On February 24, 2025, the US Food and Drug Administration (FDA) issued an informational notice on its website bearing the title “Information on Clozapine.”1 Contained within the terse 200 word notice was language long sought by providers, patients, their families, and caregivers, wording that encapsulated the frustration experienced by all who care for individuals living with schizophrenia. This frustration was rooted in the reality that access to clozapine, the only approved medication for treatment-resistant schizophrenia (TRS), was beset by administrative and dispensing burdens related to an FDA mandated risk evaluation and mitigation strategies (REMS) program created to detect and minimize morbidity and potential mortality from severe neutropenia (also known as agranulocytosis).2
Although the risk of severe neutropenia with clozapine still exists, FDA has determined that the REMS program for clozapine is no longer necessary to ensure the benefits of the medicine outweigh that risk. Eliminating the REMS is expected to decrease the burden on the health care delivery system and improve access to clozapine.
Updated clozapine prescribing information (PI) will likely eliminate references to REMS and related laboratory reporting requirements,1 but there was no indication yet from FDA of any modification to the absolute neutrophil (ANC) monitoring scheme. Inaction regarding ANC monitoring is a potential source of consternation, as the PI currently recommends ad infinitum monthly ANC monitoring starting at 1 year despite evidence that severe neutropenia risk is highest in the first 18 weeks of treatment, that this risk diminishes greatly over time and may be lower than previously estimated.3–7 A 2023 meta-analysis of clozapine related hematologic outcomes reviewed 14 studies published January 1975–April 2023 (n = 2,354), mostly retrospective, with mean observation duration 800 days and found that the rate of agranulocytosis was only 0.47%,5 roughly half that described in the early literature.8 While this paper did not provide comparative data vs nonclozapine antipsychotics (NC APs), a 2019 meta-analysis found a nonsignificant difference in severe neutropenia risk between clozapine and NC-APs (risk ratio 1.65, 95% confidence interval [CI] 0.58–4.71).4
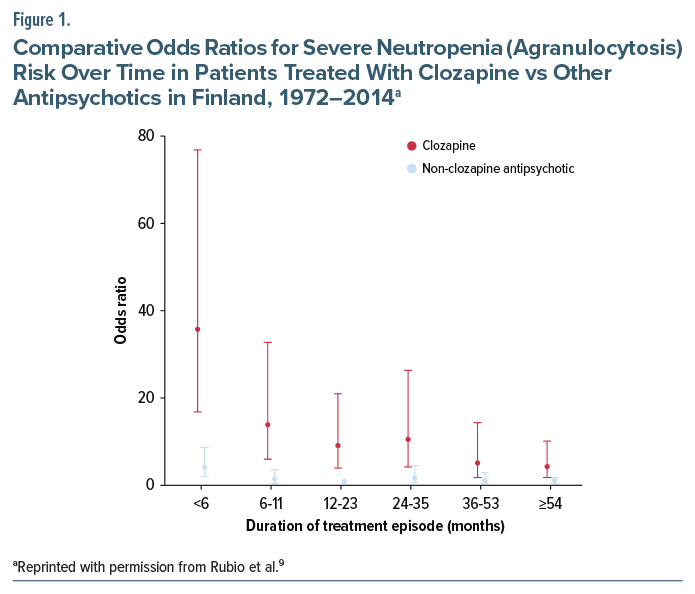
For both meta-analyses, the short mean observation period might have overlooked later onset cases, thereby distorting longitudinal severe neutropenia risk estimates. Fortunately, a 2024 study addresses those limitations by exploring comparative long-term agranulocytosis risk between clozapine and NC-APs in Finnish patients with a schizophrenia spectrum disorder (SCZ) prescribed antipsychotics 1972–2014.9 After excluding those with a history of agranulocytosis prior to the first SCZ diagnosis, the sample comprised 61,769 individuals of mean age 46.7 years followed for up to 22 years of treatment. The median duration of uninterrupted AP treatment episodes was 398 days (interquartile range 95–1,193) for clozapine and 579 days (151–1,607) for NC-APs. As seen in Figure 1, the peak agranulocytosis rate for clozapine occurs in the first 6 months and drops dramatically after that. The adjusted odds ratio 95% CIs for clozapine and NC-APs overlap at month 36, with a small numerical difference at months 54+ between the lower limit of the CI for clozapine (1.86) and the upper limit for NC-APs (1.78).9 Finally, among the 398 agranulocytosis cases (231 clozapine; 167 NC-APs), fatality rates were extremely small: 2.81 per 10,000 exposed to clozapine and 0.63 per 10,000 exposed to NC-APs.
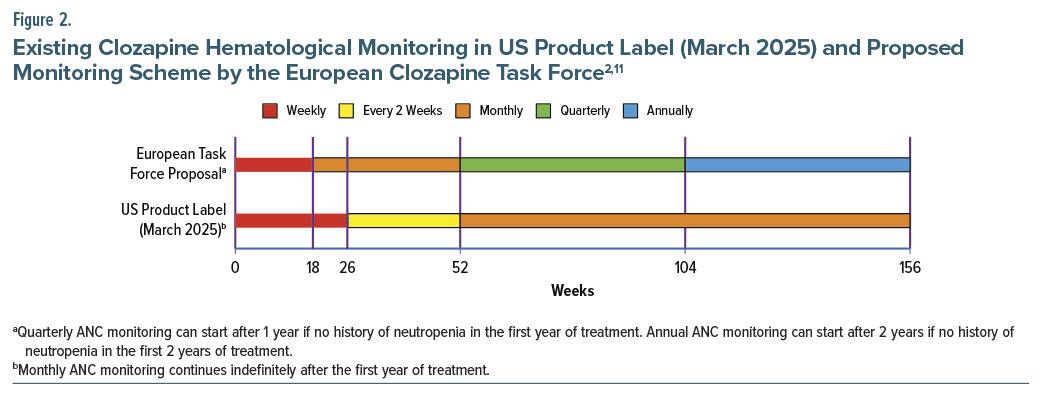
These reassessments of clozapine’s neutropenia risk have not gone unnoticed and are reflected in worldwide initiatives and proposals questioning the need for indefinite ANC monitoring. On May 18, 2022, the Chilean health ministry altered the clozapine monitoring scheme to greatly reduce ANC frequency after 1 year, the first entity to formalize this new perspective on severe neutropenia risk.10 Previously, ANC monitoring was performed weekly until 18 weeks and monthly thereafter, but this was modified with the following language: “Based on clinical judgment, in people who have been using clozapine continuously for more than one year and who have no history of neutropenia of any origin, a complete blood count with ANC may be performed at most every 56 days.” A group of Dutch authors in 2023 also suggested that, after 18 weeks of weekly ANC monitoring, a shared decision-making approach be implemented, with subsequent monitoring frequency decided together by the prescriber and the “well-informed patient.”6 As an attempt to provide formal guidance and further the worldwide discussion on this topic, an expert statement from the European Clozapine Task Force was published in early 2025.11 As shown in Figure 2, their recommendations included commencement of monthly ANC after 18 weeks of treatment, then moving to quarterly ANC monitoring after 1 year if no history of neutropenia in the first year of treatment.11 Annual ANC monitoring can start after 2 years if there is no history of neutropenia in the first 2 years of treatment. The purpose of annual monitoring is not to detect severe neutropenia, but rather as surveillance for a rare event, but one which may be numerically greater in clozapine-treated patients, a risk of hematologic malignancy.12 Adding to the chorus of entities touting evidence based criteria, a Treatment Response and Resistance in Psychosis Working Group that previously issued a Delphi consensus guideline on clozapine optimization13 has a manuscript under review on ANC monitoring (Robert O. Cotes, MD, personal communication).
This relative disconnect between recent research findings on extent and trajectory of severe neutropenia risk and the extant US PI ANC monitoring recommendation leaves clinicians in a quandary about whether deviating from the PI might be unsafe or represent substandard care. This situation is not unique to clozapine and is seen in other instances wherein studies demonstrate that certain PI precautions are not warranted. Lithium represents an extreme example in which the PI recommendation for multiple daily dosing is at odds with data indicating that single daily dosing incurs lower risk of renal insufficiency,14 and with no demonstrable efficacy difference.15 Another example of the relative mismatch between recent data and FDA recommendations is evidence that ileus and pneumonia carry greater morbidity and mortality risk than does severe neutropenia in individuals using clozapine16 despite lack of historical monitoring systems for these adverse effects. Moreover, there is no warning regarding use of alternative treatments with no evidence for efficacy in TRS in clozapine-eligible individuals despite the fact that clozapine is the only approved drug for TRS and that its use in that population is associated with reduced mortality.17
Anticipating that ANC monitoring ad infinitum may persist in the revised US clozapine PI, thus not entirely reflecting the most recent evidence, below is some guidance for providers to consider in navigating this area of clinical practice.
- Timeline of severe neutropenia risk: Unquestionably, the vast majority of events occur within the initial 18 weeks of treatment. After a sharp decline, the risk plateaus after year 2,7 and between years 3 and 4.5 is not significantly different than NC-APs (Figure 1).9 Starting at 54 months of treatment, the risk for clozapine is numerically higher than the comparison group (NC-APs between 1 and 2 years); however, this is a small increased risk of a very rare outcome.9
- Risks and alternatives to lifetime monitoring: While bloodwork in and of itself does not carry more than minimal risk, it can be a deterrent from starting or continuing clozapine treatment. Given the clear benefits of clozapine on symptoms and mortality,18–20 barriers to initiation can be associated with potential negative outcomes. In practical terms, if a patient prefers ongoing monthly monitoring, it is appropriate to do so; however, if mandated lifetime monitoring threatens the ability of the patient to initiate or stay on treatment, the risk of neutropenia should be weighed against the risk of not starting or interrupting clozapine treatment. In other words, it is important to consider the risks of getting clozapine, but also the risks of not starting or remaining on clozapine when it is indicated.
- Documentation of shared decision-making and clinical rationale: Whenever deviating from product label recommendations, it is necessary to document the clinical rationale for doing so. This is an example of language that could be considered:
Although the US clozapine product label recommends lifetime monthly ANC monitoring, upon discussion of the latest evidence in this area, and the risk, benefits, and alternatives with this patient, we have decided to forego monthly ANC monitoring. The rationale for this decision is: (1) There is no history of severe neutropenia in the prior 3 years of clozapine treatment; (2) The literature indicates that while the severe neutropenia risk from clozapine is numerically greater than that of non-clozapine antipsychotics, this risk difference is very low in absolute terms (eg, it is comparable to the risk the patient was exposed to when they initiated nonclozapine treatment in the past, with no monitoring); (3) There are concerns about the ability to continue clozapine treatment given the inconvenience of lifetime bloodwork; (4) Given the association of clozapine treatment with mortality reduction, there may be greater risk to the patient from clozapine discontinuation than from clozapine-induced severe neutropenia; (5) We will request ANC monitoring if clinically indicated, and the patient understands to call this clinician if fever or other signs of infection should occur. This patient understands this rationale and agrees with this decision.
Conclusions
The abolition of REMS means a new era in the use of clozapine. While this change is meant to expand clozapine access, it also means a transition that could result in confusion among prescribers, patients, families, pharmacies, administrators, and other stakeholders. Among the most relevant examples are some mismatches between FDA recommendations for lifetime monitoring and the literature suggesting a relaxation in monitoring after the period of highest neutropenia risk. Here, we provide practical considerations in how to address this issue. Importantly, we want to bring attention to traditionally overlooked issues beyond ANC monitoring: the risks of more common serious adverse events other than neutropenia (eg ileus, pneumonia), and the risk to eligible individuals not receiving clozapine, the only approved drug for TRS. Although there are a variety of suggested approaches to ANC monitoring after the period of highest neutropenia risk, the psychiatric field is increasingly converging on the consensus opinion that indefinite frequent ANC monitoring is not evidence based and is not the standard of care.
Article Information
Published Online: May 5, 2025. https://doi.org/10.4088/JCP.25ac15898
© 2025 Physicians Postgraduate Press, Inc.
J Clin Psychiatry 2025;86(2):25ac15898
To Cite: Meyer JM, Rubio JM. Clozapine monitoring in the post-REMS world: some guidance for clinicians. J Clin Psychiatry 2025;86(2):25ac15898.
Author Affiliations: Department of Psychiatry, University of California San Diego, San Diego, California (Meyer); Department of Psychiatry, Zucker Hillside Hospital, Glen Oaks, New York (Rubio); Institute of Behavioral Science, Feinstein Institutes of Medical Research, Manhasset, New York (Rubio); Northwell Health, New Hyde Park, New York (Rubio).
Corresponding Author: Jonathan M. Meyer, MD, University of California, San Diego, 12974 Carmel Creek Rd, Suite 180, San Diego, CA 92130 ([email protected]).
Relevant Financial Relationships: Dr Meyer reports having received speaking or advising fees in the past 24 months from 4M Therapeutics, AbbVie, Alkermes, Axsome, BioXcel, BMS, Cerevel, ITCI, Neurocrine, Otsuka USA, Relmada, Sumitomo Pharma, and Teva. Dr Rubio reports having received speaking or advising fees in the past 24 months from Bristol Myers Squibb, Teva, and Janssen; research funds from Neurocrine, Alkermes, Saladax, and the National Institutes of Health; and royalties from UpToDate.
Funding/Support: Supported by the National Institutes of Health (K23MH127300 to Dr Rubio). No funding was specifically received for this article.
ORCID: Jonathan M. Meyer: https://orcid.org/0000-0001-7294-4834
 The ASCP Corner, edited by Leslie L. Citrome, MD, MPH, is a collection of brief peer-reviewed, evidence-based articles, authored by American Society of Clinical Psychopharmacology members, that examine the practice of psychopharmacology through the lens of clinical experience. The information contained herein only represents the opinion of the author(s). See more ASCP Corner Articles at Psychiatrist.com/ASCP-Corner
The ASCP Corner, edited by Leslie L. Citrome, MD, MPH, is a collection of brief peer-reviewed, evidence-based articles, authored by American Society of Clinical Psychopharmacology members, that examine the practice of psychopharmacology through the lens of clinical experience. The information contained herein only represents the opinion of the author(s). See more ASCP Corner Articles at Psychiatrist.com/ASCP-Corner
References (20)

- Food and Drug Administration. FDA Postmarket Drug Safety Information for Patients and Providers Information on Clozapine. Food and Drug Administration; 2025. Accessed February 24, 2025. https://wwwfdagov/drugs/postmarket-drug-safetyinformation-patients-and-providers/informationclozapine.
- Clozaril Package Insert. HLS Therapeutics (USA) Inc; 2025.
- Myles N, Myles H, Xia S, et al. Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatr Scand. 2018;138(2):101–109. PubMed CrossRef
- Myles N, Myles H, Xia S, et al. A meta-analysis of controlled studies comparing the association between clozapine and other antipsychotic medications and the development of neutropenia. Aust N Z J Psychiatry. 2019;53(5):403–412. PubMed CrossRef
- Magistri C, Mellini C. Clozapine-associated agranulocytosis: a systematic review. Is it really so frighteningly common? J Clin Psychopharmacol. 2023;43(6):527–533. PubMed CrossRef
- Schulte PFJ, Veerman SRT, Bakker B, et al. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring: can the regulations be relaxed? Schizophr Res. 2024;268:74–81. PubMed CrossRef
- Northwood K, Myles N, Clark SR, et al. Evaluating the epidemiology of clozapine-associated neutropenia among people on clozapine across Australia and Aotearoa New Zealand: a retrospective cohort study. Lancet Psychiatry. 2024;11(1):27–35. PubMed CrossRef
- Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162–167. PubMed CrossRef
- Rubio JM, Kane JM, Tanskanen A, et al. Long-term persistence of the risk of agranulocytosis with clozapine compared with other antipsychotics: a nationwide cohort and case-control study in Finland. Lancet Psychiatry. 2024;11(6):443–450. PubMed CrossRef
- Gabinete del Ministro de Salud (Subsecretaría de Salud Pública - División Jurídica). Modifica Norma Técnica Para el Uso de Clozapina (Resolución Exenta No. 663 - 19 May 2022). Gobierno de Chile; 2022.
- Verdoux H, Bittner RA, Hasan A, et al. The time has come for revising the rules of clozapine blood monitoring in Europe. A joint expert statement from the European Clozapine Task Force. Eur Psychiatry. 2025;68(1):e17. PubMed CrossRef
- Hu Y, Gao L, Zhou L, et al. Rare but elevated incidence of hematological malignancy after clozapine use in schizophrenia: a population cohort study. PLoS Med. 2024;21(12):e1004457. PubMed CrossRef
- Wagner E, Siskind D, Falkai P, et al. Clozapine optimization: a Delphi consensus guideline from the treatment Response and resistance in Psychosis working group. Schizophr Bull. 2023;49(4):962–972. PubMed CrossRef
- Castro VM, Roberson AM, McCoy TH, et al. Stratifying risk for renal insufficiency among lithium-treated patients: an electronic health record study. Neuropsychopharmacology. 2016;41(4):1138–1143. PubMed CrossRef
- Singh LK, Nizamie SH, Akhtar S, et al. Improving tolerability of lithium with a once daily dosing schedule. Am J Ther. 2011;18(4):288–291. PubMed CrossRef
- Partanen JJ, Häppölä P, Kämpe A, et al. High burden of ileus and pneumonia in clozapine-treated individuals with schizophrenia: a Finnish 25-year follow-up register study. Am J Psychiatry. 2024;181(10):879–892. PubMed CrossRef
- Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, et al. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophr Bull. 2019;45(2):315–329. PubMed CrossRef
- Lee BJ, Cotes RO, Mojtabai R, et al. The protective effect of clozapine on suicide: a population mortality study of statewide autopsy records in Maryland. J Clin Psychiatry. 2023;84(3):22m14587. PubMed CrossRef
- Rafizadeh R, Danilewitz M, Bousman CA, et al. Effects of clozapine treatment on the improvement of substance use disorders other than nicotine in individuals with schizophrenia spectrum disorders: a systematic review and meta-analysis. J Psychopharmacol. 2023;37(2):135–143. PubMed CrossRef
- Taipale H, Tanskanen A, Howes O, et al. Comparative effectiveness of antipsychotic treatment strategies for relapse prevention in first-episode schizophrenia in Finland: a population-based cohort study. Lancet Psychiatry. 2025;12(2):122–130. PubMed CrossRef
This PDF is free for all visitors!