Abstract
Objective: Estimate nationwide postpartum depression (PPD) at 2 months prevalence and its related risk factors.
Methods: The representative study sample comprised 7,133 women who were included in a national perinatal population- based survey of all women who gave birth in France in March 2021. Data on maternal characteristics, course of pregnancy/delivery, and child’s health were collected from face-to-face interviews in maternity wards and/or medical records and a self-questionnaire (including the Edinburgh Postnatal Depression Scale [EPDS]) at 2 months postpartum. Women with an EPDS score ≥13 were considered to have PPD. Poisson regression models with robust variance were used to estimate adjusted prevalence ratios (aPRs) for PPD.
Results: PPD prevalence at 2 months was 16.7% (95% CI, [15.7–17.7]). Factors significantly associated with PPD were (1) age ≤29 or ≥40 years (maximum aPR=1.41 95% CI, [1.12–1.77] obtained for 15- to 24-year-olds vs 35- to 39-year-olds); (2) being born in North Africa (1.29 [1.02–1.64] vs France); (3) having a lower level of health literacy (1.23 [1.14–1.35]); (4) having a history of psychological (1.45 [1.24–1.69]) or psychiatric (1.52 [1.23–1.88]) care since adolescence (vs none); (5) receiving little/ no support or good support during pregnancy (1.80 [1.52–2.14] and 1.31 [1.15–1.48] vs receiving very good support); (6) reporting feelings of sadness (1.92 [1.65–2.25]), anhedonia (1.69 [1.36–2.11]), or both (2.61 [2.26–3.01]) during pregnancy (vs none of these feelings); and (7) having had an instrumental vaginal delivery (1.18 [1.01–1.38] vs spontaneous vaginal delivery).
Conclusion: These findings on PPD (prevalence and profile of women at higher risk) could guide clinicians and policies on early identification and preventive support for women in the perinatal period.
J Clin Psychiatry 2025;86(4):25m15818
Author affiliations are listed at the end of this article.
The months following childbirth correspond to a time of high vulnerability for women with regard to the possibility of psychiatric disorder onset or relapse.1 Postpartum depression (PPD) is one of the leading complications for women after childbirth and occurs in between 10% and 20% of mothers worldwide.2,3
PPD is a major public health issue because of its frequency and because of potentially harmful consequences for the mother (with a risk of maternal suicide4), the mother-child dyad, and the child’s development.5,6 These various consequences underline the importance of public health prevention strategies for women at higher risk of PPD and for affected mothers and the need for early detection, intervention, and support. Treatments exist and can significantly improve the health of mothers and their families.
PPD risk factors have been extensively described. Some are linked to biological changes during pregnancy.7 Others are associated with medical history (in particular psychiatric or obstetrical history8–11), medical problems during pregnancy or delivery,9,11–13 child’s characteristics (self-regulation difficulties, state of health…), self-perceived social support or from partners,8,11,13 and demographic or socioeconomic characteristics.10–12,14 As all these factors are closely interrelated, suitable models are required to measure their specific and independent role in PPD.
To date, few studies on PPD have been conducted at the national population level, and few have taken into account the interrelationships between the different risk factors described above. Most of the nationally representative cohort studies are based on relatively old national databases (≤2010).13,15,16 Thus, as they only included diagnosed PPD assigned during hospitalization or hospital outpatient care, the prevalence of PPD among mothers was underestimated.
In France, only 4 cohorts to date have been used to analyze the factors associated with PPD at 2 months. However, none was representative of all the women giving birth in the country, and 3 of the studies were carried out in a few maternity departments with small sample sizes.9,17–19 The 2021 edition of the repeated French National Population-based Perinatal Survey (2021 ENP), which produces and follows perinatal health indicators in France, provided, for the first time, the opportunity to explore the issue of depression in women at 2 months postpartum.20
The aims of the present study were to (1) estimate the prevalence of PPD in mothers in France at 2 months using the 2021 ENP and (2) identify the characteristics associated with a higher risk of PPD in mothers at this same time point.
METHODS
Survey Methodology and Data Collected
The target population was all women giving birth in France in 2021. The 2021 ENP is based on the census of women (≥15 years) who gave birth in France in the same week in March 2021 in all maternity units and birth centers. All women who delivered after at least 22 weeks of amenorrhea and/or had a newborn weighting at least 500 g were eligible. Details and inference to the target population were reported in previous publications.20,21
Data were collected at 2 times: (1) at the maternity ward in a face-to-face interview with a midwife and/or from the mother’s medical file and (2) at 2 months postpartum, via a telephone interview or an online questionnaire depending on the respondent’s preference. Two-thirds (67.5%) of the women interviewed in maternity wards responded to the 2-month postpartum questionnaire.
The interview at the maternity ward collected data on respondents’ demographic and socioeconomic characteristics, behavior before and during pregnancy, and the course of medical surveillance during the prenatal period. The data collected from medical records included maternal history, the course of the pregnancy and of the delivery, and the health status of the child. The 2-month postpartum questionnaire collected data on their experience of pregnancy and delivery, the organization of their return to home, their health (with, in particular, the Edinburgh Postnatal Depression Scale [EPDS; see below]), and that of their children.
Participants
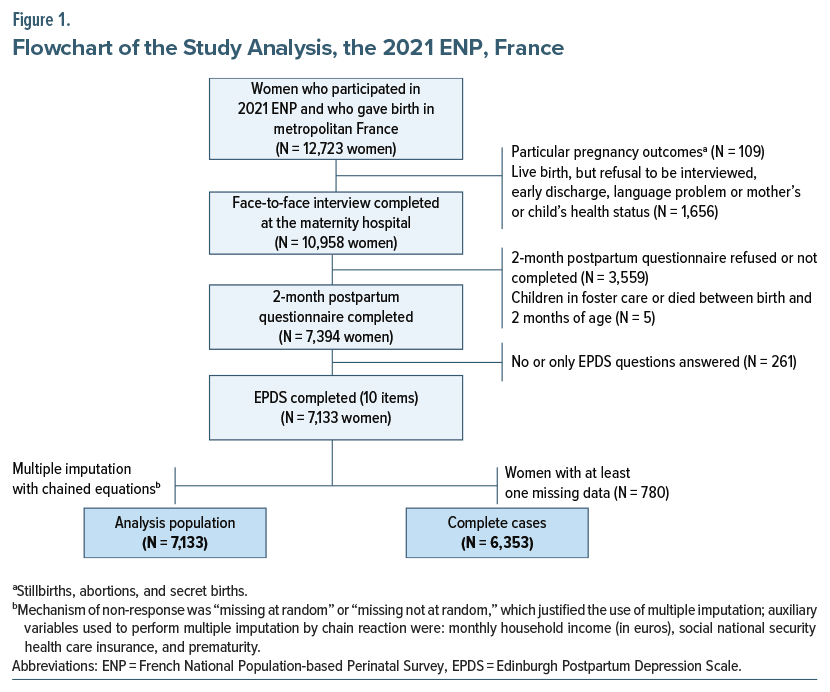
For the present analysis, among the 12,723 women included in the 2021 ENP during their maternity stay in metropolitan France, we selected the 7,133 who were interviewed at the maternity and responded to all the 10 EPDS questions of the 2-month postpartum questionnaire (Figure 1). We excluded those women (1) who had not responded to the face-to-face interview at the maternity ward (n=1,765) and/or at the 2-month postpartum questionnaire (n=3,564) and (2) who did not answer any or some of the 10 EPDS questions (given their small number: 3.5% of respondents at 2 months).
Postpartum depression (PPD). In this study, depressive symptomatology was assessed at 2 months postpartum; this time point is frequently used as mentioned in international literature.22 Indeed, international nosographies consider that perinatal psychiatric disorders occur during pregnancy and up to 4 (DSM-5) or 6 weeks (International Statistical Classification of Diseases, Eleventh Revision [ICD-11]) after delivery and whose symptoms have lasted for at least 2 weeks. Then, by testing symptomatology occurring at 2 months postpartum, we are in agreement with the ICD-11 definition. We used the EPDS, the most widely used tool for assessing perinatal depression in the world. It has been validated in French23 and comprises a 10-item self-administered questionnaire, with each item scoring from 0 to 3.24 A recent meta-analysis based on 58 studies recommended using a score ≥13 to identify women who are highly symptomatic and, therefore, are at higher risk of PPD (sensitivity: 66%, specificity: 95% compared to semi-structured interviews).25 In the present study, an EPDS score ≥13 was considered to be a proxy of PPD. Furthermore, in our study, the prevalence of PPD did not differ according to the type of questionnaire (web or telephone-based) (P = .314), suggesting that the psychometric properties of the EPDS are maintained even if administered by telephone.
Covariates
Studied variables were divided into 5 main themes (see below and Table 1 and Supplementary Table 1 for more details).
Maternal demographic and socioeconomic characteristics. Age at childbirth, country of birth, living with a partner, education level, monthly household financial income, social security health care insurance coverage, professional activity during pregnancy, and health literacy during pregnancy (mean score of the Health Literacy Questionnaire’s sixth domain, “Ability to actively engage with healthcare providers”26).
Medical history. History of medical termination of pregnancy and self-declared mental health care history since adolescence. The latter was assessed using the following 3 questionnaire items: (1) “follow-up with a psychologist for at least 3 months,” (2) “follow-up with a psychiatrist for at least 3 months,” and (3) “hospitalization for a psychological or psychiatric problem.” Respondents who answered “yes” to item 2 and/or 3 were considered to have received psychiatric care and/or hospitalization for a psychological/psychiatric reason. Those who answered “yes” only to item 1 were considered to have received psychological care. Respondents who answered “no” to all 3 questions or “no” to items 1 and 2 without providing a reply for item 3 were considered not to have any mental health care history.
Pregnancy-related characteristics. Multiparity, body mass index (BMI) before pregnancy (in kg/m2), weight change during pregnancy (difference in kg self-declared), self-reported pregnancy-related emergency or walk-in consultation, self-perceived support from loved ones, not having a pregnancy at low obstetrical risk (ie, not meeting the A, A1, and A2 criteria defined in the consensual recommendations of the French National Authority for Health), and finally, declaring feelings of “sadness, despair, hopelessness” and/or “anhedonia” for at least 2 weeks during the pregnancy.
Childbirth-related and child’s health characteristics. Mode of delivery, prematurity, birth weight according to gestational age (small, appropriate, or large, defined according to EPOPé curves adjusted for gestational age and sex27), and child re-hospitalization(s) during the 2 months after leaving the maternity ward.
Statistical Analysis
Data collected at 2 months postpartum were weighted to compensate correct the non-response bias related to the cohort attrition and to ensure the statistical inference of our results to all women who gave birth in France during the same week in March 2021 as shown in Supplementary Table 2.
As the PPD prevalence is greater than 10% in our study population, we chose to use a Poisson model with robust error variance, which provides in this case estimator more interpretable than logistic regression model.28 Crude prevalence ratios (PRs) and adjusted prevalence ratios (aPRs) of having PPD at 2 months were therefore estimated.29 The multivariable model included variables selected based on the literature or study hypotheses (conceptual model) and results from bivariate analyses (P <.2). Fractional polynomials showed a linear relationship between continuous variables included in the models (health literacy score and BMI) and the prevalence of PPD. The multicollinearity of demographic or socio-economic variables was first assessed using the generalized variance inflation factor. A manual descending stepwise procedure was then applied to identify factors independently and significantly associated with each outcome (P<.05).
We imputed missing data for variables with fewer than 15 missing data using the most frequent response modality and taking into account other related information available if necessary. Subsequently, we performed a multiple imputation with chained equations (20 data sets) to take into account the rest of missing data (10.9% global; max=7.0%; min =0.2%—see Supplementary Table 3).30 The imputation model included all variables in the final multivariable model, as well as auxiliary variables known to be associated with the variables to impute (see Figure 1 and Supplementary Table 4). In order to estimate the impact of the multiple imputation with chained equations on the association between the factors studied and PPD, we also performed the multivariable final model on the database before the multiple imputation (ie, complete case analysis) (Figure 1 and Supplementary Table 4).
The final multivariable model was performed on the whole study sample (n=7,133). As a history of mental health is a recognized major risk factor for PPD, this model was also performed on the subgroup of women with no history of psychological/psychiatric care (n =5,564).
A sensitivity analysis was performed to check the robustness of the multivariable model for each group (ie, total sample and subgroup with no history of psychological/psychiatric care) by excluding potential early markers of perinatal depression (ie, “Feeling of sadness, despair, hopelessness and/or anhedonia for at least 2 weeks during the pregnancy,” “Self-perceived support from loved ones during pregnancy,” “Self-declared emergency or walk-in consultation” [see above]).
With the exception of the raw number of respondents, all the results presented are weighted. Unless otherwise indicated, they are also imputed and include percentages and their 95% confidence intervals (95% CIs) for qualitative variables, means with standard deviations (SDs) for quantitative variables, as well as PRs and aPRs, their 95% CIs, and associated P values. PRs are interpreted in the same way as relative risks.28 PPD prevalences according to different relevant EPDS thresholds (9 to 14) are presented in Supplementary Table 5.
All statistical analyses were performed using Stata software version 16/SE (Stata Corp., College Station, TX).
Institutional and Ethical Approval
2021 ENP was approved by the following organizations: the National Council of Statistical Information (October 14, 2019), the Label Committee (Visa n°2021X701SA, November 23, 2020), the Committee of Ethics and Scientists for Research, Studies and Evaluations (June 12, 2020), a Patient Protection Committee (July 7, 2020), and the National Data Protection Authority (DR-2020-391, December 31, 2020). Mothers included in the 2021 ENP survey were informed of the purpose of the study in the maternity ward participated voluntarily. Parental information was provided to mothers who were under 18 years of age (0.1%) if they provided consent.
RESULTS
The motivations and positive effects of multiple imputations (in particular, increased reliability of the aPR) are presented in Supplementary Tables 3 and 4.
Description of the Study Sample
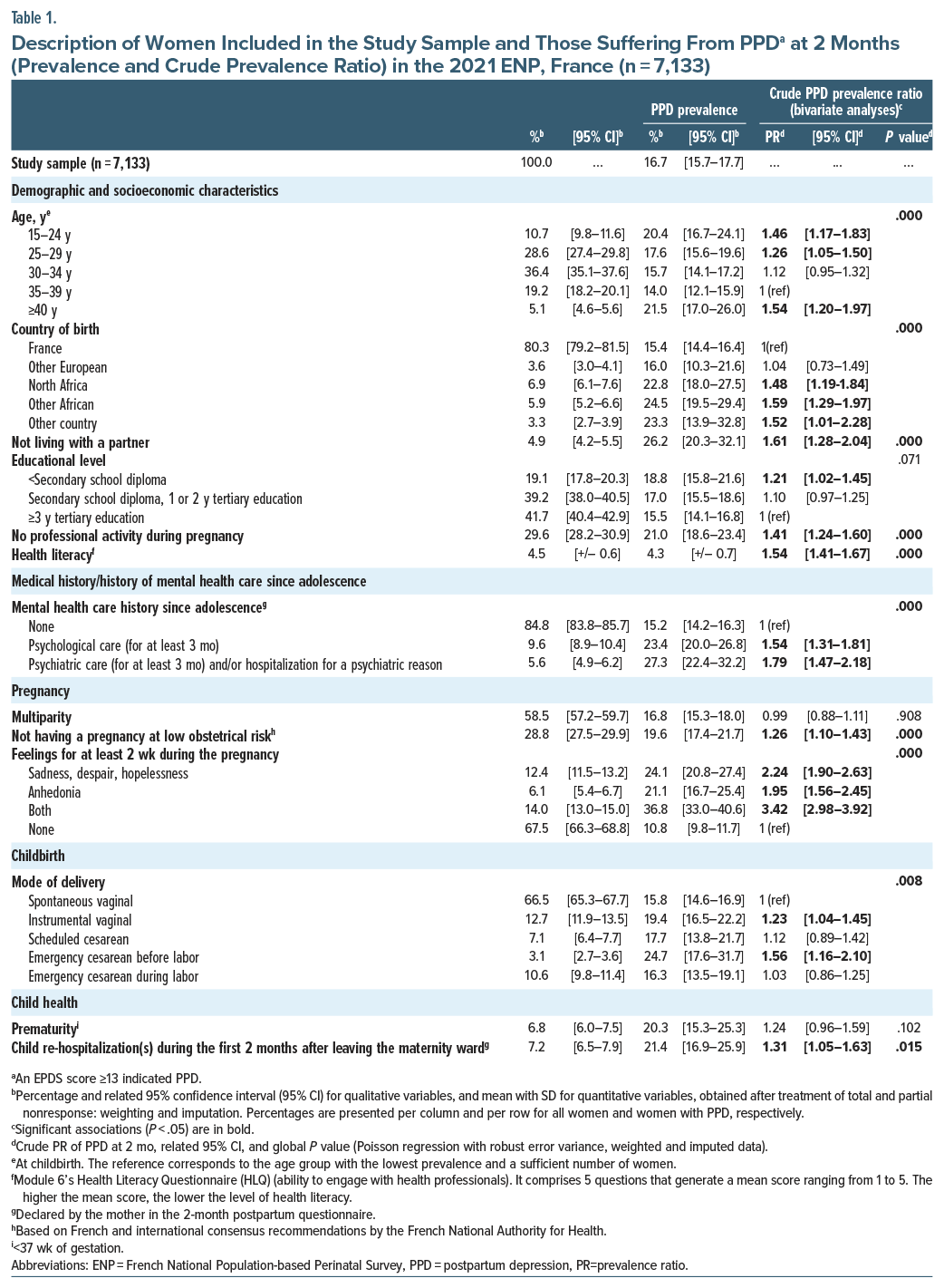
The main characteristics of the 7,133 women included in our analysis are summarized in Table 1 (Supplementary Table 1 for more characteristics).
A majority were between 25 and 34 years of age (65.0%), 80.3% were born in France, 95.1% lived with a partner, 41.7% had a high level of education (ie, ≥3 years tertiary education), and 70.4% had a professional activity during their pregnancy. Three-fifths (58.5%) of the participants were multiparous.
With regard to a history of mental health care since adolescence, 15.2% reported receiving psychological or psychiatric care (hospitalization and/or therapy for more than 3 months).
Prevalence of PPD at 2 Months in France and Associated Factors
Prevalence. In our study, 16.7% (95% CI, [15.7–17.7]) of the women (n=7,133) had PPD at 2 months.
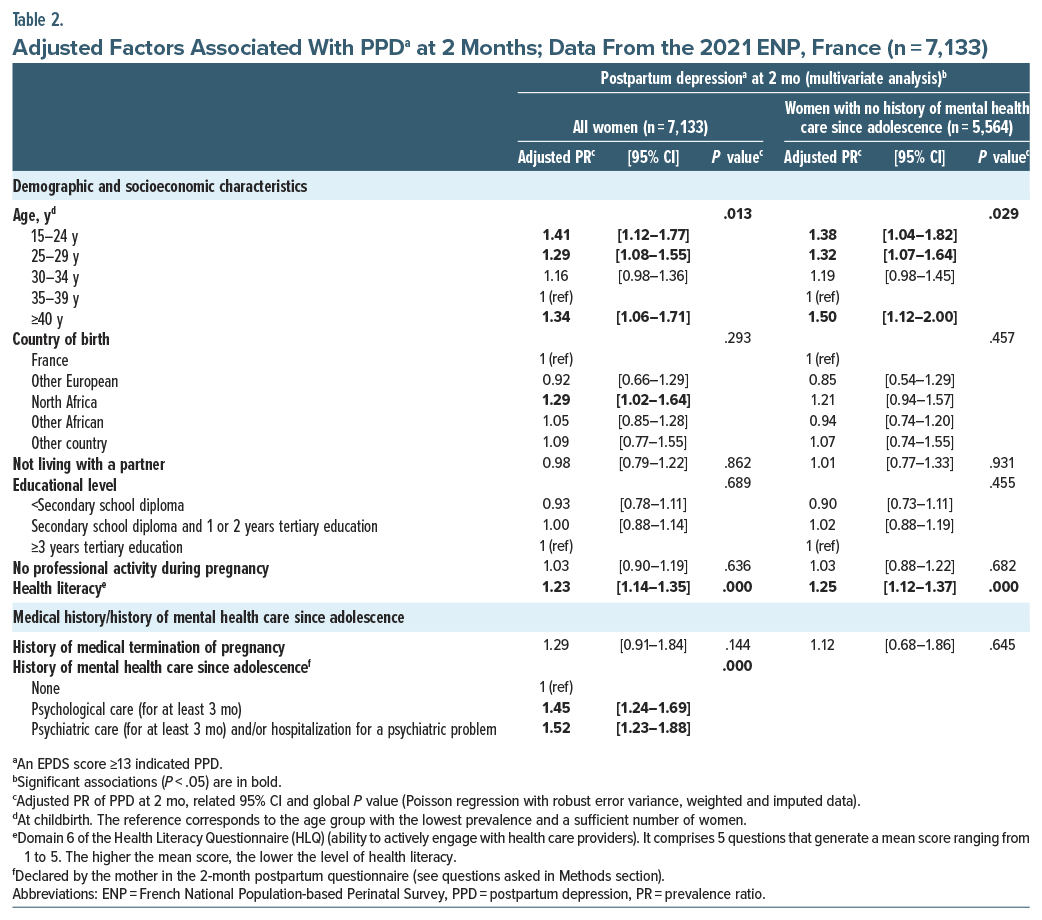
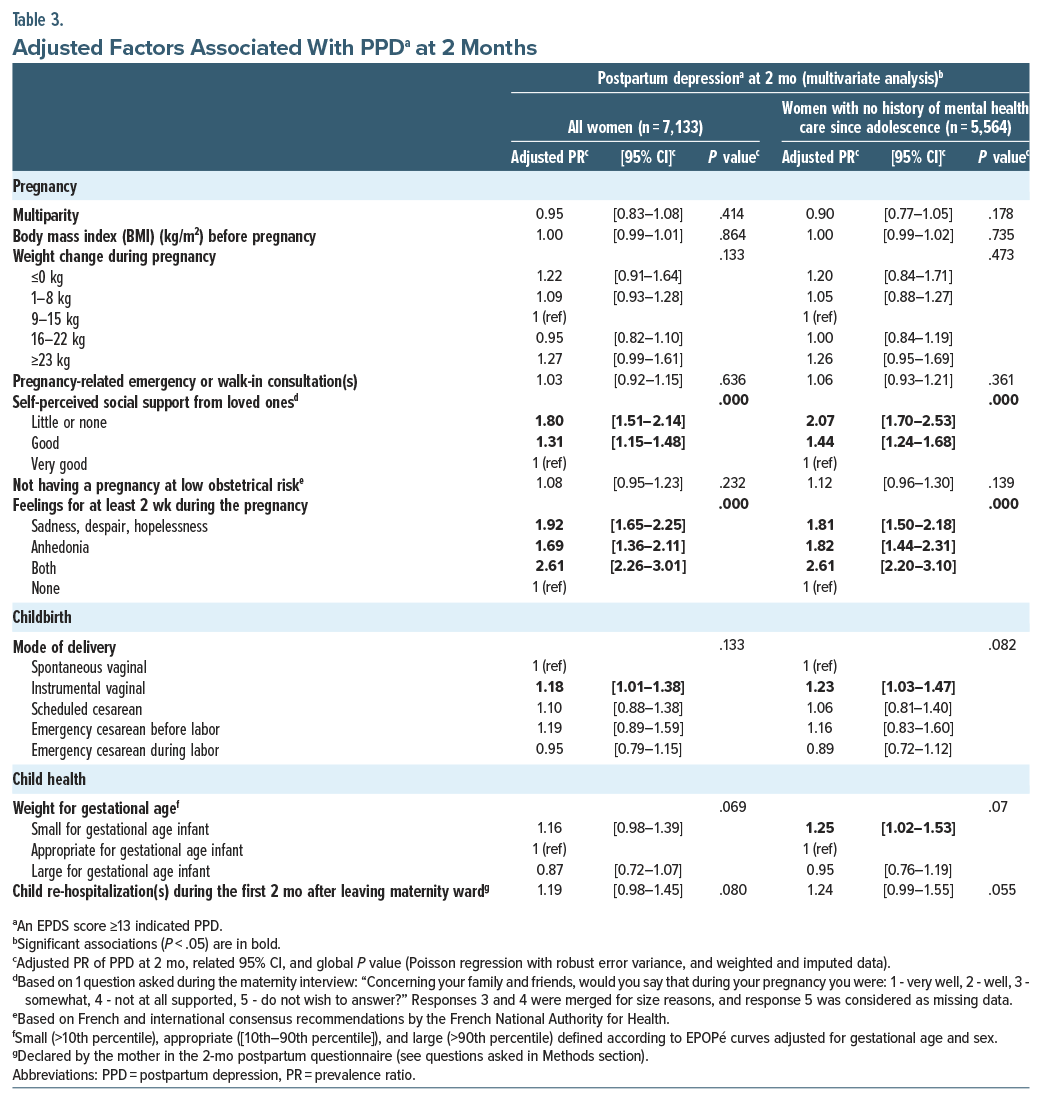
Associated factors. In the multivariable analysis, PPD was independently and significantly associated with several risk factors, which can be divided into 4 categories: demographic and socioeconomic characteristics, medical and mental health care history (Table 2), pregnancy-related characteristics, and childbirth-related characteristics (Table 3). Specifically, with regard to the first category, our analysis highlighted that younger and older age (aPR=1.41, 95% CI, [1.12–1.77] for women age ≤24 years, 1.29 [1.08–1.55] for age 25–29 years, and 1.34 [1.06–1.71] for age ≥40 years vs 35–39 years), being born in North Africa (1.29 [1.02–1.64] vs born in France), and having a poor level of health literacy (1.23 [1.14–1.35]) were all associated with PPD. In terms of medical and mental health care, PPD was more frequent in women who had a history of psychological (1.45 [1.24–1.69] vs no such history) or psychiatric (1.52 [1.23–1.88] vs no such history) care since adolescence. Among the pregnancy-related variables, self-perceived good support (1.31 [1.15–1.48] vs very good) or little or no support (1.80 [1.51–2.14] vs very good) and a feeling of sadness, despair, or hopelessness (1.92 [1.65–2.25]), anhedonia (1.69 [1.36–2.11]), or both (2.61 [2.26–3.01]), for at least 2 weeks during their pregnancy, were also associated with a higher risk of PPD. Finally, with respect to childbirth-related characteristics, instrumental vaginal delivery was associated with a higher risk of PPD (1.18 [1.01–1.38] vs a spontaneous vaginal delivery). In contrast, child health factors were not associated with a higher risk of PPD in their mother, after adjustment for other variables (Table 3).
Sensitivity analysis. The sensitivity analysis conducted on the total sample, excluding potential early markers of antenatal depression as covariates, revealed 3 additional factors associated with PPD: country of birth (sub-Saharan Africa), absence of professional activity, and excessive weight gain (≥23 kg) during pregnancy (Supplementary Table 6).
Factors Associated with PPD Among Women with No History of Mental Health Care since Adolescence
Associated factors. The analysis performed in the subgroup of women (n=5,564) with no history of mental health care since adolescence showed the same risk factors as those for the whole study sample, with the exception of country of birth. Moreover, having a small for gestational age (SGA) infant at birth appeared to be associated with PPD, despite the 95% CI being close to 1 (Table 2 and 3).
Sensitivity analysis. The sensitivity analysis conducted on this subgroup, excluding potential early markers of antenatal depression as covariates, highlighted 4 additional factors: country of birth (North Africa), excessive weight gain (≥23 kg) during pregnancy, not having a pregnancy at low obstetrical risk, and child re-hospitalization during the 2 months after leaving the maternity ward. Conversely, having an SGA infant was no longer a risk factor for PPD at 2 months (Supplementary Table 6).
DISCUSSION
This study assessed the prevalence of PPD (EPDS score ≥13) at 2 months in France in 2021 and associated factors. Of the 7,133 women studied, 16.7% had PPD. The following factors were independently associated with a higher risk of PPD: age (with a U-shaped curve: higher prevalence in younger and older women, vs 35–39 years old), being born in North Africa (vs France), a poor level of health literacy, having a history of mental health care since adolescence (vs none), not self-perceiving very good support from loved ones during their pregnancy (vs very good support), having felt sadness/despair/hopelessness, anhedonia, or both for at least 2 weeks during the pregnancy (vs none), and having had an instrumental vaginal delivery (vs spontaneous vaginal delivery).
The prevalence of PPD estimated in this study is consistent with results from the 4 previous French studies on PPD as well as those from several international studies (ie, between 10% and 20%) based on a similar screening symptoms questionnaire.1,2,9,17,18,31 However, our result was the first representative of all the women giving birth in France and gave recent data about the prevalence of PPD and its associated factors. In our study, the EPDS is used to measure depressive symptoms at 2 months postpartum and can be completed by telephone or online depending on the woman’s preference. At the end of the questionnaire, women are invited to look for information on a website dedicated to depression or to consult a health care professional if necessary. In clinical practice, women with moderate or major symptoms of depression (EPDS score ≥10) are referred to a clinician to confirm the diagnosis.
The demographic and socioeconomic characteristics associated with PPD in our study, especially maternal age and women’s migratory status (defined as country of birth here), confirm previous findings in the literature. Specifically, other studies also showed a U-shaped curve in the association between maternal age and PPD, with women under 20 and over 40 years of age appearing to be at greater risk of PPD.12 Similarly, migration was reported as a risk factor for PPD,10,32 as were other mood disorders.33 In our study, the migrant association regarded only first-generation migrants from North Africa. This may be related to a lack of power concerning migrants from other countries and/or over-fitting. The impact of migrant status on the probability of PPD could be explained by possible difficulties for immigrant women to adapt to their new environment (eg, family/cultural distance, different access to care). Another hypothesis is that healthcare professionals could provide unequal perinatal care (such as those used by Azria et al34 in the PreCARE cohort study: the timing of the initiation of care, the number of scheduled prenatal visits, and the performance of scheduled ultrasound examinations) depending on a pregnant woman’s ethnic origin.
We found that a lower level of health literacy (more specifically, the ability to actively engage with health providers) was associated with PPD at 2 months. This is not found in previous studies. It has been shown that a better understanding of PPD—in particular its symptoms, risk factors, and treatment—could make it easier to seek early and appropriate treatment.35
Our research highlighted that a pregnancy marked by negative emotions such as sadness, despair, hopelessness, and/or anhedonia was associated with PPD at 2 months; these are key symptoms of depression. This finding underlines the well-known fact that a woman presenting depressive symptoms during pregnancy is at high risk of presenting depression postpartum.13
Self-perceived support is associated with positive mental health outcomes, whether formal (support from professionals at home, childcare) or informal (support from loved ones).11,36–38 For example, recent work by Nakamura et al38 showed, in a subsample of 15,000 mothers from the national French ELFE cohort study, that self-perceived satisfactory partner support was associated with lower EPDS scores of, respectively, 7% in non-migrant women and 11% in first-generation migrant women but not in second-generation migrant women. Our study results echo this finding.
The findings from previous studies on the impact of parity on the probability of PPD are inconsistent; while some authors showed an aggravating role of parity (primiparity or multiparity, depending on the study) in PPD onset,13,39 others did not.17 For example, 2 studies carried out in the Canadian and Finnish general populations (approximately 2,500 and 511,000 postpartum women, respectively) showed that parity was a factor predisposing to PPD (adjusted odds ratio [aOR] [95% CI] of 1.59 [1.22–2.08] and 1.24 [1.08–1.41], respectively),13,39 whereas the work of Barandon et al. on nearly 16,000 women in the ELFE survey mentioned above suggested that it was not associated (aOR [95% CI], 1.04 [0.91–1.18]).17 We also found no association between parity and PPD. Perhaps, the integration of the variable “self-perceived support during the pregnancy” (and probably during the postpartum period) in our multivariable model could explain this result.
Another important aspect of the present analysis is that we found no significant difference in the probability of developing PPD at 2 months between women who had a pregnancy at low obstetrical risk and those who did not. However, the sensitivity analysis indicated a higher probability of PPD in low obstetrical risk pregnancies. This suggests that not having a pregnancy at low obstetrical risk could lead to depressive symptoms during pregnancy and finally to a higher probability of PPD at 2 months.
Finally, in our study, instrumental vaginal delivery, but not cesarean section (whether scheduled, before labor, or during labor), was associated with a higher risk of PPD. Findings from other studies are inconsistent: some showed that cesarean section increased the risk of PPD, others highlighted the aggravating role of instrumental vaginal delivery, while others highlighted the absence of any significant impact of the mode of delivery on PPD onset.13,16,40 Our results were consistent with those of a Swedish study showing a higher risk of PPD after an instrumental delivery (adjusted relative risk=1.23 95% CI, [1.09–1.38]) in nearly 694,000 mothers without personal history of depression care16 and those of a recent French study conducted on 2,811 women who had a vaginal delivery18 (aORs of PPD in case of instrumental delivery=1.4, 95% CI, [1.0–2.0]). One hypothesis is that a childbirth which does not go as expected may lead to significant postpartum stress, providing the basis for subsequent PPD.
Interestingly, prematurity was not associated with PPD at 2 months in our study, reflecting results from the 3,310 women participating in the French IGEDEPP study. This latter showed also that newborn-related onset events (preterm, SGA, and admission to neonatal intensive care unit) were not associated to early-onset or late-onset of PPD (aOR [95% CI] of 1.2 [0.8–1.8] and 1.0 [0.6–1.6], respectively).9 A recent systematic review and meta-analysis showed that when only population-based studies were considered, it was impossible to make a definitive conclusion about a link between prematurity and PPD.41
This study has some strengths: first, it was based on a large population-based national sample, representative of women who gave birth in France in 2021.20 Second, the data had a very high quality (midwives collected data).
There are some limitations of this study: first, external validity was limited by attrition; nevertheless, we corrected this issue by applying data weighting. Second, the 2021 ENP study was conducted during the third wave of the SARS-CoV-2 pandemic in France. This may have led to an overestimation of PPD prevalence but have not biased the associations found. Third, seasonality (2-month questionnaire completed in spring) could also bias the prevalence of PPD, but not the associations found.42 Thus, even if these results obtained from a nationwide general population survey are representative of all women giving birth in France, biases may exist. Then, the prevalence of PPD at 2 months should be confirmed. Fourth, we used the EPDS, which is a screening tool, which does not take account diagnostic aspect. This may also led to an overestimation of PPD prevalence. However, the high threshold used should avoid any offset of such overestimation. Fifth, in addition to PPD, which is the most frequent perinatal mental disease, women’s mental health in general and other mental health diseases would also have been important to explore. This may be the case in a future edition of the ENP survey. Sixth, self-reporting including some retrospective assessments may subject to potential social desirability and recall biases (particularly for mental health care history). Finally, at the time of these analyses, we did not have information on the use of antidepressants during pregnancy, but future project plan to study this point after linking the 2021 ENP database with the national medico-administrative database.
In 2021, almost 1 in 6 women (delivered after at least 22 weeks of amenorrhea and/or with a live born child weighting at least 500 grams) developed PPD at 2 months in France. Although this prevalence needs to be confirmed outside a pandemic period, this highlights the importance of this disorder in the postpartum period and reinforces the need to assess psychiatric manifestations in the postnatal period (screening for all women several times during the postpartum period to facilitate early detection, diagnosis, referral, and treatment) and the fundamental nature of prevention policies. Primary prevention measures could be developed or reinforced on the basis of factors that, in our study, were associated with PPD onset at 2 months: demographic and socioeconomic factors (migrants, persons self-perceiving less than very-good support), vaginal instrumental delivery, a history of mental health care since adolescence, and depressive symptoms during pregnancy.
Article Information
Published Online: October 8, 2025. https://doi.org/10.4088/JCP.25m15818
© 2025 Physicians Postgraduate Press, Inc.
Submitted: February 3, 2025; accepted July 15, 2025.
To Cite: Doncarli A, Demiguel V, Le Ray C, et al. Depression at 2 months postpartum: results from the French National Perinatal Survey. J Clin Psychiatry 2025;86(4):25m15818.
Author Affiliations: Santé Publique France, the national public health agency, Saint-Maurice, France (Doncarli, Demiguel, Lebreton, Boudet-Berquier, Regnault, Tebeka); Université Paris Cité, Obstetric, Perinatal, Paediatric Life Course Epidemiology (OPPaLE) Research Team, Center for Research in Epidemiology and Statistics (CRESS), INSERM U1153, Paris, France (Le Ray, Deneux-Tharaux, Chantry); Maternite Port-Royal, Groupe hospitalier Paris Centre, AP−HP, Université Paris Cité, FHU Prema, Paris, France (Le Ray); Service de Psychiatrie Périnatale et de l’Enfant, Groupe Hospitalier du Havre; Université de Rouen Normandie, Le Havre, France (Apter) ; Department of Gynecology and Obstetrics, Louis Mourier University Hospital, AP-HP, Colombes, France (Crenn-Hebert); Regional Health Agency of Ile de France (ARS-IDF), Saint-Denis, France (Crenn-Hebert); Audipog, Claude Bernard Lyon 1 University, Lyon, France (Crenn-Hebert); Consultation d’Information, de Conseils et d’Orientation des femmes suivies pour troubles psychiques, enceintes, ou avec désir d’enfant (CICO), GHU Paris Psychiatrie et Neurosciences, Hôpital Saint-Anne, Paris, France (Vacheron).
ENP 2021 Study Group Members: Camille Le Raya, Nathalie Lelonga, Hélène Cinellia, Béatrice Blondela, Nolwenn Regnaultb, Virginie Demiguelb, Elodie Lebretonb, Benoit Salanaveb, Jeanne Fressonc, Annick Vilainc, Thomas Deroyonc, Philippe Raynaudc, Sylvie Reyc, Khadoudja Chemlald, Nathalie Rabier-Thoreaud, Frédérique Collombet-Migeone.
aObstetric, Perinatal, Paediatric Life Course Epidemiology (OPPaLE) Research Team, Paris, France; bSanté publique France, the national public health agency, Saint-Maurice, France; cThe Directorate for Research, Studies, Assessment and Statistics (DREES), Ministry for Health and Solidarity, Paris, France; dThe Directorate-General for health (DGS), Ministry for Health and Solidarity, Paris, France; eThe Directorate of Health Care Supply (DGOS), Ministry for Health and Solidarity, Paris, France
Corresponding Author: Alexandra Doncarli, PhD, Non-Communicable Diseases and Trauma Division, Perinatology, Early Childhood and Mental Health Unit, Santé Publique France, 14, rue du Val d’Osne 94415 Saint-Maurice, France ([email protected]).
Relevant Financial Relationships: The authors declare that they have no competing interests.
Funding/Support: This work was entirely financed by public funds (Ministry of Health). The funder is a member of the ENP 2021 Study Group and as such has the right to review the articles using 2021 ENP data.
Acknowledgment: The authors thank the Maternal and Child Health Services in each of France’s regions, as well as the regional perinatal networks and regional health agencies, which are key collaborators in the repeated ENP study. We also thank all the local investigators who rigorously collect ENP data in each maternity ward and all the mothers who agreed to participate. Finally, we thank the relevant department heads and midwife coordinators for conducting the 2021 ENP in their units, Isabelle Monier for her valuable collaboration in the creation of the pregnancy at low obstetrical risk variable, and Jude Sweeney (Milan, Italy) for English revision and copyediting of the manuscript.
Data Availability Statement: The dataset contains individual data potentially identifying or sensitive patient information (childbirth’s data, age, parity, region of residence, violence, chronic diseases…). It cannot therefore be shared publicly. However, researchers who meet the criteria for access to confidential data can request access to these data by writing to [email protected].
ORCID: Alexandra Doncarli: https://orcid.org/0000-0002-0691-9136
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- One in 6 women seen in clinical practice who have given birth to a liveborn child may have postpartum depression at 2 months postpartum. The Edinburgh Postnatal Depression Scale can be used to assess this.
- Clinicians should watch for the following characteristics in postpartum women: depressive symptoms during pregnancy, history of mental health care, inadequate self-perceived support, low health literacy, young/advanced maternal age, migrant background, and non-spontaneous vaginal delivery.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Women’s Mental Health section. Please contact Marlene P. Freeman, MD, at Psychiatrist.com/contact/freeman.
References (42)

- Howard LM, Molyneaux E, Dennis CL, et al. Non-psychotic mental disorders in the perinatal period. Lancet. 2014;384(9956):1775–1788. PubMed CrossRef
- Woody CA, Ferrari AJ, Siskind DJ, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86–92. PubMed CrossRef
- Tebeka S, Le Strat Y, De Premorel Higgons A, et al. Prevalence and incidence of postpartum depression and environmental factors: the IGEDEPP cohort. J Psychiatr Res. 2021;138:366–374. PubMed CrossRef
- Vacheron MN, Tessier V, Rossignol M, Deneux-Tharaux Comité National d’Experts sur la Mortalité Maternelle, Experts sur la Mortalite. [Maternal deaths due to suicide in France 2013-2015]. Gynecol Obstet Fertil Senol. 2021;49(1):38–46. PubMed CrossRef
- Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247–253. PubMed CrossRef
- Slomian J, Honvo G, Emonts P, et al. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Womens Health (Lond). 2019;15:1745506519844044. PubMed CrossRef
- Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. 2019;52:165–180. PubMed CrossRef
- Robertson E, Grace S, Wallington T, et al. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. Jul-Aug 2004;26(4):289–295. PubMed CrossRef
- Tebeka S, Le Strat Y, Mandelbrot L, et al. Early- and late-onset postpartum depression exhibit distinct associated factors: the IGEDEPP prospective cohort study. BJOG. 2021;128(10):1683–1693. PubMed CrossRef
- van der Waerden J, Galéra C, Saurel-Cubizolles MJ, et al. Predictors of persistent maternal depression trajectories in early childhood: results from the EDEN mother- child cohort study in France. Psychol Med. 2015;45(9):1999–2012. PubMed CrossRef
- Bales M, Pambrun E, Maguet C, et al. Pathways between risk/Protective factors and maternal postnatal depressive symptoms: the ELFE cohort. J Clin Med. 2023;12(9):3204. PubMed CrossRef
- Guintivano J, Manuck T, Meltzer-Brody S. Predictors of postpartum depression: a Comprehensive review of the Last decade of Evidence. Clin Obstet Gynecol. 2018;61(3):591–603. PubMed CrossRef
- Räisänen S, Lehto SM, Nielsen HS, et al. Fear of childbirth predicts postpartum depression: a population-based analysis of 511 422 singleton births in Finland. BMJ Open. 2013;3(11):e004047. PubMed
- Wittkowski A, Patel S, Fox JR. The experience of postnatal depression in immigrant mothers living in Western countries: a meta-synthesis. Clin Psychol Psychother. 2017;24(2):411–427. PubMed CrossRef
- Munk-Olsen T, Laursen TM, Pedersen CB, et al. New parents and mental disorders: a population-based register study. JAMA. 2006;296(21):2582–2589. PubMed CrossRef
- Silverman ME, Reichenberg A, Savitz DA, et al. The risk factors for postpartum depression: a population-based study. Depress Anxiety. 2017;34(2):178–187. PubMed CrossRef
- Barandon S, Balès M, Pambrun E, et al. Maternal post-natal depressive symptoms at 2 months: effects of French antenatal preventive measures in the E.L.F.E. cohort. J Affect Disord. 2021;293:238–244. PubMed CrossRef
- Froeliger A, Deneux-Tharaux C, Loussert L, et al. Prevalence and risk factors for postpartum depression 2 months after a vaginal delivery: a prospective multicenter study. Am J Obstet Gynecol. 2024;230(3S):S1128–S1137.e6. PubMed CrossRef
- Heude B, Forhan A, Slama R, et al. Cohort Profile: the EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int J Epidemiol. 2016;45(2):353–363. PubMed CrossRef
- Le Ray C, Lelong N, Cinelli H, et al. Results of the 2021 French National Perinatal Survey and trends in perinatal health in metropolitan France since 1995. J Gynecol Obstet Hum Reprod. 2022;51(10):102509. PubMed CrossRef
- Cinelli H, Lelong N, Le Ray C. Collaborators - members of the ENPSG. In: Situation and trends since 2016. French National Perinatal Survey 2021: Births, 2-Month Follow-Up And Establishments; 2022. https://enp.inserm.fr/wp-content/uploads/2023/09/Rapport2021_Anglais.pdf
- Shorey S, Chee CYI, Ng ED, et al. Prevalence and incidence of postpartum depression among healthy mothers: a systematic review and meta-analysis. J Psychiatr Res. 2018;104:235–248. PubMed CrossRef
- Guedeney N, Fermanian J. Validation study of the French version of the Edinburgh Postnatal Depression Scale (EPDS): new results about use and psychometric properties. Eur Psychiatry. 1998;13(2):83–89. PubMed CrossRef
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression Scale. Br J Psychiatry. 1987;150:782–786. PubMed CrossRef
- Levis B, Negeri Z, Sun Y, et al. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ. 2020;371:m4022. PubMed CrossRef
- Osborne RH, Batterham RW, Elsworth GR, et al. The grounded psychometric development and initial validation of the Health Literacy Questionnaire (HLQ). BMC Public Health. 2013;13:658. PubMed CrossRef
- Ego A, Prunet C, Blondel B, et al. [Customized and non-customized French intrauterine growth curves. II - comparison with existing curves and benefits of customization]. J Gynecol Obstet Biol Reprod. 2016;45(2):165–176. PubMed CrossRef
- Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. PubMed CrossRef
- Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3(1):21. PubMed CrossRef
- van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. PubMed CrossRef
- Fritel X, Gachon B, Saurel-Cubizolles MJ, et al. Postpartum psychological distress associated with anal incontinence in the EDEN mother-child cohort. BJOG. 2020;127(5):619–627. PubMed CrossRef
- El-Khoury F, Sutter-Dallay AL, Panico L, et al. Women’s mental health in the perinatal period according to migrant status: the French representative ELFE birth cohort. Eur J Public Health. 2018;28(3):458–463. PubMed CrossRef
- Pignon B, Geoffroy PA, Thomas P, et al. Prevalence and clinical severity of mood disorders among first-second- and third-generation migrants. J Affect Disord. 2017;210:174–180. PubMed CrossRef
- Azria E, Sauvegrain P, Anselem O, et al. Racial implicit biases among obstetric care providers and associated differential care: the BiP research program. Am J Obstet Gynecol. 2023;228(1):S36–S37. CrossRef
- Branquinho M, Canavarro MC, Fonseca A. Postpartum depression in the Portuguese population: the role of Knowledge, Attitudes and Help-Seeking Propensity in Intention to Recommend professional Help-Seeking. Community Ment Health J. 2020;56(8):1436–1448. PubMed CrossRef
- Leung BM, Letourneau NL, Giesbrecht GF, et al. Predictors of postpartum depression in partnered mothers and Fathers from a Longitudinal cohort. Community Ment Health J. 2017;53(4):420–431. PubMed CrossRef
- Massoudi P, Hwang CP, Wickberg B. Fathers’ depressive symptoms in the postnatal period: prevalence and correlates in a population-based Swedish study. Scand J Public Health. 2016;44(7):688–694. PubMed CrossRef
- Nakamura A, El-Khoury Lesueur F, Sutter-Dallay AL, et al. The role of prenatal social support in social inequalities with regard to maternal postpartum depression according to migrant status. J Affect Disord. 2020;272:465–473. PubMed
- Sword W, Landy CK, Thabane L, et al. Is mode of delivery associated with postpartum depression at 6 weeks: a prospective cohort study. BJOG. 2011;118(8):966–977. PubMed CrossRef
- Lydon-Rochelle MT, Holt VL, Martin DP. Delivery method and self-reported postpartum general health status among primiparous women. Paediatr Perinat Epidemiol. 2001;15(3):232–240. PubMed CrossRef
- de Paula Eduardo JAF, de Rezende MG, Menezes PR, et al. Preterm birth as a risk factor for postpartum depression: a systematic review and meta-analysis. J Affect Disord. 2019;259:392–403. PubMed CrossRef
- Henriksson HE, Sylvén SM, Kallak TK, et al. Seasonal patterns in self-reported peripartum depressive symptoms. Eur Psychiatry. 2017;43:99–108. PubMed CrossRef
This PDF is free for all visitors!