jan 2025;48:101135. doi.org/10.1016/j.lanepe.2024.101135
- Tohen M, Vieta E, Gonzalez-Pinto A, Reed C, Lin D. Baseline characteristics and outcomes in patients with first episode or multiple episodes of acute mania. J Clin Psychiatry. Mar 2010;71(3):255–61. doi.org/10.4088/JCP.08m04580
- Ramsey CM, Spira AP, Mojtabai R, Eaton WW, et al. Lifetime manic spectrum episodes and all-cause mortality: 26-year follow-up of the NIMH Epidemiologic Catchment Area Study. J Affect Disord. Oct 2013;151(1):337–42. doi.org/10.1016/j.jad.2013.06.019
- Vieta E, Tohen M, McIntosh D, et al. Early use of long-acting injectable antipsychotics in bipolar disorder type I: An expert consensus. Bipolar Disord. Oct 22 2024;doi:10.1111/bdi.13498
- D’Agostino A, Aguglia A, Barbui C, et al. Off-label long acting injectable antipsychotics in real-world clinical practice: a cross-sectional analysis of prescriptive patterns from the STAR Network DEPOT study. BMC Psychiatry. Jun 30 2022;22(1):442. doi.org/10.1186/s12888-022-04071-2
- Lähteenvuo M, Tanskanen A, Taipale H, et al. Real-world effectiveness of pharmacologic treatments for the prevention of rehospitalization in a Finnish nationwide cohort of patients with bipolar disorder. JAMA Psychiatry. Apr 1 2018;75(4):347–355. doi.org/10.1001/jamapsychiatry.2017.4711
- Gigante AD, Lafer B, Yatham LN. Long-acting injectable antipsychotics for the maintenance treatment of bipolar disorder. CNS Drugs. May 1 2012;26(5):403–20. doi.org/10.2165/11631310-000000000-00000
- Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analyses of randomized controlled trials. Int J Neuropsychopharmacol. Sep 2016;19(9)doi:10.1093/ijnp/pyw038
- Lin CH, Chan HY, Hsu CC, et al. Time to rehospitalization in patients with bipolar mania discharged on long-acting injectable or oral antipsychotics. J Affect Disord. Jan 15 2021;279:292–298. doi.org/10.1016/j.jad.2020.10.023
- Bartoli F, Cavaleri D, Nasti C, et al. Long-acting injectable antipsychotics for the treatment of bipolar disorder: evidence from mirror-image studies. Ther Adv Psychopharmacol. 2023;13:20451253231163682. doi.org/10.1177/20451253231163682
- Lohman MC, Scott V, Verma M, et al. Distribution and correlates of long-acting injectable antipsychotic use among community mental health center patients. Psychiatry Res. Mar 2025;345:116378. doi.org/10.1016/j.psychres.2025.116378
- de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs. Sep 2015;29(9):773–99. doi.org/10.1007/s40263-015-0278-3
- Kumar A, Singh H, Mishra A, et al. Aripiprazole: an FDA approved bioactive compound to treat schizophrenia- a mini review. Curr Drug Discov Technol. 2020;17(1):23–29. doi.org/10.2174/1570163815666181008151718
- Cookson J, Pimm J. Partial agonists of dopamine receptors: mechanisms and clinical effects of aripiprazole, brexpiprazole and cariprazine. BJPsych Advances. 2023;29(2):145–150. doi.org/10.1192/bja.2021.49
- Taylor D, Chithiramohan R, Grewal J, et al. Dopamine partial agonists: a discrete class of antipsychotics. Int J Psychiatry Clin Pract. Sep 2023;27(3):272–284. doi.org/10.1080/13651501.2022.2151473
- Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–777. doi.org/10.2147/TCRM.S117321
- Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. Oct 2018;17(3):330–340. doi.org/10.1002/wps.20579
- Citrome L. Aripiprazole long-acting injectable formulations for schizophrenia: aripiprazole monohydrate and aripiprazole lauroxil. Expert Rev Clin Pharmacol. 2016;9(2):169–86. doi.org/10.1586/17512433.2016.1121809
- Pacchiarotti I, Tiihonen J, Kotzalidis GD, et al. Long-acting injectable antipsychotics (LAIs) for maintenance treatment of bipolar and schizoaffective disorders: A systematic review. Eur Neuropsychopharmacol. Apr 2019;29(4):457–470. doi.org/10.1016/j.euroneuro.2019.02.003
- Kotzalidis GD, Rapinesi C, Chetoni C, et al. Aripiprazole IM depot as an option for the treatment of bipolar disorder. Expert Opin Pharmacother. Aug 2021;22(11):1407–1416. doi.org/10.1080/14656566.2021.1910236
- Dratcu L, Bobmanuel S, Davies W, et al. A UK panel consensus on the initiation of aripiprazole for the treatment of bipolar mania. Int J Psychiatry Clin Pract. Oct 2012;16(4):244–58. doi.org/10.3109/13651501.2012.709865
- Fountoulakis KN, Vieta E, Schmidt F. Aripiprazole monotherapy in the treatment of bipolar disorder: a meta-analysis. J Affect Disord. Oct 2011;133(3):361–70. doi.org/10.1016/j.jad.2010.10.018
- McIntyre RS, Such P, Yildirim M, et al. Safety and efficacy of aripiprazole 2-month ready-to-use 960 mg: secondary analysis of outcomes in adult patients with bipolar I disorder in a randomized, open-label, parallel-arm, pivotal study. Curr Med Res Opin. Jul 2023;39(7):1021–1030. doi.org/10.1080/03007995.2023.2219155
- Ratheesh A, Hett D, Ramain J, et al. A systematic review of interventions in the early course of bipolar disorder I or II: a report of the International Society for Bipolar Disorders Taskforce on early intervention. Int J Bipolar Disord. Jan 3 2023;11(1):1. doi.org/10.1186/s40345-022-00275-3
- Bell Lynum KS, Castro CF, Zhang Z, et al. Aripiprazole once-monthly for the treatment of adult patients with earlier-stage bipolar I disorder: a post hoc analysis of data from a double-blind, placebo-controlled, 52-week randomized withdrawal trial. Int J Bipolar Disord. Oct 27 2024;12(1):37. doi.org/10.1186/s40345-024-00358-3
- Paans O, Tilborg JL, Kamperman AM, et al. Psychotropic comedication trends in long-term lithium treatment for older adults with bipolar disorder: A 10-year analysis. J Affect Disord. Jul 1 2025;380:366–374. doi.org/10.1016/j.jad.2025.03.091
- Kim AM, Salstein L, Goldberg JF. A systematic review of complex polypharmacy in bipolar disorder: prevalence, clinical features, adherence, and preliminary recommendations for practitioners. J Clin Psychiatry. Jun 1 2021;82(3)doi:10.4088/JCP.20r13263
- Yan T, Greene M, Chang E, et al. medication adherence and discontinuation of aripiprazole once-monthly 400 mg (aom 400) versus oral antipsychotics in patients with schizophrenia or bipolar I disorder: a real-world study using US claims data. Adv Ther. Oct 2018;35(10):1612–1625. doi.org/10.1007/s12325-018-0785-y
- Bell Lynum K, Awasthi S, Huang S, et al. The impact of aripiprazole once-monthly initiation and persistence on concomitant psychiatric medications in adults: diagnosed with bipolar I disorder: a retrospective analysis of claims data from the United States. 2024:
- Harlin M, Yildirim M, Such P, et al. A randomized, open-label, multiple-dose, parallel-arm, pivotal study to evaluate the safety, tolerability, and pharmacokinetics of aripiprazole 2-month long-acting injectable in adults with schizophrenia or bipolar I disorder. CNS Drugs. Apr 2023;37(4):337–350. doi.org/10.1007/s40263-023-00996-8
- McIntyre RS, Such P, Yildirim M, et al. Use of an injection of aripiprazole given once every 2 months (Abilify Asimtufii((R))) in people with bipolar I disorder: a plain language summary of publication. Ther Adv Psychopharmacol. 2024;14:20451253241278830. doi.org/10.1177/20451253241278830
- Goldberg JF, Achtyes ED, Correll CU, et al. Optimizing treatment with aripiprazole monohydrate: pharmacokinetic advantages of long-acting injectable formulations, a consensus panel report. J Clin Psychiatry. Jun 13 2025;86(2)doi:10.4088/JCP.plunlai2424ah1
- Sajatovic M. Bipolar disorder: disease burden. Am J Manag Care. Jun 2005;11(3 Suppl):S80–4.
- Bessonova L, Ogden K, Doane MJ, et al. The economic burden of bipolar disorder in the United States: a systematic literature review. Clinicoecon Outcomes Res. 2020;12:481–497. doi.org/10.2147/CEOR.S259338
- Semahegn A, Torpey K, Manu A, et al. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: a systematic review and meta-analysis. Systematic Reviews. 2020/01/16 2020;9(1):17. doi.org/10.1186/s13643-020-1274-3
- Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449–468. doi.org/10.2147/ppa.S124658
- Belete H, Ali T, Legas G. Relapse and clinical characteristics of patients with bipolar disorders in central ethiopia: a cross-sectional study. Psychiatry J. 2020;2020:8986014. doi.org/10.1155/2020/8986014
- Rosenblat JD, Simon GE, Sachs GS, et al. Factors that impact treatment decisions: results from an online survey of individuals with bipolar and unipolar depression. Prim Care Companion CNS Disord. Nov 1 2018;20(6)doi:10.4088/PCC.18m02340
- Rosenblat JD, Simon GE, Sachs GS, et al. Treatment effectiveness and tolerability outcomes that are most important to individuals with bipolar and unipolar depression. J Affect Disord. Jan 15 2019;243:116–120. doi.org/10.1016/j.jad.2018.09.027
- Youn H, Lee MS, Jeong HG, et al. Evaluation of factors associated with medication adherence in patients with bipolar disorder using a medication event monitoring system: a 6-month follow-up prospective study. Ann Gen Psychiatry. Aug 23 2022;21(1):33. doi.org/10.1186/s12991-022-00411-4
- Arnold MJ. Management of bipolar disorder: guidelines from the VA/DoD. Am Fam Physician. Jun 2024;109(6):585–587.
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 03 2018;20(2):97–170. doi.org/10.1111/bdi.12609
- Achtyes E, Simmons A, Skabeev A, et al. Patient preferences concerning the efficacy and side-effect profile of schizophrenia medications: a survey of patients living with schizophrenia. BMC Psychiatry. Sep 12 2018;18(1):292. doi.org/10.1186/s12888-018-1856-y
- Novick D, Montgomery W, Treuer T, et al. Relationship of insight with medication adherence and the impact on outcomes in patients with schizophrenia and bipolar disorder: results from a 1-year European outpatient observational study. BMC Psychiatry. Aug 5 2015;15:189. doi.org/10.1186/s12888-015-0560-4
- Sajatovic M, Ignacio RV, West JA, et al. Predictors of nonadherence among individuals with bipolar disorder receiving treatment in a community mental health clinic. Compr Psychiatry. Mar-Apr 2009;50(2):100–7. doi.org/10.1016/j.comppsych.2008.06.008
- Read J. The experiences of 585 people when they tried to withdraw from antipsychotic drugs. Addict Behav Rep. Jun 2022;15:100421. doi.org/10.1016/j.abrep.2022.100421
- Correll CU, Detraux J, De Lepeleire J, et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. Jun 2015;14(2):119–36. doi.org/10.1002/wps.20204
- Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. Oct 2015;14(3):339–47. doi.org/10.1002/wps.20252
- Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. Nov 14 2017;15(8):1192–1207. doi.org/10.2174/1570159x15666170413115754
- Schneider-Thoma J, Chalkou K, Dörries C, et al. Comparative efficacy and tolerability of 32 oral and long-acting injectable antipsychotics for the maintenance treatment of adults with schizophrenia: a systematic review and network meta-analysis. Lancet. Feb 26 2022;399(10327):824–836. doi.org/10.1016/s0140-6736(21)01997-8
- Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophrenia research. 2003;61(2-3):123–136.
- Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. Mar 2017;78(3):e264-e278. doi.org/10.4088/JCP.16r10832
- Solmi M, Fornaro M, Caiolo S, et al. Efficacy and acceptability of pharmacological interventions for tardive dyskinesia in people with schizophrenia or mood disorders: a systematic review and network meta-analysis. Mol Psychiatry. Mar 2025;30(3):1207–1222. doi.org/10.1038/s41380-024-02733-z
- Solmi M, Pigato G, Kane JM, et al. Treatment of tardive dyskinesia with VMAT-2 inhibitors: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2018;12:1215–1238. doi.org/10.2147/dddt.S133205
- Cahling L, Berntsson A, Bröms G, et al. Perceptions and knowledge of antipsychotics among mental health professionals and patients. BJPsych Bull. Oct 2017;41(5):254–259. doi.org/10.1192/pb.bp.116.055483
- Blackwood C, Sanga P, Nuamah I, et al. Patients’ preference for long-acting injectable versus oral antipsychotics in schizophrenia: results from the Patient-Reported Medication Preference Questionnaire. Patient Prefer Adherence. 2020;14:1093–1102. doi.org/10.2147/PPA.S251812
- Chakrabarti S. Treatment-adherence in bipolar disorder: A patient-centred approach. World J Psychiatry. Dec 22 2016;6(4):399–409. doi.org/10.5498/wjp.v6.i4.399
- Doane MJ, Raymond K, Saucier C, et al. Unmet needs with antipsychotic treatment in schizophrenia and bipolar I disorder: patient perspectives from qualitative focus groups. BMC Psychiatry. Apr 12 2023;23(1):245. doi.org/10.1186/s12888-023-04746-4
- Fitzgerald HM, Shepherd J, Bailey H, et al. Treatment goals in schizophrenia: a real-world survey of patients, psychiatrists, and caregivers in the United States, with an analysis of current treatment (long-acting injectable vs oral antipsychotics) and goal selection. Neuropsychiatr Dis Treat. 2021;17:3215–3228. doi.org/10.2147/NDT.S330936
- Pappa S, Yildirim M, Loomer S, et al. Exploring patient, caregiver, and prescriber preferences for an injectable antipsychotic administered every 2 months for the maintenance treatment of schizophrenia: a multicenter qualitative interview study conducted in Europe. Patient Prefer Adherence. 2025;19:1179–1195. doi.org/10.2147/PPA.S520160
- Vieta E, Tohen M, McIntosh D, et al. Early use of long-acting injectable antipsychotics in bipolar disorder type I: An expert consensus. Bipolar Disord. Feb 2025;27(1):7–16. doi.org/10.1111/bdi.13498
- Yildiz A, Sachs GS, Turgay A. Pharmacological management of agitation in emergency settings. Emerg Med J. Jul 2003;20(4):339–46. doi.org/10.1136/emj.20.4.339
- Currier GW. Atypical antipsychotic medications in the psychiatric emergency service. J Clin Psychiatry. 2000;61 Suppl 14:21–6.
- Weiden PJ, Roma RS, Velligan DI, et al. The challenge of offering long-acting antipsychotic therapies: a preliminary discourse analysis of psychiatrist recommendations for injectable therapy to patients with schizophrenia. J Clin Psychiatry. Jun 2015;76(6):684–90. doi.org/10.4088/JCP.13m08946
- Priebe S, Conneely M, McCabe R, et al. What can clinicians do to improve outcomes across psychiatric treatments: a conceptual review of non-specific components. Epidemiol Psychiatr Sci. Aug 15 2019;29:e48. doi.org/10.1017/S2045796019000428
- Gignac A, McGirr A, Lam RW, et al. Course and outcome following a first episode of mania: four-year prospective data from the Systematic Treatment Optimization Program (STOP-EM). J Affect Disord. Apr 1 2015;175:411–7. doi.org/10.1016/j.jad.2015.01.032
- Cerullo MA, Strakowski SM. The prevalence and significance of substance use disorders in bipolar type I and II disorder. Subst Abuse Treat Prev Policy. Oct 1 2007;2:29. doi.org/10.1186/1747-597x-2-29
- Kupka R, Hillegers M. Early intervention and staging bipolar disorder: conceptual and clinical dilemmas. Eur Neuropsychopharmacol. Oct 2022;63:9–11. doi.org/10.1016/j.euroneuro.2022.07.010
- Macfadden W, Alphs L, Haskins JT, et al. A randomized, double-blind, placebo-controlled study of maintenance treatment with adjunctive risperidone long-acting therapy in patients with bipolar I disorder who relapse frequently. Bipolar Disord. Dec 2009;11(8):827–39. doi.org/10.1111/j.1399-5618.2009.00761.x
- McIntyre RS, Such P, Yildirim M, et al. Use of an injection of aripiprazole given once every 2 months (Abilify Asimtufii(®)) in people with bipolar I disorder: a plain language summary of publication. Ther Adv Psychopharmacol. 2024;14:20451253241278830. doi.org/10.1177/20451253241278830
This PDF is free for all visitors!